Abstract
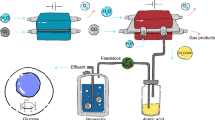
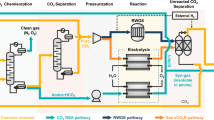
Solar-powered electrochemical reduction of CO2 and H2O to syngas, followed by fermentation, could lead to sustainable production of useful chemicals. However, due to insufficient electric current densities and instabilities of current CO2-to-CO electrolysers, a practical, scalable artificial photosynthesis remains a major challenge. Here, we address these problems using a commercially available silver-based gas diffusion electrode (used in industrial-scale chlorine–alkaline electrolysis) as the cathode in the CO2 electrolyser. Electric current densities up to 300 mA cm–2 were demonstrated for more than 1,200 hours with continuous operation. This CO2 electrolyser was coupled to a fermentation module, where the out-coming syngas from the CO2 electrolyser was converted to butanol and hexanol with high carbon selectivity. Conversion of photovoltaic electricity, CO2 and H2O to the desired alcohols achieved close to 100% Faradaic efficiency. Industrial production of useful and high-value chemicals via artificial photosynthesis is closer than expected with the proposed scalable hybrid system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Malveda, M. P., Liu, S., Passararat, S. & Sesto, B. Chemical Economics Handbook: Plasticizer Alcohols (C 4 –C 13 ) 8,86 (IHS Chemical, 2015).
Kim, D., Sakimoto, K. K., Hong, D. C. & Yang, P. D. Artificial photosynthesis for sustainable fuel and chemical production. Angew. Chem. Int. Ed. 54, 3259–3266 (2015).
Ganesh, I. Solar fuels vis-a-vis electricity generation from sunlight: the current state-of-the-art (a review). Renew. Sust. Energ. Rev. 44, 904–932 (2015).
Karkas, M. D., Verho, O., Johnston, E. V. & Akermark, B. Artificial photosynthesis: molecular systems for catalytic water oxidation. Chem. Rev. 114, 11863–12001 (2014).
Scott, E. L., Bruins, M. E. & Sanders, J. P. M. Rules for the Bio-Based Production of Bulk Chemicals on a Small Scale: Can the Production of Bulk Chemicals on Small Scale be Competitive? 1–36 (Agrotechnology and Food Science Group, Wageningen UR/Biobased Commodity Chemistry, 2013).
Clomburg, J. M., Crumbley, A. M. & Gonzalez, R. Industrial biomanufacturing: the future of chemical production. Science 355, aag0804 (2017).
Woo, H. M. Solar-to-chemical and solar-to-fuel production from CO2 by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 45, 1–7 (2017).
Jiao, F. et al. Selective conversion of syngas to light olefins. Science 351, 1065–1068 (2016).
Polman, A., Knight, M., Garnett, E. C., Ehrler, B. & Sinke, W. C. Photovoltaic materials: present efficiencies and future challenges. Science 352, aad4424 (2016).
Liew, F. et al. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metabol. Eng. 40, 104–114 (2017).
Mock, J. et al. Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum involving electron bifurcation. J. Bacteriol. 197, 2965–2980 (2015).
Dürre, P. Butanol formation from gaseous substrates. FEMS Microbiol. Lett. 363, fnw040 (2016).
von der Assen, N., Muller, L. J., Steingrube, A., Voll, P. & Bardow, A. Selecting CO2 sources for CO2 utilization by environmental-merit-order curves. Environ. Sci. Technol. 50, 1093–1101 (2016).
Hori, Y. & Suzuki, S. Electrolytic reduction of bicarbonate ion at a mercury-electrode. J. Electrochem. Soc. 130, 2387–2390 (1983).
Asadi, M. et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 353, 467–470 (2016).
Gao, S. et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 529, 68–71 (2016).
Neubauer, S. S., Krause, R. K., Schmid, B., Guldi, D. M. & Schmid, G. Overpotentials and Faraday efficiencies in CO2 electrocatalysis-the impact of 1-ethyl-3-methylimidazolium trifluoromethanesulfonate. Adv. Energy Mater. 6, 1502231 (2016).
