Abstract
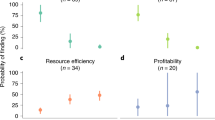
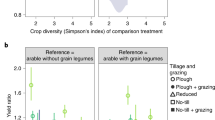
How we manage farming and food systems to meet rising demand is pivotal to the future of biodiversity. Extensive field data suggest that impacts on wild populations would be greatly reduced through boosting yields on existing farmland so as to spare remaining natural habitats. High-yield farming raises other concerns because expressed per unit area it can generate high levels of externalities such as greenhouse gas emissions and nutrient losses. However, such metrics underestimate the overall impacts of lower-yield systems. Here we develop a framework that instead compares externality and land costs per unit production. We apply this framework to diverse data sets that describe the externalities of four major farm sectors and reveal that, rather than involving trade-offs, the externality and land costs of alternative production systems can covary positively: per unit production, land-efficient systems often produce lower externalities. For greenhouse gas emissions, these associations become more strongly positive once forgone sequestration is included. Our conclusions are limited: remarkably few studies report externalities alongside yields; many important externalities and farming systems are inadequately measured; and realizing the environmental benefits of high-yield systems typically requires additional measures to limit farmland expansion. Nevertheless, our results suggest that trade-offs among key cost metrics are not as ubiquitous as sometimes perceived.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Code availability
The R codes used for the analyses are available from the corresponding author upon request.
References
Poore, J. & Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 360, 987–992 (2018).
Green, R. E., Cornell, S. J., Scharlemann, J. P. W. & Balmford, A. Farming and the fate of wild nature. Science 307, 550–555 (2005).
Tilman, D., Balzer, C., Hill, J. & Befort, B. L. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA 108, 20260–20264 (2011).
Hunter, M. C., Smith, R. G., Schipanski, M. E., Atwood, L. W. & Mortensen, D. A. Agriculture in 2050: recalibrating targets for sustainable intensification. Bioscience 67, 386–391 (2017).
Godfray, H. C. J. et al. Food security: the challenge of feeding 9 billion people. Science 327, 812–818 (2010).
Bajželj, B. et al. Importance of food-demand management for climate mitigation. Nat. Clim. Change 4, 924–929 (2014).
Foley, J. A. et al. Solutions for a cultivated planet. Nature 478, 337–342 (2011).
Ripple, W. J. et al. Ruminants, climate change and climate policy. Nat. Clim. Change 4, 2–5 (2014).
Phalan, B., Onial, M., Balmford, A. & Green, R. E. Reconciling food production and biodiversity conservation: land sharing and land sparing compared. Science 333, 1289–1291 (2011).
Balmford, A., Green, R. & Phalan, B. Land for food & land for nature? Daedalus 144, 57–75 (2015).
Hulme, M. F. et al. Conserving the birds of Uganda’s banana-coffee arc: land sparing and land sharing compared. PLoS ONE 8, e54597 (2013).
Kamp, J. et al. Agricultural development and the conservation of avian biodiversity on the Eurasian steppes: a comparison of land-sparing and land-sharing approaches. J. Appl. Ecol. 52, 1578–1587 (2015).
Dotta, G., Phalan, B., Silva, T. W., Green, R. & Balmford, A. Assessing strategies to reconcile agriculture and bird conservation in the temperate grasslands of South America: grasslands conservation and agriculture. Conserv. Biol. 30, 618–627 (2016).
Williams, D. R. et al. Land‐use strategies to balance livestock production, biodiversity conservation and carbon storage in Yucatán, Mexico. Glob. Change Biol. 23, 5260–5272 (2017).
Phalan, B. et al. How can higher-yield farming help to spare nature? Science 351, 450–451 (2016).
Pretty, J. Agricultural sustainability: concepts, principles and evidence. Phil. Trans. R. Soc. B 363, 447–465 (2008).
Matson, P. A., Parton, W. J., Power, A. G. & Swift, M. J. Agricultural intensification and ecosystem properties. Science 277, 504–509 (1997).
Tilman, D., Cassman, K. G., Matson, P. A., Naylor, R. & Polasky, S. Agricultural sustainability and intensive production practices. Nature 418, 671–677 (2002).
