Abstract
Mobile health (mHealth) interventions hold promise for addressing the epidemic of noncommunicable diseases (NCDs) in low- and middle-income countries (LMICs) by assisting healthcare providers managing these disorders in low-resource settings. We aimed to systematically identify and assess provider-facing mHealth applications used to screen for, diagnose, or monitor NCDs in LMICs. In this systematic review, we searched the indexing databases of PubMed, Web of Science, and Cochrane Central for studies published between January 2007 and October 2019. We included studies of technologies that were: (i) mobile phone- or tablet-based, (ii) able to screen for, diagnose, or monitor an NCD of public health importance in LMICs, and (iii) targeting health professionals as users. We extracted disease type, intervention purpose, target population, study population, sample size, study methodology, technology stage, country of development, operating system, and cost. Our initial search retrieved 13,262 studies, 315 of which met inclusion criteria and were analyzed. Cardiology was the most common clinical domain of the technologies evaluated, with 89 publications. mHealth innovations were predominantly developed using Apple’s iOS operating system. Cost data were provided in only 50 studies, but most technologies for which this information was available cost less than 20 USD. Only 24 innovations targeted the ten NCDs responsible for the greatest number of disability-adjusted life years lost globally. Most publications evaluated products created in high-income countries. Reported mHealth technologies are well-developed, but their implementation in LMICs faces operating system incompatibility and a relative neglect of NCDs causing the greatest disease burden.
Similar content being viewed by others
Introduction
The rapid rise of noncommunicable disease (NCD) prevalence in low- and middle-income countries (LMICs, as defined by the World Bank 2021 country classifications)1 has become a critical public health challenge, triggered by multiple global demographic trends such as population aging, economic development, and dietary/lifestyle transitions2,3,4,5. In 2019, NCDs, which include major chronic diseases such as hypertension, diabetes, depression, and their sequelae, were responsible for an estimated 42 million deaths globally, 77% of which were in LMICs6. People living in poor nations are particularly susceptible to NCDs due to health system vulnerabilities and high socioeconomic inequality7,8,9. In particular, healthcare provider shortages in low-resource settings exacerbate these challenges, fueling the urgent need for innovative solutions in these locations10,11.LMIC
One potential solution to stemming the stresses that NCDs place upon healthcare systems has been the incorporation of mHealth (mobile health) technologies10,12,13,14. By standardizing complex protocols and adapting diagnostic and monitoring equipment for simplified use in ubiquitous mobile devices such as cellular telephones and tablets, the efficiency and practice range of physicians and nurses can be extended, while other tasks can be shifted to community health workers (CHWs). Indeed, over the past several decades, there has been an unprecedented increase in the number of mobile phone and Internet users in LMICs, owing to a steep decline in the price of these devices and their connectivity services15. As of 2021, there were an estimated 5.27 billion unique mobile-phone users worldwide and 4.72 billion Internet users16; LMICs account for 2.9 billon of these users17, with cell phone and mobile internet connectivity penetration in these countries over 90% and around 40%, respectively18,19. It is no surprise that CHWs are increasingly being equipped with smartphones and tablets, as many mHealth applications represent focused, protocolized programs that are ideal for CHW use20,21.
Despite these advantages, challenges such as limited internet connectivity, lack of technical support, disparity in clinical resources between technology development and usage settings, and insufficient training of users can limit the use and expansion of mHealth in resource-constrained regions22,23. As such, maximizing the benefits of mobile technologies for healthcare workers in LMICs will require healthcare professionals, program managers, researchers, and policymakers to understand the context and limitations of these tools. To our knowledge, there is currently no up-to-date, comprehensive systematic overview of mHealth technologies for NCD management targeted for healthcare provider use in LMIC settings. This systematic review thus aims to identify and summarize existing provider-facing mobile-phone and tablet-based applications that can be used to screen for, diagnose, or monitor NCDs in LMICs.