Verma, S., Lu, X., Ma, S. C., Masel, R. I. & Kenis, P. J. A. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag-based gas diffusion electrodes. Phys. Chem. Chem. Phys. 18, 7075–7084 (2016).
Aoi, S., Mase, K., Ohkubo, K. & Fukuzumi, S. Selective electrochemical reduction of CO2 to CO with a cobalt chlorin complex adsorbed on multi-walled carbon nanotubes in water. Chem. Commun. 51, 10226–10228 (2015).
Kang, P., Chen, Z. F., Brookhart, M. & Meyer, T. J. Electrocatalytic reduction of carbon dioxide: let the molecules do the work. Top. Catal. 58, 30–45 (2015).
Dufek, E. J., Lister, T. E., Stone, S. G. & McIlwain, M. E. Operation of a pressurized system for continuous reduction of CO2. J. Electrochem. Soc. 159, F514–F517 (2012).
Schreier, M. et al. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. Nat. Commun. 6, 7326 (2015).
Schreier, M. et al. Solar conversion of CO2 to CO using Earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat. Energy 2, 17087 (2017).
Turek, T., Moussallem, I., Bulan, A., Schmitz, N. & Weuta, P. Oxygen-consuming electrode with multilayer catalytic coating and process for the production thereof. US patent 9,243,337 B2 (2016).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 355, eaad4998 (2017).
Hatsukade, T., Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. Insights into the electrocatalytic reduction of CO2 on metallic silver surfaces. Phys. Chem. Chem. Phys. 16, 13814–13819 (2014).
Mazloomi, K., Sulaiman, N. B. & Moayedi, H. Electrical efficiency of electrolytic hydrogen production. Int. J. Electrochem. Sci. 7, 3314–3326 (2012).
Liew, F. et al. Gas fermentation: a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front. Microbiol. 7, 27242719 (2016).
Angenent, L. T. et al. Chain elongation with reactor microbiomes: open-culture biotechnology to produce biochemicals. Environ. Sci. Technol. 50, 2796–2810 (2016).
Bertsch, J. & Müller, V. Bioenergetic constraints for conversion of syngas to biofuels in acetogenic bacteria. Biotechnol. Biofuels 8, 26692897 (2015).
Wang, S. N. et al. NADP-specific electron-bifurcating [FeFe]-hydrogenase in a functional complex with formate dehydrogenase in Clostridium autoethanogenum grown on CO. J. Bacteriol. 195, 4373–4386 (2013).
Jones, S. W. et al. CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion. Nat. Commun. 7, 12800 (2016).
Torella, J. P. et al. Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system. Proc. Natl Acad. Sci. USA 112, 2337–2342 (2015).
Liu, C., Colon, B. C., Ziesack, M., Silver, P. A. & Nocera, D. G. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352, 1210–1213 (2016).
Li, H. et al. Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335, 1596–1596 (2012).
Seedorf, H. et al. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc. Natl Acad. Sci. USA 105, 2128–2133 (2008).
Li, F. et al. Coupled ferredoxin and crotonyl coenzyme a (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. J. Bacteriol. 190, 843–850 (2008).
Perez, J. M., Richter, H., Loftus, S. E. & Angenent, L. T. Biocatalytic reduction of short-chain carboxylic acids into their corresponding alcohols with syngas fermentation. Biotechnol. Bioeng. 110, 1066–1077 (2013).
Phillips, J. R. et al. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: medium development and culture techniques. Bioresource Technol. 190, 114–121 (2015).
Isom, C. E., Nanny, M. A. & Tanner, R. S. Improved conversion efficiencies for n-fatty acid reduction to primary alcohols by the solventogenic acetogen “Clostridium ragsdalei”. J. Ind. Microbiol. Biotechnol. 42, 29–38 (2015).
Napora-Wijata, K., Strohmeier, G. A. & Winkler, M. Biocatalytic reduction of carboxylic acids. Biotechnol. J. 9, 822–843 (2014).
Choi, J. I. & Lee, S. Y. Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess. Eng. 17, 335–342 (1997).
Hermann, T. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 104, 155–172 (2003).
Bohlmann, G. M. & Bray, R. Biobutanol Report No. 264 (SRI Consulting, Menlo Park, CA, 2008).