Didham, R. K. et al. Agricultural intensification exacerbates spillover effects on soil biogeochemistry in adjacent forest remnants. PLoS ONE 10, e0116474 (2015).
Seufert, V. & Ramankutty, N. Many shades of gray – the context-dependent performance of organic agriculture. Sci. Adv. 3, e1602638 (2017).
Kirchmann, H., Bergström, L., Kätterer, T., Andrén, O. & Andersson, R. in Organic Crop Production – Ambitions and Limitations (eds Kirchmann, H. & Bergström, L.) 39–72 (Springer, Dordrecht, 2008).
Madhusudan, M. D. The global village: linkages between international coffee markets and grazing by livestock in a South Indian wildlife reserve. Conserv. Biol. 19, 411–420 (2005).
Nijdam, D., Rood, T. & Westhoek, H. The price of protein: review of land use and carbon footprints from life cycle assessments of animal food products and their substitutes. Food Policy 37, 760–770 (2012).
Clark, M. & Tilman, D. Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice. Environ. Res. Lett. 12, 64016 (2017).
Yan, X., Yagi, K., Akiyama, H. & Akimoto, H. Statistical analysis of the major variables controlling methane emission from rice fields. Glob. Change Biol. 11, 1131–1141 (2005).
Pittelkow, C. M., Adviento-Borbe, M. A., van Kessel, C., Hill, J. E. & Linquist, B. A. Optimizing rice yields while minimizing yield-scaled global warming potential. Glob. Change Biol. 20, 1382–1393 (2014).
Carrijo, D. R., Lundy, M. E. & Linquist, B. A. Rice yields and water use under alternate wetting and drying irrigation: a meta-analysis. Field Crop Res. 203, 173–180 (2017).
Herrero, M. et al. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proc. Natl Acad. Sci. USA 110, 20888–20893 (2013).
Beauchemin, K., McAllister, T. A. & McGinn, S. M. Dietary mitigation of enteric methane from cattle. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 4, 1–18 (2009).
Wilkinson, J. M. & Garnsworthy, P. C. Dietary options to reduce the environmental impact of milk production. J. Agric. Sci. 155, 334–347 (2017).
IPCC 2006 IPCC Guidelines for National Greenhouse Gas Inventories (eds Eggleston, H. S. et al.) (IGES, 2006).
Gilroy, J. J. et al. Optimizing carbon storage and biodiversity protection in tropical agricultural landscapes. Glob. Change Biol. 20, 2162–2172 (2014).
Lamb, A. et al. The potential for land sparing to offset greenhouse gas emissions from agriculture. Nat. Clim. Change 6, 488–492 (2016).
Cui, Z. et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature 555, 363–366 (2018).
Notarnicola, B. et al. The role of life cycle assessment in supporting sustainable agri-food systems: a review of the challenges. J. Clean. Prod. 140, 399–409 (2017).
Bravo, V. et al. Monitoring pesticide use and associated health hazards in Central America. J. Int. J. Occup. Environ. Heal. 173, 1077–3525 (2011).
Lambin, E. F. & Meyfroidt, P. Global land use change, economic globalization, and the looming land scarcity. Proc. Natl Acad. Sci. USA 108, 3465–3472 (2011).
Ewers, R. M., Scharlemann, J. P. W., Balmford, A. & Green, R. E. Do increases in agricultural yield spare land for nature? Glob. Change Biol. 15, 1716–1726 (2009).
Byerlee, D., Stevenson, J. & Villoria, N. Does intensification slow crop land expansion or encourage deforestation? Glob. Food Sec. 3, 92–98 (2014).
Tilman, D. & Clark, M. Global diets link environmental sustainability and human health. Nature 515, 518–522 (2014).
Yang, Q. et al. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 174, 516 (2014).
FAOSTAT: Food and Agriculture Data (Food and Agriculture Organization of the United Nations, 2017); http://fao.org/faostat
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
R: a Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2016); https://www.r-project.org
Guinée, J. B., Heijungs, R. & Huppes, G. Economic allocation: examples and derived decision tree. Int. J. Life Cycle Assess. 9, 23–33 (2004).
Shang, Q. et al. Net annual global warming potential and greenhouse gas intensity in Chinese double rice-cropping systems: a 3-year field measurement in long-term fertilizer experiments. Glob. Change Biol. 17, 2196–2210 (2011).