Results
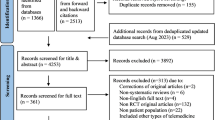
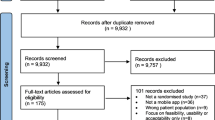
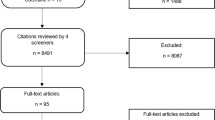
Our initial search of all databases retrieved 13,262 results. After duplicates were removed, abstracts screened, full texts reviewed, and articles identified from reference lists of included publications were added, 315 studies met our inclusion criteria (Fig. 1).
Articles were excluded if they described or evaluated: (i) interventions not meant for diagnosis, screening and/or monitoring (n = 49); ii) non-mobile technology-based interventions (n = 85); (iii) interventions targeting patients instead of health professionals as users (n = 43); (iv) the general status of current technologies (n = 48); (v) communicable diseases (n = 34), or that vi) did not have a full text available (n = 42); (vii) were not available in English (n = 14); (viii) were systematic reviews (n = 36); (ix) were study protocols or involved non-human testing (n = 6); (x) presented technology that merely digitalized protocols, scores or other procedures that could be done on paper (n = 103). A detailed overview of our results is presented in Table 1.
Epidemiology
Most studies described technologies to screen for, diagnose or monitor conditions within the clinical domains of cardiovascular medicine (89/315), ophthalmology and otorhinolaryngology (51/315), neurology (39/315), general medicine (22/315) and maternal and child health (17/315). Development of products occurred predominantly in North America (118/315), followed by Asia (77/315) and Europe (60/315).
Among those studies that were included in our review, the specific NCDs that were most frequently targeted by the study intervention, in order of descending frequency, were arrhythmias (n = 40), Parkinson’s disease (n = 20), retinal pathologies (n = 14), hearing loss (n = 13), melanoma and other skin cancers (n = 11), diabetes (n = 10), anemia (n = 7), and visual impairment (n = 7). Studies focusing on these conditions are summarized in Supplementary Table 3.
Technology
The most popular device platform for studied technologies was the smartphone (253/315), followed by tablets (31/315) and “conventional” mobile phones (21/315), i.e., phones without internet capabilities. Applications were predominantly developed using Apple iOS (113/315) and Android (98/315), with 21 working across different operating systems. Very few were tailored specifically for BlackBerry (2/315), Windows (5/315), or other operating systems such as NetBeans, Tiny OS platform, or Symbian (3/315). In terms of connectivity, some of them required internet connection to work as intended (56/315), while most did not (258/315) and two did not specify. Similarly, a minority required a Bluetooth connection (59/315). However, the majority required the use of accessories (209/315), such as additional lenses to cameras and wired sensors to detect movement and electrical activity developed specifically for the technologies.
Due to the incipient nature of the technologies, costs were not readily available for the vast majority (266/315) of them. For products with price information (50/315), most cost less than 20 USD (32/50), followed by between 21 and 100 USD (4/50) and over 100 USD (14/50).
Methodology
Most of the included publications focused on technologies in an advanced development stage, i.e., already validated and/or commercially available (211/315) (Supplementary Table 2), with the remaining studies describing prototypes (50/315), proof of concept/principle studies (20/315), validation tests (6/315), and pilots (6/315). 16 analyses did not specify the development stage of the products. Publications presenting applications that were already developed or available as described by the authors are summarized in Supplementary Table 4. There was wide variation in studies’ assessment of technology depending on the developmental stage: early-development innovations were assessed through product or technical descriptions (65/315), whereas those further in development were subjected to observational cohort or case–control studies (102/315) or diagnostic accuracy studies (98/315), and a few of them tended to be evaluated via more rigorous experimental methodologies (12/315), such as randomized clinical trials. A small number evaluated qualitative aspects of the product, assessing the attitudes of healthcare workers towards mHealth applications (5/315). To assess the studied innovations, most analyses recruited cohorts between 101 and 500 subjects (125/315), followed by several over 1000 participants (14/315) and those with fewer than 30 subjects (84/315). Some publications did not need to specify a sample size due to the study design used (89/315).
