Abstract
The procurement and provision of expert-driven, evidence-informed, and independent science advice is integral to timely decision-making during public health emergencies. The 2019 coronavirus disease (COVID-19) pandemic has underscored the need for sound evidence in public health policy and exposed the challenges facing government science advisory mechanisms. This paper is a jurisdictional case study describing (i) the federal science advice bodies and mechanisms for public health in Canada (i.e., the federal science advice “ecosystem”); and (ii) how these bodies and mechanisms have mobilized and evolved to procure expertise and evidence to inform decisions during the first two years of the COVID-19 pandemic. We reviewed publicly accessible Government of Canada documents, technical reports, and peer-reviewed articles available up to December 2021. Canada’s federal landscape of science advisory bodies for public health within the Health Portfolio was largely shaped by Canada’s experiences with the 2003 severe acute respiratory syndrome and 2009 H1N1 outbreaks. In parallel, Canada has a designated science advisory apparatus that has seen frequent reforms since the early 2000s, with the current Office of the Chief Science Advisor created within the Science Portfolio in 2018. The COVID-19 pandemic has further complicated Canada’s science advice ecosystem, with involvement from departments, expert advisory groups, and partnerships within both the federal Health and Science Portfolios. Although the engagement of federal departments outside the health sector is promising, the COVID-19 experience in Canada supports the need to institutionalize science advisory bodies for public health to improve pandemic preparedness and ensure rapid mobilization of well-coordinated and independent advice in future emergencies. This review also identified pressing areas for further inquiry to strengthen science advice for public health in Canada, including to assess the independence of science advisory actors and the interaction between federal and subnational authorities.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has placed significant pressures on the science-policy interface due to its rapidly changing trajectory, considerable health, social, and economic impacts, and constantly evolving evidence base (Allin et al., 2021). The World Health Organization (WHO) declared COVID-19 a public health emergency of international concern on January 30, 2020 and a global pandemic on March 11, 2020 (World Health Organization, 2020). In Canada, the first case of COVID-19 was confirmed on January 25, 2020 in a traveler from Wuhan, China (Public Health Agency of Canada, 2020b). As of December 2021, Canada entered a fifth wave of the disease, with a total of nearly 2 million cases and over 30 thousand deaths (Public Health Agency of Canada, 2020b, 2021k). While Canada’s overall COVID-19 mortality rate has been lower than that of other comparable nations, such as the United States (US) and the United Kingdom (UK) (Our World in Data, 2022; Unruh et al., 2021), much of the burden of COVID-19 infections and deaths has been borne by low income, racialized, migrant, and long-term care populations (Canadian Institute for Health Information, 2020; Guttmann et al., 2020; Public Health Agency of Canada, 2021k). Similar to other countries, Canada has relied on different public health measures to mitigate the spread of COVID-19, including state of emergency declarations, border closures, physical distancing guidelines, movement and gathering restrictions, school and workplace closures, and mask mandates (Allin et al., 2021; McCoy et al., 2020; Public Health Agency of Canada, 2021k; Unruh et al., 2021). Public vaccination efforts began in December 2020, with 83% of eligible individuals (aged ≥5 years) having received at least one dose and 77% at least two doses of an approved COVID-19 vaccine in Canada by December 2021 (Government of Canada, 2022). The administration of a third vaccine dose among adults began in December 2021 (Government of Canada, 2022).
Canada’s federal science advice landscape for public health emergencies was shaped by two infectious disease outbreaks. In 2003, Canada had the greatest burden of the severe acute respiratory syndrome (SARS) cases than any other country outside of Asia, where the virus was initially identified (Naylor, 2003). Between February and July of 2003, Canada experienced two waves of the disease across two provinces (Ontario and British Columbia) (Naylor, 2003). The SARS outbreak triggered major public health reforms at the federal level, including the creation of a national public health agency in 2004, the introduction of a new mechanism to coordinate public health response across the federal and subnationalFootnote 1 governments, and the release of pandemic preparedness and response plans (Naylor, 2003). The ensuing two waves of the H1N1 influenza virus between April 2009 and January 2010 presented the first test of these structures (Eggleton et al., 2010; Pan-Canadian Public Health Network, 2018a; Public Health Agency of Canada, 2011). Post-H1N1 government inquiries revealed that similar issues persisted from SARS through to the H1N1 outbreak, including the availability of public health expertise, surge capacity, and rapid evidence to inform government decisions and guidance (Canadian Public Health Association, 2021; Eggleton et al., 2010; Naylor, 2003; Public Health Agency of Canada, 2011).