Liu, Y. et al. Net global warming potential and greenhouse gas intensity from the double rice system with integrated soil–crop system management: a three-year field study. Atmos. Environ. 116, 92–101 (2015).
Chen, Z., Chen, F., Zhang, H. & Liu, S. Effects of nitrogen application rates on net annual global warming potential and greenhouse gas intensity in double-rice cropping systems of the Southern China. Environ. Sci. Pollut. Res. Int. 23, 24781–24795 (2016).
Xue, J. F. et al. Assessment of carbon sustainability under different tillage systems in a double rice cropping system in Southern China. Int. J. Life Cycle Assess. 19, 1581–1592 (2014).
Shen, J. et al. Contrasting effects of straw and straw-derived biochar amendments on greenhouse gas emissions within double rice cropping systems. Agric. Ecosyst. Environ. 188, 264–274 (2014).
Ma, Y. C. et al. Net global warming potential and greenhouse gas intensity of annual rice–wheat rotations with integrated soil–crop system management. Agric. Ecosyst. Environ. 164, 209–219 (2013).
Zhang, X., Xu, X., Liu, Y., Wang, J. & Xiong, Z. Global warming potential and greenhouse gas intensity in rice agriculture driven by high yields and nitrogen use efficiency. Biogeosciences 13, 2701–2714 (2016).
Yang, B. et al. Mitigating net global warming potential and greenhouse gas intensities by substituting chemical nitrogen fertilizers with organic fertilization strategies in rice–wheat annual rotation systems in China: a 3-year field experiment. Ecol. Eng. 81, 289–297 (2015).
Zhang, Z. S., Guo, L. J., Liu, T. Q., Li, C. F. & Cao, C. G. Effects of tillage practices and straw returning methods on greenhouse gas emissions and net ecosystem economic budget in rice–wheat cropping systems in central China. Atmos. Environ. 122, 636–644 (2015).
Xiong, Z. et al. Differences in net global warming potential and greenhouse gas intensity between major rice-based cropping systems in China. Sci. Rep. 5, 17774 (2015).
Xu, Y. et al. Improved water management to reduce greenhouse gas emissions in no-till rapeseed–rice rotations in Central China. Agric. Ecosyst. Environ. 221, 87–98 (2016).
Xu, Y. et al. Effects of water-saving irrigation practices and drought resistant rice variety on greenhouse gas emissions from a no-till paddy in the central lowlands of China. Sci. Total Environ. 505, 1043–1052 (2015).
Yao, Z. et al. Nitrous oxide and methane fluxes from a rice–wheat crop rotation under wheat residue incorporation and no-tillage practices. Atmos. Environ. 79, 641–649 (2013).
Xia, L., Wang, S. & Yan, X. Effects of long-term straw incorporation on the net global warming potential and the net economic benefit in a rice–wheat cropping system in China. Agric. Ecosyst. Environ. 197, 118–127 (2014).
Zhang, A. et al. Change in net global warming potential of a rice–wheat cropping system with biochar soil amendment in a rice paddy from China. Agric. Ecosyst. Environ. 173, 37–45 (2013).
Zou, J., Huang, Y., Zong, L., Zheng, X. & Wang, Y. Carbon dioxide, methane, and nitrous oxide emissions from a rice–wheat rotation as affected by crop residue. Adv. Atmos. Sci. 21, 691–698 (2004).
Zhou, M. et al. Nitrous oxide and methane emissions from a subtropical rice–rapeseed rotation system in China: a 3-year field case study. Agric. Ecosyst. Environ 212, 297–309 (2015).
Yao, Z. et al. Improving rice production sustainability by reducing water demand and greenhouse gas emissions with biodegradable films. Sci. Rep. 7, 39855 (2017).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. WorldClim – Global Climate Data: WorldClim Version 2 (2017); http://www.worldclim.org/version2
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. WorldClim – Global Climate Data: Bioclimatic Variables (2017); http://www.worldclim.org/bioclim
Heuzé, V., Tran, G. & Hassoun, P. Feedipedia: Rough Rice (Paddy Rice) (Feedipedia, a programme by INRA, CIRAD, AFZ and FAO, 2015); https://www.feedipedia.org/node/226
Liang, K. et al. Grain yield, water productivity and CH4 emission of irrigated rice in response to water management in south China. Agric. Water Manag. 163, 319–331 (2016).