Bohlmann, G. M. & Cesar, M. A. Ethanol Production in Brazil Report No. 149A (SRI Consulting, Menlo Park, CA, 2006).
Bell, S. Bio-Based Succinic Acid (IHS, 2014).
Thauer, R. K., Jungerma., K., Henninge., H., Wenning, J. & Decker, K. Energy metabolism of Clostridium kluyveri. Eur. J. Biochem 4, 173–180 (1968).
Reports & Markets: Global and Chinese Natural Hexyl Alcohols Report No. CAS 111-27-3 (360 Market Updates, Pune, 2016).
Camara Greiner, E. O., Blagoev, M. & Yamaguchi, Y. Chemical Economics Handbook: Linear Alpha-Olefines (IHS Chemical, 2013).
Schink, B., Kremer, D. R. & Hansen, T. A. Pathway of propionate formation from ethanol in Pelobacter propionicus. Arch. Microbiol. 147, 321–327 (1987).
Hu, P. et al. Integrated bioprocess for conversion of gaseous substrates to liquids. Proc. Natl Acad. Sci. USA 113, 3773–3778 (2016).
Li, X., Trevethick, S. & Cossey, B. J. Improved fermentation of gaseous substrates. Patent WO 2015/016722 A1 (2015).
Jörissen, J., Turek, T. & Weber, R. A silver-based oxygen depolarized cathode (ODC). Chemie in unserer Zeit 45, 172–183 (2011).
Dufek, E. J., Lister, T. E. & McIlwain, M. E. Influence of S-contamination on CO2 reduction at Ag electrodes. J. Electrochem. Soc. 158, B1384–B1390 (2011).
Thauer, R. K., Jungermann, K. & Decker, K. Energy-conservation in chemotropic anaerobic bacteria. Bacteriol. Rev. 41, 100–180 (1977).
Haegel, N. M. et al. Terawatt-scale photovoltaics: trajectories and challenges. Science 356, 141–143 (2017).
Xu, G. et al. An improved CO2 separation and purification system based on cryogenic separation and distillation theory. Energies 7, 3484–3502 (2014).
Service, R. F. Cost of carbon capture drops, but does anyone want it? Science 354, 1362–1363 (2016).
Diender, M., Stams, A. J. M. & Sousa, D. Z. Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels 9, 27042211 (2016).
Acknowledgements
The authors thank R. K. Thauer (Max Planck Institute for Terrestrial Microbiology, Marburg) for his help in preparing the manuscript. Evonik Creavis GmbH (T.H. and M.D.), Siemens AG (R.K. and G.S.) and Covestro AG (R.W.) thank the German Federal Ministry of Education and Research (BMBF) for funding part of this work within the Kopernikus Initiative ‘Power-to-X’ under contract number P2X-03SFK2J0.
Author information
Authors and Affiliations
Contributions
G.S. and R.W. discovered the potential of oxygen depolarized cathodes (ODC) for electrochemical CO2-reduction. T.H. and M.D. are responsible for the fermentation part. G.S. and R.K. are responsible for the electrochemical part. M.D. and R.K. performed the laboratory work. G.S. and T.H. are heading the corresponding technology programmes at Siemens AG and Evonik Creavis GmbH, respectively, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Supplementary Table 1
Rights and permissions
About this article
Cite this article
Haas, T., Krause, R., Weber, R. et al. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat Catal 1, 32–39 (2018). https://doi.org/10.1038/s41929-017-0005-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-017-0005-1
This article is cited by
-
Tandem reactors and reactions for CO2 conversion
Nature Chemical Engineering (2024)
-
Cascade electrolysis and thermocatalysis: a reliable system for upgrading C1 to C4 hydrocarbons
Rare Metals (2024)
-
Identifying and alleviating the durability challenges in membrane-electrode-assembly devices for high-rate CO electrolysis
Nature Catalysis (2023)
-
In operando NMR investigations of the aqueous electrolyte chemistry during electrolytic CO2 reduction
Communications Chemistry (2023)
-
A CO2 electrolyzer tandem cell system for CO2-CO co-feed valorization in a Ni-N-C/Cu-catalyzed reaction cascade
Nature Communications (2023)