The science advice “ecosystem” plays a crucial role in supporting the management of pandemic emergencies, which require timely government decisions carrying significant population impacts in the context of uncertainty. Despite accumulating knowledge about the virus over the first two years of the pandemic, COVID-19 remains a whole-of-society crisis necessitating multidisciplinary expertise and evidence on issues ranging from the effectiveness of clinical therapeutics and vaccines to public uptake of protective public health measures amid disease resurgences driven by novel viral variants. As one of the most decentralized federations among high-income countries, Canada presents an interesting case study of mobilizing science advice in a pandemic emergency, since the development of technical guidance is largely within the remit of the federal governmentFootnote 2, while the implementation of public health measures is primarily the responsibility of subnational governments. In this paper, we identify opportunities for strengthening federal science advice for public health emergencies in Canada. To do so, we describe (i) Canada’s federal science advice ecosystem established before the COVID-19 pandemic (the pandemic “playbook”); and (ii) how Canada’s federal science advice ecosystem has evolved and mobilized in the first two years of the COVID-19 pandemic.
Methods
We conducted a jurisdictional descriptive case study of Canada’s federal science advice ecosystem, reliant on a literature review of publicly accessible primary (i.e., Government of Canada technical reports and guidance) and secondary (i.e., peer-reviewed literature) documents. Study methodology is detailed in Supplementary file 1, including concept definitions, document retrieval processes, and synthesis approaches. Briefly, the document search, using iterative snowballing techniques, focused on the period between the 2003 SARS outbreak in Canada and December 2021. Local public health experts were contacted to verify the completeness of the retrieved information. We identified key advisory bodies in the federal science advice ecosystem, including those involved in evidence generation, brokerage, communication, and decision-making. We considered advisory bodies to be part of the federal science advice ecosystem if evidence that the body has an advisory relationship with the federal government could be established in the literature.
The manuscript findings are organized as follows: first, we document the advisory bodies that constituted the federal science advice ecosystem before the COVID-19 pandemic; then, we discuss how these bodies have been mobilized to inform the COVID-19 response, highlighting new science advisory bodies created for convening experts and generating evidence to inform federal decision-making. In the discussion section, we reflect on the mobilization and evolution of Canada’s federal science advice ecosystem during the first two years of the COVID-19 pandemic in the context of the broader literature on science advice to provide policy and research recommendations. This study was conducted as part of the Evaluation of Science Advice in a Pandemic Emergency (EScAPE) international case series. Box 1 presents the full list of acronyms used in this paper.
Findings
Canada’s science advice ecosystem before the COVID-19 pandemic
The role of the federal government in health and science policy
The Canadian federation is composed of the Parliament of Canada and 13 subnational governments (10 provinces and three territories). Authority on matters of health is not covered by a specific provision of the 1867 Constitution Act and federal, provincial, and territorial (FPT) governments may all legislate on matters of health (Braën, 2004; Brideau and Brosseau, 2019; Government of Canada, 2019; Martin et al., 2018). Through the Constitution Act, PT governments have de jure authority to establish, maintain, and manage hospitals, asylums, and charitable institutions, while the federal government is responsible for quarantine, establishing marine hospitals, and leveraging criminal law to regulate hazardous substances (Braën, 2004; Brideau and Brosseau, 2019; Government of Canada, 2019; Martin et al., 2018). PT governments eventually became the de facto authorities for planning, organizing, and delivering health services, with the 1982 Canada Health Act setting out the criteria that must be met by the PT universal health insurance plans to receive federal funds (Braën, 2004; Brideau and Brosseau, 2019; Government of Canada, 2019; Martin et al., 2018). Municipal and regional governments also hold some de jure and de facto responsibilities on health matters (Bana et al., 2018; Di Ruggiero et al., 2022). Science policy in Canada does not have delineated roles and FPT governments are all involved in science matters to varied extents (Council of Canadian Academies, 2017).
The Federal Health Portfolio
Canada’s Minister of Health oversees five federal departments, including Health Canada, the Canadian Institutes of Health Research (CIHR), and the Public Health Agency of Canada (PHAC)—together comprising the federal Health Portfolio (Fig. 1) (Government of Canada, 2017). Health Canada regulates the safety and efficacy of substances, including medical devices, treatments, and vaccines (Government of Canada, 2014; Marchildon et al., 2020). CIHR is composed of 13 institutes that provide health research funding (Canadian Institutes of Health Research, 2018; Marchildon et al., 2020). PHAC provides national public health leadership within the Health Portfolio and, as Canada’s national contact point for the WHO on International Health Regulations matters, has a prominent role in a pandemic response (Pan-Canadian Public Health Network, 2018a; Public Health Agency of Canada, 2021k).