Kreye, C. et al. Fluxes of methane and nitrous oxide in water-saving rice production in north China. Nutr. Cycl. Agroecosyst. 77, 293–304 (2007).
Lu, W., Cheng, W., Zhang, Z., Xin, X. & Wang, X. Differences in rice water consumption and yield under four irrigation schedules in central Jilin Province, China. Paddy Water Environ. 14, 473–480 (2016).
Jin, X. et al. Water consumption and water-saving characteristics of a ground cover rice production system. J. Hydrol. 540, 220–231 (2016).
Sun, H. et al. CH4 emission in response to water-saving and drought-resistance rice (WDR) and common rice varieties under different irrigation managements. Water Air Soil Pollut. 227, 47 (2016).
Wang, X. et al. The positive impacts of irrigation schedules on rice yield and water consumption: synergies in Jilin Province, Northeast China. Int. J. Agric. Sustain. 14, 1–12 (2016).
Xiong, Y., Peng, S., Luo, Y., Xu, J. & Yang, S. A paddy eco-ditch and wetland system to reduce non-point source pollution from rice-based production system while maintaining water use efficiency. Environ. Sci. Pollut. Res. 22, 4406–4417 (2015).
Shao, G.-C. et al. Effects of controlled irrigation and drainage on growth, grain yield and water use in paddy rice. Eur. J. Agron. 53, 1–9 (2014).
Liu, L. et al. Combination of site-specific nitrogen management and alternate wetting and drying irrigation increases grain yield and nitrogen and water use efficiency in super rice. Field Crop Res. 154, 226–235 (2013).
Chen, Y., Zhang, G., Xu, Y. J. & Huang, Z. Influence of irrigation water discharge frequency on soil salt removal and rice yield in a semi-arid and saline-sodic area. Water 5, 578–592 (2013).
Ye, Y. et al. Alternate wetting and drying irrigation and controlled-release nitrogen fertilizer in late-season rice. Effects on dry matter accumulation, yield, water and nitrogen use. Field Crop Res. 144, 212–224 (2013).
Peng, S. et al. Integrated irrigation and drainage practices to enhance water productivity and reduce pollution in a rice production system. Irrig. Drain. 61, 285–293 (2012).
Bell, M. J. et al. Nitrous oxide emissions from fertilised UK arable soils: fluxes, emission factors and mitigation. Agric Ecosyst Environ 212, 134–147 (2015).
Bell, M. J. et al. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory: Fertiliser Experimental Site in East Lothian, 2011 Version: 1 [data set] (Freshwater Biological Association, 2017); https://doi.org/10.17865/ghgno606
Cardenas, L. M., Webster, C. & Donovan, N. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory: Fertiliser Experimental Site in Bedfordshire, 2011 Version: 1 [data set] (Freshwater Biological Association, 2017); https://doi.org/10.17865/ghgno613
Williams, J. R. et al. Agricultural Greenhouse Gas Inventory Research Inventory Research Platform - InveN2Ory: Fertiliser Experimental Site in Herefordshire, 2011 Version: 1 [data set] (Freshwater Biological Association, 2017); https://doi.org/10.17865/ghgno675
Goulding, K. W. T., Poulton, P. R., Webster, C. P. & Howe, M. T. Nitrate leaching from the Broadbalk Wheat Experiment, Rothamsted, UK, as influenced by fertilizer and manure inputs and the weather. Soil Use Manag. 16, 244–250 (2000).
Cardoso, A. S. et al. Impact of the intensification of beef production in Brazil on greenhouse gas emissions and land use. Agric. Syst. 143, 86–96 (2016).
de Figueiredo, E. B. et al. Greenhouse gas balance and carbon footprint of beef cattle in three contrasting pasture-management systems in Brazil. J. Clean. Prod. 142, 420–431 (2017).
Dick, M., Abreu Da Silva, M. & Dewes, H. Life cycle assessment of beef cattle production in two typical grassland systems of southern Brazil. J. Clean. Prod. 96, 426–434 (2015).