NCDs of high importance
Table 2 summarizes the 24 studies that focused on one of the ten diseases responsible for the most disability-adjusted life years (DALYs) lost in LMICs in 2019:24 ischemic heart disease, chronic obstructive pulmonary disease, neonatal preterm birth, Type 2 diabetes, lower back pain, ischemic stroke, age-related hearing loss, falls, and other musculoskeletal concerns. The uses of such mHealth applications ranged from diagnosing and screening to monitoring these ten disorders. Nearly all of these interventions were smartphone-based (20/24) and most were already available or fully developed (18/24). On the whole, authors of the studies did not specify the cost of the technologies; for those that did, most were under 5 USD (5/6). The majority of mHealth products reviewed were developed either exclusively in high-income countries (HICs, as defined by the World Bank 2021 country classifications) (13/24), or HICs in association with LMIC organizations (3/24). Eight were developed exclusively in LMICs (8/24).
Discussion
Our systematic review of mHealth interventions for NCDs relevant to LMIC healthcare providers identified several important characteristics of this current landscape. We found that most of these interventions were relatively affordable (in the small number of cases where cost data were provided), generally at an advanced stage of development, and designed to be employed using smartphone devices while favoring Apple iOS as the preferred operating system, consistent with the fact that most of the interventions were developed in HICs—albeit occasionally in partnerships with LMIC organizations. Concerningly, most applications (92%) focused on diseases other than the ten responsible for the most DALYs lost globally, and only a small minority employed rigorous randomized clinical trial methodology.
In further detail, our first key finding was that most of the reported technologies were developed in North America, Europe, and Asia—specifically from HICs within these continents. Seldom were LMIC institutions listed as leading the research of the mobile applications identified in our search; even when they were, it was typically in partnership with HIC organizations. This phenomenon has previously been detailed by analyses25,26 which conclude that the poor local health research capacities of LMIC institutions are due to uneven power relations between them and international funders, weak links between research policy and practice, and the lack of a systems approach to research capacity development. In fact, this observation has been called the “10/90 gap”, whereby LMICs possess ninety percent of the global disease burden but only ten percent of global funding for health. This disparity is critical, as many mHealth applications created in high-income country settings may not be applicable for use in LMICs due to factors such as local disease prevalence and availability of diagnostics and therapeutics in low-resource settings. “North-South Partnerships”, project-specific collaborations between HIC and LIC research institutions which were identified in our study, may hold promise for promoting more sustainable research models, though they remain controversial25.
The disconnect between the HICs generating mHealth and LMICs utilizing mHealth becomes more apparent when examining applications’ choice of operating systems. Even though the Android operating system is the most popular in the world, holding ~73–87% of market share27,28, our study found that Apple iOS-based mobile technologies are almost twice as prevalent as Android-based technologies. This incongruence in operating systems may represent another access barrier: people can only use tools that are supported by their devices.
Most concerningly, we found that only 24 of the 315 technologies included in our sample focus on the ten diseases responsible for the greatest number of DALYs lost globally. Many of these conditions have historically relied on expensive specialized equipment for screening, diagnosis, and monitoring; for example, many cardiovascular diseases require such tools as electrocardiograms, echocardiograms, and rhythm monitors for optimal management29, yet these technologies can cost thousands to tens of thousands of dollars. This represents a cost-prohibitive barrier to access for many LMICs, particularly in the setting of compounding systemic issues such as sociopolitical instability, low health expenditures, and corruption30,31,32. As mHealth technologies hold promise for lower-cost management of these illnesses of great public health importance, the relatively low number of technologies devoted to innovatively disrupting these fields remains disappointing. Rather, the focus on disorders that affect quality of life in HICs appears to be reflected in the choice of target diseases selected by their investigators. It is also noteworthy that researchers seldom attempted to extend clinician-oriented findings to questions of specific interest to policymakers or program managers. We believe an effort to make these findings more policy-relevant is one of several potential steps forward (Box 1).