ACOA Atlantic Canada Opportunities Agency, BDC Business Development Bank of Canada, CED Canada Economic Development for Quebec Regions, CIHR Canadian Institutes for Health Research, CanNor Canadian Northern Economic Development Agency, FedDev Ontario Federal Economic Development Agency for Southern Ontario, FedNor Federal Economic Development Agency for Northern Ontario, ISED Innovation, Science and Economic Development Canada, NRC National Research Council Canada, NSERC Natural Sciences and Engineering Research Council Canada, PacificCan Pacific Economic Development Canada, SSHRC Social Sciences and Humanities Research Council of Canada.
Public Health Agency of Canada
In October 2003, the National Advisory Committee on SARS and Public Health, chaired by Dr. David Naylor, released the “Learning from SARS: Renewal of Public Health in Canada” report (“the Naylor Report”), recommending the creation of a federal public health agency, helmed by a Chief Public Health Officer of Canada (CPHO) (Naylor, 2003). This recommendation was the primary driver for the creation of PHAC in September 2004 (Public Health Agency of Canada et al., 2008). The Public Health Agency of Canada Act (2006) subsequently (i) confirmed PHAC as a legal entity responsible for governing public health functions, including emergency preparedness and response; and (ii) established the position of the CPHO—the federal government’s lead public health expert (Government of Canada, 2006, 2016; Public Health Agency of Canada et al., 2008).
The CPHO (a health professional with public health qualifications) ranks at the deputy ministerFootnote 3 level and has two primary functions: first, as a public health advisor to the Minister of Health, the President of PHAC, and the federal CabinetFootnote 4; and second, as a public health communicator with the public, other governments, public health authorities, and non-governmental organizations in Canada and internationally (Government of Canada, 2006). The CPHO is legally required to submit an annual report to the Minister of Health on the state of public health in Canada, which is tabled in Parliament and made public (Government of Canada, 2006). While outside the scope of the present paper, public health within each PT is governed, in part, by a similar role (the Chief Medical Officer of Health, CMOH). The responsibilities and authorities of CMOHs in advising PT governments, managing public health programs and resources, communicating with the public, and advocating on behalf of public health vary substantially across PTs (Bana et al., 2018; Eggleton et al., 2010; Fafard et al., 2018).
The PHAC departments and advisory groups that are relevant to a pandemic response are presented in Table 1. PHAC has intramural research scientists and the capacity to convene expert advisory groups to evaluate evidence for decision-making (Public Health Agency of Canada, 2021g). PHAC also funds the National Collaborating Centres (NCCs) for Public Health, which were established in 2005 and are housed within independent organizations external to PHAC to support Canada’s public health systems through evidence synthesis and knowledge translation services (National Collaborating Centres for Public Health, 2021). There are six NCCs based within universities, public health agencies, and research centers across five provinces, with each NCC working on a specific public health area of focus (National Collaborating Centres for Public Health, 2021).
Pan-Canadian Public Health Network
Following the 2003 Naylor Report recommendations, the Pan-Canadian Public Health Network (PHN) was established in 2005 to serve as the formal intergovernmental mechanism for FPT collaboration on routine public health issues and public health threats (Pan-Canadian Public Health Network, 2016). The structure of the PHN (Fig. 2) aligns with the principle of collaborative federalism, whereby national policies are co-determined by FPT authorities as equal partners (Cameron and Simeon, 2002; Fierlbeck, 2010; Pan-Canadian Public Health Network, 2016). PHN is governed by the 17-member PHN Council, composed of senior appointed FPT civil servants that lead the health portfolios within their respective jurisdictions (such as the CPHO and CMOHs) (Pan-Canadian Public Health Network, 2020a, 2020b).
The 17-member PHN Council is co-chaired by the Chief Public Health Officer and a provincial or territorial Chief Medical Officer of Health and is accountable to the Conference of Deputy Ministers of Health. The Council of Chief Medical Officers of Health (a technical advisory forum composed of the Chief Public Health Officer and Chief Medical Officer of Health from provincial, territorial, and Indigenous health authorities) and three Steering Committees (each dedicated to a PHN area of focus)—(i) Healthy People and Communities, (ii) Communicable and Infectious Disease, and (iii) Public Health Infrastructure—support the PHN Council. Note: reporting relationships are depicted in solid lines, while supporting relationships are depicted in dashed lines.