Florindo, T. J., de Medeiros Florindo, G. I. B., Talamini, E., da Costa, J. S. & Ruviaro, C. F. Carbon footprint and life cycle costing of beef cattle in the Brazilian midwest. J. Clean. Prod. 147, 119–129 (2017).
Mazzetto, A. M., Feigl, B. J., Schils, R. L. M., Cerri, C. E. P. & Cerri, C. C. Improved pasture and herd management to reduce greenhouse gas emissions from a Brazilian beef production system. Livest. Sci. 175, 101–112 (2015).
Pashaei Kamali, F. et al. Environmental and economic performance of beef farming systems with different feeding strategies in southern Brazil. Agric. Syst. 146, 70–79 (2016).
Ruviaro, C. F., De Léis, C. M., Lampert, V. D. N., Barcellos, J. O. J. & Dewes, H. Carbon footprint in different beef production systems on a southern Brazilian farm: a case study. J. Clean. Prod. 96, 435–443 (2015).
Ruviaro, C. F. et al. Economic and environmental feasibility of beef production in different feed management systems in the Pampa biome, southern Brazil. Ecol. Indic. 60, 930–939 (2016).
Dick, M., Da Silva, M. A. & Dewes, H. Mitigation of environmental impacts of beef cattle production in southern Brazil - evaluation using farm-based life cycle assessment. J. Clean. Prod. 87, 58–67 (2015).
Lesnoff, M. DynMod: a Tool for Demographic Projections of Tropical Livestock Populations Under Microsoft Excel, User’s Manual - Version 1 (CIRAD, Montpelier, Cedex; ILRI, Nairobi, Kenya, 2008).
Broom, D. M., Galindo, F. A. & Murgueitio, E. Sustainable, efficient livestock production with high biodiversity and good welfare for animals. Proc. R. Soc. B 280, 20132025 (2013).
Junior, C. C. et al. Brazilian beef cattle feedlot manure management: a country survey. J. Anim. Sci. 91, 1811–1818 (2013).
Garnsworthy, P. C. The environmental impact of fertility in dairy cows: a modelling approach to predict methane and ammonia emissions. Anim. Feed Sci. Technol. 112, 211–223 (2004).
Collins, A. L. & Zhang, Y. Exceedance of modern ‘background’ fine-grained sediment delivery to rivers due to current agricultural land use and uptake of water pollution mitigation options across England and Wales. Environ. Sci. Policy 61, 61–73 (2016).
Chadwick, D. et al. Manure management: implications for greenhouse gas emissions. Anim. Feed Sci. Technol. 166–167, 514–531 (2011).
Organic Dairy Cows: Milk Yield and Lactation Characteristics in Thirteen Established Herds and Development of a Herd Simulation Model for Organic Milk Production Project Report OF0170 (DEFRA, 2000); https://randd.defra.gov.uk/Default.aspx?Menu=Menu&Module=More&Location=None&Completed=0&ProjectID=8431
Wilkinson, J. M. Re-defining efficiency of feed use by livestock. Animal 5, 1014–1022 (2011).
Webb, J., Audsley, E., Williams, A., Pearn, K. & Chatterton, J. Can UK livestock production be configured to maintain production while meeting targets to reduce emissions of greenhouse gases and ammonia? J. Clean. Prod. 83, 204–211 (2014).
de Ponti, T., Rijk, B. & van Ittersum, M. K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 108, 1–9 (2012).
Gerber, P, Vellinga, T, Opio, C, Henderson, B. & Steinfeld, H. Greenhouse Gas Emissions from the Dairy Sector: A Life Cycle Assessment (Food and Agriculture Organization of the United Nations: 2010); http://www.fao.org/docrep/012/k7930e/k7930e00.pdf
Brown, K. et al. UK Greenhouse Gas Inventory, 1990 to 2010: Annual Report for Submission under the Framework Convention on Climate Change (DEFRA, 2012); https://uk-air.defra.gov.uk/assets/documents/reports/cat07/1204251149_ukghgi-90-10_main_chapters_issue2_print_v1.pdf
Misselbrook, T. H., Sutton, M. A. & Scholefield, D. A simple process-based model for estimating ammonia emissions from agricultural land after fertilizer applications. Soil Use Manag. 20, 365–372 (2006).
