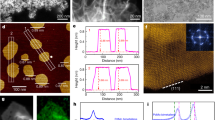
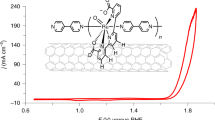
Abstract
Water oxidation is a key reaction for the conversion of solar energy into chemical fuels, but effective water-oxidation catalysts are often based on rare and costly precious metals such as Pt, Ir or Ru. Developing strategies based on earth-abundant metals is important to explore critical aspects of this reaction, and to see whether different and more efficient applications are possible for energy systems. Herein, we present an approach to tuning a redox-active electrocatalyst based on the doping of molybdenum into the tungsten framework of [Co4(H2O)2(PW9O34)2]10–, known as the Weakley sandwich. The Mo-doped framework was confirmed by X-ray crystallography, electrospray ionization mass spectrometry and inductively coupled plasma optical emission spectrometry studies. The doping of molybdenum into the robust Weakley sandwich framework leads to the oxidation of water at a low onset potential, and with no catalyst degradation, whereby the overpotential of the oxygen evolution reaction is lowered by 188 mV compared with the pure tungsten framework.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 231, 1072–1075 (2008).
Young, K. J. et al. Light-driven water oxidation for solar fuels. Coord. Chem. Rev. 256, 2503–2520 (2012).
Meyer, T. J. Catalysis: the art of splitting water. Nature 451, 778–779 (2008).
Eisenberg, R. & Gray, H. B. Preface on making oxygen. Inorg. Chem. 47, 1697–1699 (2008).
Mazloomi, K. & Gomes, C. Hydrogen as an energy carrier: prospects and challenges. Renew. Sustain. Energy Rev. 16, 3024–3033 (2012).
Dau, H. et al. The mechanism of water oxidation: from electrolysis via homogeneous to biological catalysis. ChemCatChem 2, 724–761 (2010).
Zou, X. & Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 44, 5148–5180 (2015).
Slavcheva, E. et al. Sputtered iridium oxide films as electrocatalysts for water splitting via PEM electrolysis. Electrochim. Acta 52, 3889–3894 (2007).
Fillol, J. L. et al. Efficient water oxidation catalysts based on readily available iron coordination complexes. Nat. Chem. 3, 807–813 (2011).
Ruttinger, W. & Dismukes, G. C. Synthetic water-oxidation catalysts for artificial photosynthetic water oxidation. Chem. Rev. 97, 1–24 (1997).
Wasylenko, D. J., Palmer, R. D. & Berlinguette, C. P. Homogeneous water oxidation catalysts containing a single metal site. Chem. Commun. 49, 218–227 (2013).
Cole-Hamilton, D. J. Homogeneous catalysis—new approaches to catalyst separation, recovery, and recycling. Science 299, 1702–1706 (2003).
Evangelisti, F., Güttinger, R., Moré, R., Luber, S. & Patzke, G. R. Closer to photosystem II: a Co4O4 cubane catalyst with flexible ligand architecture. J. Am. Chem. Soc. 135, 18734–18737 (2013).
Brimblecombe, R., Swiegers, G. F., Dismukes, G. C. & Spiccia, L. Sustained water oxidation photocatalysis by a bioinspired manganese cluster. Angew. Chem. Int. Ed. 47, 7335–7338 (2008).
Dismukes, G. C. et al. Development of bioinspired Mn4O4-cubane water oxidation catalysts: lessons from photosynthesis. Acc. Chem. Res. 42, 1935–1943 (2009).
Enthaler, S., Junge, K. & Beller, M. Sustainable metal catalysis with iron: from rust to a rising star? Angew. Chem. Int. Ed. 47, 3317–3321 (2008).
Ellis, W. C., McDaniel, N. D., Bernhard, S. & Collins, T. J. Fast water oxidation using iron. J. Am. Chem. Soc. 132, 10990–10991 (2010).
Evangelisti, F., Car, P. E., Blacque, O. & Patzke, G. R. Photocatalytic water oxidation with cobalt-containing tungstobismutates: tuning the metal core. Catal. Sci. Technol. 3, 3117–3129 (2013).
Lv, H. et al. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 41, 7572–7589 (2012).
Yin, Q. et al. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328, 342–345 (2010).
Huang, Z. et al. Efficient light-driven carbon-free cobalt-based molecular catalyst for water oxidation. J. Am. Chem. Soc. 133, 2068–2071 (2011).
Weakley, T. J. R., Evans, H. T., Showell, J. S., Tourné, G. F. & Tourné, C. M. 18-Tungstotetracobalto(11)diphosphate and related anions: a novel structural class of heteropolyanions. J. Chem. Soc. Chem. Commun. 139, 140 (1973).
Han, X. B. et al. Polyoxometalate-based cobalt-phosphate molecular catalysts for visible light-driven water oxidation. J. Am. Chem. Soc. 136, 5359–5366 (2014).
Goberna-Ferrón, S., Vigara, L., Soriano-López, J. & Galán-Mascarós, J. R. Identification of a nonanuclear {Coii 9} polyoxometalate cluster as a homogeneous catalyst for water oxidation. Inorg. Chem. 46, 11707–11715 (2012).
Zhu, G. et al. Water oxidation catalyzed by a new tetracobalt-substituted polyoxometalate complex: [{Co4(μ-OH)(H2O)3}(Si2W19O70)]11−. Dalton Trans. 41, 2084–2090 (2012).