A key function of the PHN is to activateFootnote 5 a time-limited Special Advisory Committee (SAC) during public health emergencies to advise the Conference of FPT Deputy Ministers of Health (Fig. 3) (Pan-Canadian Public Health Network, 2018b, 2020a, 2020b). Upon activation, the SAC is composed of all members of the PHN Council and the Council of Chief Medical Officers of Health (Pan-Canadian Public Health Network, 2018b, 2020a). In its current structure (devised following the H1N1 pandemic) (Public Health Agency of Canada, 2011), the first SAC was activated in December 2016 to focus on the ongoing epidemic of opioid overdoses (Pan-Canadian Public Health Network, 2020b, 2021). A 2017 internal operational review of the PHN identified the SAC as an effective structure for managing public health emergencies (Di Ruggiero et al., 2022; Dyke, 2017).
The Special Advisory Committee (SAC) may activate and deactivate three advisory groups: (i) the Technical Advisory Committee (TAC, chaired by the Communicable and Infectious Disease Steering Committee), (ii) the Logistics Advisory Committee (LAC, chaired by the Public Health Infrastructure Steering Committee), and (iii) the Pan-Canadian Public Health Network (PHN) Communication Group. Each province and territory selects representatives (typically, senior civil servants within the subnational health portfolios that have decision-making authority) to serve on the TAC and LAC. The SAC Secretariat manages the intersection between the SAC and the three response streams of the governance (the TAC, LAC, and PHN Communication Group). The TAC and LAC may convene task groups and working groups on, respectively, technical (e.g., laboratory testing, disinfection and decontamination, and case and contact surveillance) and logistical (e.g., funding allocation, engagement of external resources, use of designated sites, surge worker planning) issues and approve the protocols and guidance developed by the task groups. The PHN Communication Group coordinates public communication across the federal, provincial, and territorial authorities. Note: reporting relationships are depicted in solid lines, while supporting relationships are depicted in dashed lines.
The Federal Science Portfolio
The Minister of Innovation, Science and Economic Development (ISED) oversees the Science Portfolio, which is composed of 18 federal departments and agencies (Fig. 1), including ISED, National Research Council Canada (NRC), Natural Sciences and Engineering Research Council of Canada (NSERC), Social Sciences and Humanities Research Council of Canada (SSHRC), and Statistics Canada (Innovation, Science and Economic Development Canada, 2021f). ISED leads the Science Portfolio and is mandated to support business innovation and scientific research (Innovation, Science and Economic Development Canada, 2018). The NRC is Canada’s largest federal research and development organization, responsible for bringing research innovations to market, as legislated in the National Research Council Act (1985) (Government of Canada, 1985a; National Research Council of Canada, 2019). NSERC and SSHRC are funding agencies for natural sciences and technology and humanities and social sciences, respectively. Together, CIHR (in the Health Portfolio), NSERC, and SSHRC are referred to as the Tri-Agencies or the Government’s granting councils, which are coordinated by the Canada Research Coordinating Committee, created in 2018 (Canada Research Coordinating Committee, 2020). Statistics Canada is Canada’s central statistical office that is governed by the Statistics Act (1985) and is responsible for conducting the Census and over 350 other surveys on the health, social, and economic activities of the public (Government of Canada, 1985b; Statistics Canada, 2020).
Each Science Portfolio department and agency may conduct stakeholder consultations and public opinion research, and convene expert advisory groups (Innovation, Science and Economic Development Canada, 2020). Several arm’s-length research organizations are funded by the Science Portfolio, such as Genome Canada (a non-profit organization focused on genomics-based technologies) (Genome Canada, 2021a) and the Council of Canadian Academies (CCA) (a non-profit organization focused on synthesis and expert appraisal of the best available evidence on public policy matters) (Council of Canadian Academies, 2021; Quirion et al., 2016).
Office of the Chief Science Advisor
Prior to 2018, Canada did not have a formalized apparatus for providing science advice to inform the federal government’s decision-making (Office of the Chief Science Advisor, 2020a). For a comprehensive account of the history of science advisory systems in Canada, we direct interested readers to the work by Quirion et al. (2016). Briefly, between 2003 and 2008, the Office of the National Science Advisor was instituted to, first, advise the Prime Minister of Canada on broad policy issues (Quirion et al., 2016), and then, to advise the Minister of Industry on science and technology research and development policy (Industry Canada, 2008; Quirion et al., 2016). The Office of the National Science Advisor also supported the Royal Society of Canada, the Canadian Academy of Engineering, and the Canadian Academy of Health Sciences in founding the CCA in 2005, which is seen as one of the Office’s major accomplishments (CBC News, 2008; Quirion et al., 2016).
