Abstract
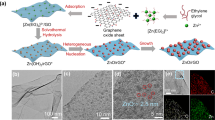
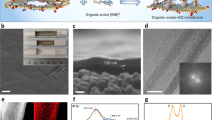
The demands of clean water production and wastewater recycling continue to drive nanofiltration membrane development. Graphene oxide (GO) membranes have exhibited the potential to revolutionize nanofiltration, but sustaining high solute rejections at realistic concentrations remains a major challenge. Here we show that a series of membranes based on GO bound to polycyclic π-conjugated cations such as toluidine blue O show substantially enhanced rejections for salts and neutral solutes over a wide concentration range. The observed solute rejection behaviours in these π-intercalated GO membranes can be understood by a dual mechanism of interlayer spacing modulation and creation of diffusion barriers in the two-dimensional interlayer galleries. These membranes are easily scalable and possess good chemical and mechanical robustness in desalination of a multicomponent industrial stream at elevated pH, temperature, stream velocity and solids content.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information files. Raw instrumental characterization data (X-ray diffraction, UV-vis and fluorescence spectra) are shown graphically. The numerical versions of these data are available from the corresponding author upon request.
References
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017).
Nair, R. R., Wu, H. A., Jayaram, P. N., Grigorieva, I. V. & Geim, A. K. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 335, 442–444 (2012).
Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 343, 740–742 (2014).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Abraham, J. et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 12, 546–550 (2017).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 380–383 (2017).
Homaeigohar, S. & Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 9, e427 (2017).
Morelos-Gomez, A. et al. Effective NaCl and dye rejection of hybrid graphene oxide/graphene layered membranes. Nat. Nanotechnol. 12, 1083–1088 (2017).
Rashidi, F., Kevlich, N. S., Sinquefield, S. A., Shofner, M. L. & Nair, S. Graphene oxide membranes in extreme operating environments: concentration of Kraft black liquor by lignin retention. ACS Sustain. Chem. Eng. 5, 1002–1009 (2017).
Wang, Z., Ma, C., Sinquefield, S. A., Shofner, M. L. & Nair, S. High-Performance graphene oxide nanofiltration membranes for black liquor concentration. ACS Sustain. Chem. Eng. 7, 14915–14923 (2019).
Yang, Q. et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nat. Mater. 16, 1198–1202 (2017).
Akbari, A. et al. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat. Commun. 7, 10891 (2016).
Han, Y., Jiang, Y. & Gao, C. High-Flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes. ACS Appl. Mater. Interfaces 7, 8147–8155 (2015).
Yuan, S. et al. Minimizing non-selective nanowrinkles of reduced graphene oxide laminar membranes for enhanced NaCl rejection. Environ. Sci. Technol. Lett. 7, 273–279 (2020).
Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 86, 119–126 (2012).
Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 88, 1475–1498 (1988).
Han, Y., Xu, Z. & Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 23, 3693–3700 (2013).
Hu, M. & Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 47, 3715–3723 (2013).
Yang, J. et al. Self-assembly of thiourea-crosslinked graphene oxide framework membranes toward separation of small molecules. Adv. Mater. 30, 1705775 (2018).
Xu, X.-L. et al. Graphene oxide nanofiltration membranes stabilized by cationic porphyrin for high salt rejection. ACS Appl. Mater. Interfaces 8, 12588–12593 (2016).
Jia, Z., Wang, Y., Shi, W. & Wang, J. Diamines cross-linked graphene oxide free-standing membranes for ion dialysis separation. J. Membr. Sci. 520, 139–144 (2016).
El-Kady, M. F., Shao, Y. & Kaner, R. B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 1, 16033 (2016).
Sun, P., Wang, K. & Zhu, H. Recent developments in graphene-based membranes: structure, mass-transport mechanism and potential applications. Adv. Mater. 28, 2287–2310 (2016).
Fornasiero, F. et al. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl Acad. Sci. USA 105, 17250–17255 (2008).
Childress, A. E. & Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 119, 253–268 (1996).
Bartels, C., Franks, R., Rybar, S., Schierach, M. & Wilf, M. The effect of feed ionic strength on salt passage through reverse osmosis membranes. Desalination 184, 185–195 (2005).
Wu, Y., Tam, N. F. Y. & Wong, M. H. Effects of salinity on treatment of municipal wastewater by constructed mangrove wetland microcosms. Mar. Pollut. Bull. 57, 727–734 (2008).
Konkena, B. & Vasudevan, S. Understanding aqueous dispersibility of graphene oxide and reduced graphene oxide through pKa measurements. J. Phys. Chem. Lett. 3, 867–872 (2012).
Xu, Y. et al. Chemically converted graphene induced molecular flattening of 5,10,15,20-tetrakis(1-methyl-4-pyridinio)porphyrin and its application for optical detection of cadmium(II) ions. J. Am. Chem. Soc. 131, 13490–13497 (2009).
Zheng, S., Tu, Q., Urban, J. J., Li, S. & Mi, B. Swelling of graphene oxide membranes in aqueous solution: characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano 11, 6440–6450 (2017).
Matassa, R., Sadun, C., D’Ilario, L., Martinelli, A. & Caminiti, R. Supramolecular organization of toluidine blue dye in solid amorphous phases. J. Phys. Chem. B 111, 1994–1999 (2007).