Our results further reveal that smartphones are the main device for mHealth development and highlight the importance of connectivity (both device-to-device and to the internet) in the functionality of mobile technologies. This has important implications for ongoing efforts to equip CHWs with tools to maximize their essential role in resource-constrained settings—i.e., smartphones, rather than tablets, may be more useful devices.
Lastly, our results suggest that the overall validation process of these mHealth technologies is still in its early stages. Whereas many of the technologies are already available commercially, most of the testing and evaluation has been done through pretesting and pilot studies, without the rigor of randomized controlled trials to determine their clinical efficacy compared to current standards of care. That said, a significant number of these innovations have been subjected to initial diagnostic accuracy studies with modest sample sizes, often in comparison to clinical gold standards, paving the way for future analyses of their effect on measurable patient outcomes.
Limitations
Our present study has several limitations. First, the considerable design and population heterogeneity of the publications analyzed precluded the systematic assessment of study quality (by validated tools such as the GRADE framework33). We thus attempted to quantify the quality of evidence in simpler terms by extracting cohort size, general study design, and measurement instruments. Future analyses focusing on more granular subgroups within our study can clarify these findings. Similarly, a fine-grained technical analysis of the phase of product development was beyond the scope of the present work. Hence, our lack of granularity in the appraisal of early-development technologies, e.g., differentiating between diffusion and refinement phases, is also a limitation and area for potential further investigation. Similarly, we have categorized development stage as “advanced” for technologies that were validated, commercially available, or both, a simplification given that not all commercially available technologies have been validated. Finally, the studies included in this review were not necessarily targeted or designed for the populations in LMICs, and hence these findings represent a bare minimum for provider-focused mHealth technologies potentially usable for these conditions. Demonstrating that such technology transfer is feasible will be essential to realize the promise of these technologies.
Our results indicate that mHealth holds promise to equip LMIC healthcare providers with powerful tools to improve population NCD health. However, widespread implementation of these technologies still faces barriers, in particular unbalanced health research development between HICs and LMICs that translates into a disconnect between developer and user software choice, priority diseases being addressed, missing product cost reporting, and lack of rigorous randomized clinical trials to detect improvements in patient outcome. These limitations must be acknowledged and addressed for the full potential of mHealth to be achieved in LMICs.
Methods
Search strategy
We searched for English-language studies published between January 2007 and October 2019 in the following indexing databases: Cochrane Central (searched on September 30th, 2019), PubMed (searched on October 7th, 2019), and Web of Science (searched on October 7th, 2019). Keywords and medical subject headings (MeSH) used included “smartphones”, “tablets”, “diagnosis”, “screening”, and “monitoring”. The full list of search terms for each database are shown in Supplementary Table 1.
No restrictions were placed on study design, sample size, or publication type. Additionally, the reference lists of all included studies and relevant review articles and commentaries were screened for additional references. The search process is graphically summarized in Fig. 1. Our systematic review was registered in The International Prospective Register of Systematic Reviews (PROSPERO; Registration number: CRD42020193945)34.
Inclusion and exclusion criteria
Titles and abstracts were screened for relevance, and then the full‐text versions of retrieved publications were assessed using the following three inclusion criteria: (i) the technology reported must be mobile phone- or tablet-based, (ii) the technology reported must be able to screen for, diagnose, or monitor a disease, and (iii) the disease the technology is designed to address must be an NCD of public health importance for LMICs, defined as conditions that are estimated to cause more than 1% of deaths in any 5-year-age group in the general population or among neonates, or diseases that have a prevalence of greater than 0.1% in any 5-year-age group in the general population or among neonates. The Global Burden of Disease Project’s 2019 estimates were used for the assessment as to whether a condition is an NCD of public health importance in LMICs35.