Following a change in the national governing party, the Office of the National Science Advisor was terminated in March 2008 and replaced with the Science, Technology and Innovation Council (STIC)—an advisory committee within the Department of Industry (CBC News, 2008; Quirion et al., 2016). Considering the narrow remit of the STIC, limited to technological innovation and development (Royal Society of Canada, 2015), many scientists and the public viewed the closure of the Office of the National Science Advisor as a dissolution of a necessary ‘voice at the table’ for science within the government and a crucial line of communication between Canada’s scientific community and the federal government (CBC News, 2008; Quirion et al., 2016). The STIC was wound down in 2018 following the recommendations of the 2017 Fundamental Science Review (an independent review of the federal science and research ecosystem) (Canada’s Fundamental Science Review and Naylor, 2017; Evidence for Democracy, 2019).
The Office of the Chief Science Advisor (OCSA) (currently in operation) was established by the Liberal government in 2018 within the ISED department, reporting to the Minister of ISED and the Prime Minister (Office of the Chief Science Advisor, 2020a). The mandate of the Chief Science Advisor is (i) to develop and implement guidelines to ensure that government scientists are able to speak freely about their work and that government science is available to the public; (ii) to ensure that scientific analyses are considered in the federal government’s decisions; (iii) to recommend ways for improving the science advisory function within the federal government; and (iv) to recommend ways for the federal government to better support quality scientific research (Office of the Chief Science Advisor, 2018). In addition, the Chief Science Advisor delivers an annual report, which is tabled in ParliamentFootnote 6 and made public, to the Prime Minister and to the Minister of ISED on OCSA activities; coordinates expert advice to the federal Cabinet and the Minister of ISED; and supports the dialogue between scientists within and outside of government in Canada and internationally (Office of the Chief Science Advisor, 2018).
In its first year, the OCSA established the Departmental Science Advisors (DSA) Network—a network of lead science officials and subject matter experts that work closely with senior officials within each federal department and support the mandate of the Chief Science Advisor (Office of the Chief Science Advisor, 2019, 2020a, 2021b). The DSA Network, chaired by the Chief Science Advisor, aims to coordinate science advice across the federal portfolios (including the Health Portfolio), and, as of 2021, has eight members from different federal departments, including PHAC, Health Canada, and the NRC (Office of the Chief Science Advisor, 2021b). The DSA Network meets on a monthly basis to collaborate and serve as peer-reviewers on multi-departmental government science initiatives (Office of the Chief Science Advisor, 2021b).
Federal guidelines for science advice in a pandemic emergency
Emergency management in Canada falls within the National Security Portfolio, which is managed by the Minister of Public Safety. Canada’s first National Security Policy (“Securing an Open Society: Canada’s National Security Policy”) was released after SARS in 2004 and served to position pandemics among the key national security priorities (alongside intelligence, emergency planning and management, and transport, border, and international security) (Canada and Privy Council Office, 2004). The first National Security Policy also supported the implementation of PHAC and the Office of the CPHO (Canada and Privy Council Office, 2004). The federal government’s “all-hazards” emergency management approach is guided by the Federal Emergency Response Plan (2011) (Public Safety Canada, 2011), with the legal and policy scaffolding provided by, respectively, the Emergency Management Act (2007) (Government of Canada, 2007) and the Federal Policy on Emergency Management (2009) (Public Safety Canada, 2009). In a national emergency, one of 13 federal departments is designated as the “primary institution” that convenes and coordinates national stakeholders in emergency response (Public Safety Canada, 2011). The Health Portfolio is the primary federal institution for all public health emergencies, including pandemics (Pan-Canadian Public Health Network, 2018a; Public Safety Canada, 2011). The Health Portfolio Operations Centre (HPOC) within the Centre for Emergency Preparedness and Response (first created in 2000 and moved to PHAC in 2004) (Canada and Privy Council Office, 2004; Public Health Agency of Canada, 2006) acts as the focal point for coordinating emergency management across federal departments, PT authorities, and other actors during public health events (Pan-Canadian Public Health Network, 2018b, 2018a). The HPOC supports the PHN SAC and its committees via the SAC Secretariat (Pan-Canadian Public Health Network, 2018b).