Tanaka, S. et al Visible light-induced water oxidation catalyzed by molybdenum-based polyoxometalates with mono- and dicobalt(iii) cores as oxygen-evolving centers. Chem. Commun. 48, 1653–16559 (2012).
Sartorel, A., McDaniel, N. D., Bernhard, S. & Bonchio, M. Polyoxometalate embedding of a tetraruthenium(iv)-oxo-core by template-directed metalation of [γ-SiW10O36]8−: a totally inorganic oxygen-evolving catalyst. J. Am. Chem. Soc. 130, 5006–5007 (2008).
Sartorel, A. et al. Water oxidation at tetraruthenate core stabilized by polyoxometalated ligands: experimental and computational evidence to trace the competente intermediates. J. Am. Chem. Soc. 131, 16051–16053 (2009).
Zhang., B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016).
Stracke, J. J. & Finke, R. G. Electrocatalytic water oxidation beginning with the cobalt polyoxometalate [Co4(H2O)2(PW9O34)2]10–: identification of heterogeneous CoO x as the dominant catalyst. J. Am. Chem. Soc. 133, 14872–14875 (2011).
Concepcion, J. J., Binstead, R. A., Alibabaei, L. & Meyer, T. J. Application of the rotating ring-disc-electrode technique to water oxidation by surface-bound molecular catalysts. Inorg. Chem. 52, 10744–10746 (2013).
Zhong, D. K., Zhao, S. L., Polyansky, D. E. & Fujita, E. Diminished photoisomerization of active ruthenium water oxidation catalyst by anchoring to metal oxide electrodes. J. Catal. 307, 140–147 (2013).
Klepser, B. M. & Bartlett, B. M. Anchoring a molecular iron catalyst to solar-responsive WO3 improves the rate and selectivity of photoelectrochemical water oxidation. J. Am. Chem. Soc. 136, 1694–1697 (2014).
Soriano-López, J. et al. Cobalt polyoxometalates as heterogeneous water oxidation catalysts. Inorg. Chem. 52, 4753–4755 (2013).
Soriano-López, J. et al. Tetracobalt-polyoxometalate catalysts for water oxidation: key mechanistic details. J. Catal. 350, 56–63 (2017).
Song, F. et al. {Co4O4} and {Co x Ni4−xO4} cubane water oxidation catalysts as surface cut-outs of cobalt oxides. J. Am. Chem. Soc. 139, 14198–14208 (2017).
Li, S. et al. Rare sandwich-type polyoxomolybdates constructed form di/tetra-nuclear transition-metal clusters and trivacant keggin germanomolybdate fragments. Inorg. Chem. 48, 9819–9830 (2009).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A64, 112–122 (2008).
Müller, P. Crystal Structure Refinement: A Crystallographer’s Guide to SHELXL (Oxford Univ. Press, Oxford, 2006).
Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 45, 849–854 (2012).
Acknowledgements
We acknowledge J. Mathieson and D. Castro-Spencer for their kind help and support in acquiring the ESI-IM-MS data. We thank L. MacDonald for offering supportive discussion on the electrochemistry. We also thank J. M. Poblet and J. Carbò for helpful discussion on the POM design, characterization and electrocatalysis. We gratefully acknowledge financial support from the Engineering and Physical Sciences Research Council (grant nos EP/H024107/1, EP/J015156/1, EP/K021966/1, EP/L015668/1, EP/L023652/1), the European Research Council (project 670467 SMART-POM) and the University of Glasgow. This work was partially funded by the Spanish Ministerio de Economia y Competitividad (MINECO) through projects CTQ2015-71287-R and the Severo Ochoa Excellence Accreditation 2014-2018 SEV-2013-0319; the Generalitat de Catalunya (2014-SGR-797) and the Centres de Recera de Catalunya Programme/Generalitat de Catalunya. We also thank Chemistry and Molecular Sciences and Technologies European Cooperation in Science & Technology Action CM1203.
Author information
Authors and Affiliations
Contributions
L.C. conceived the original concept and both L.C. and J.R.G.-M. designed the project and together with L.V.-N. coordinated the efforts of the research team. M.M.-S. and J.S.-L. contributed equally. M.M.-S. and J.S.-L. synthesized the compounds, M.M.-S. characterized the compounds electrochemically, analysed the ESI-IM-MS and determined the formula for each compound. J.S.-L. measured the oxygen evolution. R.S.W. and J.-J.C. supervised directly the electrochemistry and the synthetic work and characterization analysis. D.-L.L. finalized the X-ray structures. M.M.-S., L.V.-N. and L.C. co-wrote the paper with input from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–10, Supplementary Tables 1–19, Supplementary References
Rights and permissions
About this article
Cite this article
Martin-Sabi, M., Soriano-López, J., Winter, R.S. et al. Redox tuning the Weakley-type polyoxometalate archetype for the oxygen evolution reaction. Nat Catal 1, 208–213 (2018). https://doi.org/10.1038/s41929-018-0037-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0037-1
This article is cited by
-
Polyoxometalates for continuous power generation by atmospheric humidity
Nano Research (2024)
-
Single-dispersed polyoxometalate clusters embedded on multilayer graphene as a bifunctional electrocatalyst for efficient Li-S batteries
Nature Communications (2022)
-
Spontaneously separated intermetallic Co3Mo from nanoporous copper as versatile electrocatalysts for highly efficient water splitting
Nature Communications (2020)
-
Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation
Nature Communications (2020)
-
High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics
Nature Catalysis (2020)