Data extraction
The following data were extracted from each included article: author(s), title, disease or risk factor, clinical domain by MeSH36, intervention name, intervention type, purpose and aim of the intervention, target population, type of mobile device utilized, type of software, operating system used by intervention, study population, sample size, study methods, stage of development, cost, country of development and/or testing (based on the authors’ institutional affiliations and/or the study population country of residence), year of publication, and a summary of the tool. These data were extracted qualitatively using Microsoft Excel (Redmond, WA).
Analysis
The extracted data were synthesized into three themes based on study characteristics: epidemiology, technology, and methodology. Supplementary Table 2 describes these themes and associated categories, subcategories, and definitions. We subsequently constructed tables crossing clinical domains with all the subthemes to identify trends across the studies.
Due to the large degree of heterogeneity in study designs, outcome measures, and reporting of outcomes, meta-analysis techniques were unable to be used to further summarize the studies.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Material. Any further data analysis information is available from the corresponding author by request.
References
World Bank Country and Lending Groups—World Bank Data Help Desk. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (2022).
Sudharsanan, N. & Geldsetzer, P. Impact of coming demographic changes on the number of adults in need of care for hypertension in Brazil, China, India, Indonesia, Mexico, and South Africa. Hypertension 73, 770–776 (2019).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
NCDs | Third United Nations High-level Meeting on NCDs. WHO. World Health Organization. http://www.who.int/ncds/governance/third-un-meeting/en/ (2021).
Action Plan for the Prevention and Control of Noncommunicable Diseases in the WHO European Region 2016–2025. https://www.euro.who.int/en/health-topics/noncommunicable-diseases/pages/policy/publications/action-plan-for-the-prevention-and-control-of-noncommunicable-diseases-in-the-who-european-region-20162025 (2021).
Bennett, J. E. et al. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 392, 1072–1088 (2018).
Sommer, I. et al. Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC Public Health 15, 914 (2015).
Coates, M. M. et al. Burden of non-communicable diseases from infectious causes in 2017: a modelling study. Lancet Glob. Health 8, e1489–e1498 (2020).
Roth, G. A. et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788 (2018).
Beratarrechea, A. et al. The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemed. e-Health 20, 75–82 (2013).
Bhattacharyya, O. et al. Innovative health service delivery models in low and middle income countries - what can we learn from the private sector? Health Res. Policy Syst. 8, 24 (2010).
WHO | Impacts of e-health on the outcomes of care in low- and middle-income countries: where do we go from here? WHO. World Health Organization. https://www.who.int/bulletin/volumes/90/5/11-099069/en/ (2021).
Mechael P. Barriers and gaps affecting mHealth in low and middle income countries: policy white paper. http://bibalex.org/baifa/en/resources/document/452419 (2010).
Chan, C. V. & Kaufman, D. R. A technology selection framework for supporting delivery of patient-oriented health interventions in developing countries. J. Biomed. Inform. 43, 300–306 (2010).
Lewis, T., Synowiec, C., Lagomarsino, G. & Schweitzer, J. E-health in low-and middle-income countries: findings from the Center for Health Market Innovations. Bull. World Health Organ 90, 332–340 (2012).
Digital 2021 April Statshot Report. DataReportal—Global Digital Insights. https://datareportal.com/reports/digital-2021-april-global-statshot (2021).
The State of Mobile Internet Connectivity Report 2020—Mobile for Development. https://www.gsma.com/r/somic/ (2021).
Chen, L. et al. Human resources for health: overcoming the crisis. Lancet 364, 1984–1990 (2004).
Hongoro, C. & McPake, B. How to bridge the gap in human resources for health. Lancet 364, 1451–1456 (2004).