Under the Emergency Management Act, each federal Minister is responsible for developing, testing, and maintaining an emergency plan specific to their portfolio (Pan-Canadian Public Health Network, 2018a). Two such documents outline the roles and responsibilities of the Health Portfolio in a pandemic emergency response: the FPT Public Health Response Plan for Biological Events (2018) and the Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector (CPIP) (2018). In alignment with the principles outlined in the “all-hazards” Federal Emergency Response Plan, the FPT Public Health Response Plan for Biological Events provides the overarching FPT governance framework for responding to public health events, including the activation of the Health Portfolio emergency management structure (Pan-Canadian Public Health Network, 2018b, 2018a). The CPIP provides complementary pandemic-specific guidance, viewed as applicable to both influenza and other respiratory viruses (Pan-Canadian Public Health Network, 2018a, 2018b; Public Health Agency of Canada, 2021f). The CPIP was first published after the SARS outbreak in 2004, majorly revised after the 2009 H1N1 pandemic, and is reviewed every five years (Pan-Canadian Public Health Network, 2018a). PTs also have their own emergency management and pandemic response plans that function in concert with the federal plans.
Evidence-informed decision-making is cited as a core principle in Canada’s national pandemic response plans (Pan-Canadian Public Health Network, 2018a, 2018b). The CPIP outlines four components of a pandemic knowledge generation and dissemination strategy: rapidly identifying research needs in specific areas of the response; leveraging existing collaborations to mount rapid primary research; performing evidence syntheses for decision-making; and forming collaborations between PHAC, Health Canada, and other FPT, academic, and public health institutions to learn from the pandemic in the post-pandemic period (Pan-Canadian Public Health Network, 2018a). Notably, the CPIP does not provide specific guidance on the procurement of expertise or the role of the Science Portfolio.
Canada’s science advice ecosystem during the COVID-19 pandemic
Mobilization of pre-established science advisory bodies
At the outset of the COVID-19 pandemic, the federal response was coordinated by the Incident Response Group—a time-limited Cabinet group of federal ministers chaired by the clerk of the Privy Council Office/Secretary of the Cabinet that is convened by the Prime Minister in national crises, such as domestic and international terrorism threats (Cappe, 2020; Prime Minister of Canada Office, 2021b). A pandemic-specific federal decision-making group—the Sub-Committee on the Federal Response to COVID-19Footnote 7, mandated to provide “whole-of-government leadership, coordination, and preparedness for a response to, and recovery from, COVID-19”—was announced on March 4, 2020 by the Prime Minister (Cappe, 2020; Prime Minister of Canada Office, 2021c).
The Health Portfolio emergency management structure for the COVID-19 pandemic was established in January 2020 and followed the existing pandemic playbook. The HPOC and the FPT Public Health Response Plan for Biological Events were triggered mid-January 2020 (Office of Audit and Evaluation, Health Canada and Public Health Agency of Canada, 2020). Upon official confirmation of the first COVID-19 case in Canada by the National Microbiology Laboratory (NML), the Council of Chief Medical Officers of Health and the FPT SAC on COVID-19, along with its committees and their supporting task forces and working groups (e.g., Surveillance Expert Working Group, Canadian Pandemic Influenza Plan Task Group, and the Public Health Working Group on Remote and Isolated CommunitiesFootnote 8), were activated on January 28, 2020 (Office of Audit and Evaluation, Health Canada and Public Health Agency of Canada, 2020; Pan-Canadian Public Health Network, 2022; Public Health Agency of Canada, 2021f). Over 14 public statements had been issued by the Council of Chief Medical Officers of Health on behalf of the SAC by December 2021, with 10 statements focused on COVID-19 vaccination, endorsing the National Advisory Committee on Immunization (NACI) COVID-19 vaccination guidance (Pan-Canadian Public Health Network, 2022; Public Health Agency of Canada, 2022a). Since the start of the pandemic, the NML has provided leadership in developing COVID-19 testing assays, scaling up testing capacity across the country and sharing epidemiological and surveillance information through the Canadian Public Health Laboratory Network (CPHLN), and supporting research on medical countermeasures and the impact of emerging variants of concern (VOC) (Public Health Agency of Canada, 2021f, 2022c).