Misselbrook, T. H., Gilhespy, S. L., Cardenas, L. M., Williams, J. & Dragosits, U. Inventory of Ammonia Emissions from UK Agriculture2015: DEFRA Contract Report (SCF0102) (DEFRA, 2016); https://uk-air.defra.gov.uk/library/reports?report_id=928
Vellinga, T. V et al. Methodology Used in FeedPrint: a Tool Quantifying Greenhouse Gas Emissions of Feed Production and Utilization Report 674 (Wageningen UR Livestock Research, 2013).
Anthony, S., Quinn, P. & Lord, E. Catchment scale modelling of nitrate leaching. Asp. Appl. Biol. 46, 23–32 (1996).
Wang, L. et al. The changing trend in nitrate concentrations in major aquifers due to historical nitrate loading from agricultural land across England and Wales from 1925 to 2150. Sci. Total Environ. 542, 694–705 (2016).
Davison, P. S., Lord, E. I., Betson, M. J. & Strömqvist, J. PSYCHIC – A process-based model of phosphorus and sediment mobilisation and delivery within agricultural catchments. Part 1: Model description and parameterisation. J. Hydrol. 350, 290–302 (2008).
Koponen, K. & Soimakallio, S. Foregone carbon sequestration due to land occupation - the case of agro-bioenergy in Finland. Int. J. Life Cycle Assess. 20, 1544–1556 (2015).
Guo, L. B. & Gifford, R. M. Soil carbon stocks and land use change: a meta analysis. Glob. Change Biol. 8, 345–360 (2002).
Acknowledgements
We are grateful for funding from the Cambridge Conservation Initiative Collaborative Fund and Arcadia, the Grantham Foundation for the Protection of the Environment, the Kenneth Miller Trust, the UK-China Virtual Joint Centre for Agricultural Nitrogen (CINAg, BB/N013468/1, financed by the Newton Fund via BBSRC and NERC), BBSRC (BBS/E/C/000I0330), DEVIL (NE/M021327/1), U-GRASS (NE/M016900/1), Soils-R-GRREAT (NE/P019455/1), N-Circle (BB/N013484/1), BBSRC Soil to Nutrition (S2N) strategic programme (BBS/E/C/000I0330), UNAM-PAPIIT (IV200715), the Belmont Forum/FACEE-JPI (NE/M021327/1 ‘DEVIL’) and the Cambridge Earth System Science NERC DTP (NE/L002507/1); A.B. is supported by a Royal Society Wolfson Research Merit award. We thank F. Brendrup, E. Caton, A. Dobermann, T. J. Florindo, E. Fonte, O. Leyser, A. Mazzetto, J. Murthwaite, F. P. Kamali, R. Olea-Perez, S. Ramsden, C. Ruviaro, J. Storkey, B. Strassburg, M. Topliff, J. N. V. da Silva, D. Williams, X. Yan and Y. Zhang for advice, data or analysis, and K. Willott for much practical support.
Author information
Authors and Affiliations
Contributions
A.B., T.A., H.B., D.C., D.E., R.F., P.G., R.G., P.S., H.W., A.W. and R.E. designed the study and performed the research; D.M.B., A.C., J.C., T.F., E.G., A.G.-H., J.H.-M., M.H., F.H., A.L., T.M., B.P., B.I.S., T.T., J.V. and E.z.E. contributed and analysed data and results; and all authors contributed substantially to the analysis and interpretation of results and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–3, Supplementary Tables 1–5
Rights and permissions
About this article
Cite this article
Balmford, A., Amano, T., Bartlett, H. et al. The environmental costs and benefits of high-yield farming. Nat Sustain 1, 477–485 (2018). https://doi.org/10.1038/s41893-018-0138-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-018-0138-5
This article is cited by
-
Reconciling trade-offs in pig farming requires a change in mitigation approach
Nature Food (2024)
-
Potential unintended consequences of agricultural land use change driven by dietary transitions
npj Sustainable Agriculture (2024)
-
Trade-offs in the externalities of pig production are not inevitable
Nature Food (2024)
-
Energy, economic, and environmental (3E) assessment of the major greenhouse crops: MFCA-LCA approach
Environmental Science and Pollution Research (2024)
-
Identifying ways of producing pigs more sustainably: tradeoffs and co-benefits in land and antimicrobial use
Scientific Reports (2023)