Feroz, A. S., Khoja, A. & Saleem, S. Equipping community health workers with digital tools for pandemic response in LMICs. Arch. Public Health 79, 1 (2021).
Geniets A, O’Donovan J, Winters N, Hakimi L. Training for Community Health: Bridging the global health care gap. p. 257 (Oxford University Press, 2021).
Feroz, A., Jabeen, R. & Saleem, S. Using mobile phones to improve community health workers performance in low-and-middle-income countries. BMC Public Health 20, 49 (2020).
Pallas, S. W. et al. Community Health Workers in Low- and Middle-Income Countries: What Do We Know About Scaling Up and Sustainability? Am. J. Public Health 103, e74–e82 (2013).
GBD Compare | IHME Viz Hub. http://vizhub.healthdata.org/gbd-compare (2021).
Franzen, S. R. P., Chandler, C. & Lang, T. Health research capacity development in low and middle income countries: reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open 7, e012332 (2017).
Ali N, Hill C, Kennedy A, IJsselmuiden C. What factors influence national health research agendas in low and middle income countries? Perspectives of health research stakeholders from six countries and 11 international agencies. (Council on Health Research for Development (COHRED), 2006).
Mobile Operating System Market Share Worldwide. StatCounter Global Stats. https://gs.statcounter.com/os-market-share/mobile/worldwide (2021).
Android vs iOS market share 2023. Statista. https://www.statista.com/statistics/272307/market-share-forecast-for-smartphone-operating-systems/ (2021).
Guidelines & Clinical Documents. American College of Cardiology. http://www.acc.org/fguidelines (2021).
Grimes, C. E., Bowman, K. G., Dodgion, C. M. & Lavy, C. B. D. Systematic Review of Barriers to Surgical Care in Low-Income and Middle-Income Countries. World J. Surg. 35, 941–950 (2011).
Sayani, S. et al. Addressing cost and time barriers in chronic disease management through telemedicine: an exploratory research in select low- and middle-income countries. Therapeutic Adv. Chronic Dis. 10, 2040622319891587 (2019).
Barros, A. J. D. et al. Are the poorest poor being left behind? Estimating global inequalities in reproductive, maternal, newborn and child health. BMJ Glob. Health 5, e002229 (2020).
Guyatt, G. H. et al. What is “quality of evidence” and why is it important to clinicians? BMJ 336, 995–998 (2008).
Geldsetzer, P. & Flores, B. A systematic review of mobile applications to diagnose, screen or monitor diseases of public health importance in low-resource settings. PROSPERO 2020 CRD42020193945. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020193945 (2020).
About GBD [Internet]. Institute for Health Metrics and Evaluation. http://www.healthdata.org/gbd/about (2014).
Medicine—MeSH—NCBI. https://www.ncbi.nlm.nih.gov/mesh/?term=medical+specialties (2021).
Acknowledgements
P.G. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR003143. R.L.T. was supported by the Veterans Administration (VA) Office of Academic Affairs Advanced Fellowship in Health Services Research. A.B. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—project number 427397439. A.B. was also supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professor award to Till Bärnighausen, funded by the German Federal Ministry of Education and Research.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: P.G. and B.F.; data collection and extraction: B.F. and S.F.; analysis and interpretation of results: P.G., S.F., A.C., R.T., A.B.; draft paper preparation: P.G., S.F., A.C., R.T., G.W., A.B., A.R. All authors reviewed the results and approved the final version of the paper. All authors meet the following four criteria: (1) Substantial contributions to the conception or design of the work or the acquisition, analysis or interpretation of the data, (2) Drafting the work or revising it critically for important intellectual content, (3) Final approval of the completed version, and (4) Accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Geldsetzer, P., Flores, S., Wang, G. et al. A systematic review of healthcare provider-targeted mobile applications for non-communicable diseases in low- and middle-income countries. npj Digit. Med. 5, 99 (2022). https://doi.org/10.1038/s41746-022-00644-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-022-00644-3