New collaborations between the Health Portfolio and other federal departments emerged during the COVID-19 pandemic. For example, in March 2020, PHAC sought the expertise of behavioral scientists at the Impact and Innovation Unit (situated within the Privy Council Office since 2017) to inform PHAC’s pandemic messaging to promote public uptake of public health protective measures (Impact and Innovation Unit, 2018, 2020; Impact Canada Initiative, 2022b). The Impact and Innovation Unit was explicitly acknowledged by the CPHO for the first time in February 2021 in the context of the federal government’s efforts to promote vaccine confidence (Delacourt, 2021); however, the CPHO’s statements generally aligned with the Impact and Innovation Unit’s public messaging campaigns since the first wave of the pandemic (Impact Canada Initiative, 2022c, 2022a; McCoy et al., 2020). Another notable example is the Wastewater Surveillance Program for COVID-19, comprised of scientists at the NML and Statistics Canada (National Collaborating Centre for Infectious Diseases, 2021a; Public Health Agency of Canada, 2021h, 2021i). Wastewater surveillance has supported targeted outbreak prevention and detection of circulating VOCs in several communities in Canada—particularly where widespread testing may not have been available (National Collaborating Centre for Infectious Diseases, 2021a; Public Health Agency of Canada, 2021h, 2021i). As of December 2021, PHAC was leading four working groups on VOCs in wastewater, wastewater laboratory detection methods, and data modeling and epidemiological interpretation (National Collaborating Centre for Infectious Diseases, 2021a).
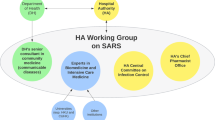
The interim FPT Public Health Response Plan for Ongoing Management of COVID-19 was released in August 2020 (Public Health Agency of Canada, 2020a) and revised in April 2021 (Public Health Agency of Canada, 2021f) to outline the common near-, mid-, and long-term goals for COVID-19 management in Canada in the context of the overall pandemic response objectiveFootnote 9 and a “reasonable worst-case scenario”. This interim plan “draws extensively” from the 2018 FPT Public Health Response Plan for Biological Events and the CPIP to provide guidance for the federal COVID-19 response as a living document, updated with evolving scientific knowledge until COVID-19 activity in Canada has reached a “low, manageable, and tolerable level” (Public Health Agency of Canada, 2021f). The August 2020 version of the interim plan described the federal government’s prioritization of investigations related to COVID-19 mathematical modeling, social and behavioral sciences, serological and wastewater surveillance, and genomic innovation (Public Health Agency of Canada, 2020a); this was also reiterated in the April 2021 updated version of the plan (Public Health Agency of Canada, 2021f). As detailed next, these priorities were pursued by establishing new science advisory bodies between the spring and fall of 2020 (i.e., the first two waves of the COVID-19 pandemic in Canada) (Fig. 4) to (i) convene relevant experts, and (ii) support real-time primary data collection and rapid evidence syntheses.
Note: dashed borders represent bodies established by the Health Portfolio; solid borders represent bodies established by the Science Portfolio. COVID-19 case data (reporting dates: January 25, 2020-December 31, 2021) was retrieved from the COVID-19 Canada Open Data Working Group: https://opencovid.ca/work/dataset/.
Procurement of expertise through new science advisory bodies
Expert advisory groups established by the Health Portfolio
The time-limited expert advisory groups convened by the Health and Science Portfolios during the COVID-19 pandemic are detailed in Table 2. The PHAC External COVID-19 Modeling Expert Group was created in February 2020 to support PHAC’s internal modeling group (formed in January 2020 and largely composed of modelers and epidemiologists at the NML) (National Collaborating Centre for Infectious Diseases, 2022; Public Health Agency of Canada, 2022c) in developing epidemiological models to estimate the infection burden, impact of public health measures, and factors contributing to chains of transmission (Public Health Agency of Canada, 2021f, 2022b). The Pan-Canadian Health Data Strategy Expert Advisory Group, convened by PHAC in December 2020, was mandated to provide recommendations to the Conference of Deputy Ministers of Health on the creation, collection, storage, and use of health data across FPTs during and beyond public health emergencies like COVID-19 (Public Health Agency of Canada, 2021m). The Group was expected to conclude its mandate by winter 2022, and by December 2021, the Group had held 10 monthly meetings and released two public reports identifying challenges and opportunities for modernizing Canada’s health data ecosystem (Public Health Agency of Canada, 2021e, 2022d). The Ad-hoc COVID-19 Clinical Pharmacology Task Group was convened in June 2020 to advise PHAC’s Director General of the Centre for Immunization and Respiratory Infectious Diseases on pharmaceutical products for treatment and chemoprophylaxis of COVID-19 (Government of Canada, 2020c, 2020d). Owing to the entry of COVID-19 vaccines in Canada, the Task Group concluded its mandate on March 30, 2021, and its recommendation statements are no longer publicly accessible (Government of Canada, 2020c).