D’Ilario, L. & Martinelli, A. Toluidine blue: aggregation properties and structural aspects. Model. Simul. Mater. Sci. Eng. 14, 581–595 (2006).
Zhang, M. et al. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 10, 1253 (2019).
Chen, X., Qiu, M., Ding, H., Fu, K. & Fan, Y. A reduced graphene oxide nanofiltration membrane intercalated by well-dispersed carbon nanotubes for drinking water purification. Nanoscale 8, 5696–5705 (2016).
Wei, Y. et al. Declining flux and narrowing nanochannels under wrinkles of compacted graphene oxide nanofiltration membranes. Carbon 108, 568–575 (2016).
Hung, W.-S. et al. Graphene-induced tuning of the d-spacing of graphene oxide composite nanofiltration membranes for frictionless capillary action-induced enhancement of water permeability. J. Mater. Chem. A 6, 19445–19454 (2018).
Li, W., Wu, W. & Li, Z. Controlling interlayer spacing of graphene oxide membranes by external pressure regulation. ACS Nano 12, 9309–9317 (2018).
Zhang, Z. et al. Interfacial force-assisted in-situ fabrication of graphene oxide membrane for desalination. ACS Appl. Mater. Interfaces 10, 27205–27214 (2018).
Li, Y. et al. Thermally reduced nanoporous graphene oxide membrane for desalination. Environ. Sci. Technol. 53, 8314–8323 (2019).
Rajesh, S. & Bose, A. B. Development of graphene oxide framework membranes via the “from” and “to” cross-linking approach for ion-selective separations. ACS Appl. Mater. Interfaces 11, 27706–27716 (2019).
Chen, L. et al. A large-area free-standing graphene oxide multilayer membrane with high stability for nanofiltration applications. Chem. Eng. J. 345, 536–544 (2018).
Jimbo, T., Tanioka, A. & Minoura, N. Pore-surface characterization of poly(acrylonitrile) membrane having amphoteric charge groups by means of zeta potential measurement. Colloids Surf. A 159, 459–466 (1999).
Yang, Y. et al. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration. Science 364, 1057–1062 (2019).
Kevlich, N. S., Shofner, M. L. & Nair, S. Membranes for Kraft black liquor concentration and chemical recovery: current progress, challenges, and opportunities. Sep. Sci. Technol. 52, 1070–1094 (2017).
Ding, L. et al. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain 3, 296–302 (2020).
Ritt, C. L., Werber, J. R., Deshmukh, A. & Elimelech, M. Monte carlo simulations of framework defects in layered two-dimensional nanomaterial desalination membranes: implications for permeability and selectivity. Environ. Sci. Technol. 53, 6214–6224 (2019).
Thomas, T. E., Aani, S. A., Oatley-Radcliffe, D. L., Williams, P. M. & Hilal, N. Laser Doppler electrophoresis and electro-osmotic flow mapping: a novel methodology for the determination of membrane surface zeta potential. J. Membr. Sci. 523, 524–532 (2017).
Masuko, T. et al. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 339, 69–72 (2005).
Acknowledgements
We acknowledge the following individuals at Georgia Tech: E. Reichmanis and Y. Deng for instrumentation access; N. Hooshmand, A. Korde and S. Liang for useful discussions. We acknowledge financial support by the DOE-RAPID Institute (#DE-EE0007888-7-5) and an industrial consortium comprising Georgia-Pacific, International Paper, SAPPI and WestRock. Z.W. acknowledges the additional support from the Georgia Tech Renewable Bioproducts Institute for a PhD Fellowship. XRD, XPS and SEM characterizations were performed at the Georgia Tech Institute for Electronics and Nanotechnology, home to one of the 16 sites of the National Nanotechnology Coordinated Infrastructure (NNCI), which was supported by the National Science Foundation (grant no. ECCS-1542174).
Author information
Authors and Affiliations
Contributions
S.N., Z.W. and M.L.S. conceived this work. Z.W., C.M., S.A.S. and C.X. designed and conducted synthesis, structure characterization and membrane coupon permeation experiments. C.M. and S.A.S. performed membrane size scale-up and crossflow measurements. All authors participated in the interpretation of data and in writing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Sustainability thanks Juergen Caro and Yanying Wei for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17 and Tables 1–5.
Rights and permissions
About this article
Cite this article
Wang, Z., Ma, C., Xu, C. et al. Graphene oxide nanofiltration membranes for desalination under realistic conditions. Nat Sustain 4, 402–408 (2021). https://doi.org/10.1038/s41893-020-00674-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-020-00674-3
This article is cited by
-
Graphene oxide-based membranes for water desalination and purification
npj 2D Materials and Applications (2024)
-
Bio-inspired design of next-generation ultrapermeable membrane systems
npj Clean Water (2024)
-
Nanowire-assisted electrochemical perforation of graphene oxide nanosheets for molecular separation
Nature Communications (2024)
-
Nanoconfinement enabled non-covalently decorated MXene membranes for ion-sieving
Nature Communications (2023)
-
Covalent organic framework membrane for efficient removal of emerging trace organic contaminants from water
Nature Water (2023)