Health Canada established the COVID-19 Testing and Screening Expert Advisory Panel in November 2020 to advise the Minister of Health on science and policy issues related to COVID-19 testing and screening, including strategies for different testing technologies, populations, and settings (Health Canada, 2021a; Public Health Agency of Canada, 2021a, 2021n). Between November 2020 and April 2021, the Panel had held 24 meetings and published five reports (Government of Canada, 2021). At the time of writing, the Panel has not been disbanded; however, it has also not been asked to provide advice since the publication of its fifth report in August 2021. The Industry Advisory Roundtable on COVID-19 Testing, Screening, Tracing and Data Management was launched in October 2020 to complement the technical work of the COVID-19 Testing and Screening Expert Advisory Panel and advise on implementing COVID-19 testing in industry workplace settings (Health Canada, 2021c). The Roundtable was formed by Health Canada in consultation with ISED’s Industry Strategy Council, created in May 2020 “to coordinate business communities’ input on the impact of COVID-19” (Innovation, Science and Economic Development Canada, 2021a).
Expert advisory groups established by the Science Portfolio
In March 2020, the OCSA established the COVID-19 Expert Panel, composed of 19 experts internal and external to the federal government, to support the Chief Science Advisor in developing evidence-informed advice for federal decision-makers (Office of the Chief Science Advisor, 2020a, 2021a). Subgroups of the Expert Panel—the Expert Group on Health Systems and the Expert Group on Modeling Approaches—were formed in mid-to-end of March 2020 (Office of the Chief Science Advisor, 2020a, 2020b, 2020c). The Expert Group on Health Systems was assembled to procure information on health service needs and system planning at the request of Health Canada (Office of the Chief Science Advisor, 2020a, 2020b). The objective of the Expert Group on Modeling Approaches is to review mathematical modeling methodologies to predict COVID-19 spread and identify hot spots, recovery strategies, and data gaps (Office of the Chief Science Advisor, 2020a, 2020c). As the pandemic evolved, the Chief Science Advisor drew on the Expert Panel and the two Expert Groups to develop public reports on scientific standards and best practices on emerging issues, including respirators, ventilators, virtual care, long-term care, data analytics, optimal use of health system capacity, COVID-19 in children, vaccine-associated cardiovascular complications, vaccination certificates, and the role of bioaerosols and indoor ventilation in COVID-19 transmission (Office of the Chief Science Advisor, 2020a, 2022).
Three expert advisory groups were convened by other Science Portfolio departments. In July 2020, the Government of Canada began rolling out the COVID-19 exposure notification smartphone application (“app”) developed through Health Canada—COVID Alert (Bhatia et al., 2020). The COVID-19 Exposure Notification App Advisory Council, established by ISED to “ensure that the app meets the highest standards in public health outcomes, technology, and privacy”, had its first meeting on August 5, 2020 (Innovation, Science and Economic Development Canada, 2021e). By May 26, 2021, the Council had held 15 meetings and released its first interim report on the social and economic determinants of COVID Alert adoption, retention, and use in February 2021 (Innovation, Science and Economic Development Canada, 2021e). The COVID-19 Therapeutics Task Force was formed by the NRC in July 2020 to advise the Minister of ISED on the procurement, prioritization, and manufacturing of COVID-19 therapeutic products seeking to enter the Canadian market, “until [it is possible to] immunize Canadians on a national scale with an effective vaccine” (Innovation, Science and Economic Development Canada, 2021b). The Therapeutics Task Force concluded its mandate on February 28, 2021 (Innovation, Science and Economic Development Canada, 2021b). The COVID-19 Vaccine Task Force, convened to advise the Minister of ISED on the prioritization, development, manufacturing, and supply chain coordination of domestic and international vaccine projects, was first publicly acknowledged by the NRC in August 2020 (Government of Canada, 2020b). No outputs of the Therapeutics and Vaccine Task Forces have been made public; however, the Ministers of ISED and Public Services and Procurement cited the recommendations of both Task Forces in their August 2020 announcements regarding new contracts with vaccine candidate suppliers and investments in domestic clinical trials of COVID-19 vaccines and therapeutics (Government of Canada, 2020a).
Procurement of evidence through new science advisory bodies
Primary data collection
The federal government funded three major research partnerships during the COVID-19 pandemic to scale up primary data collection to inform decision-making at FPT levels. In February 2021, the Government of Canada announced the VOC Strategy, which included an investment of $53 million to support and scale up VOC surveillance and sequencing efforts (Public Health Agency of Canada, 2021b). The Canadian COVID-19 Genomics Network (CanCOGeN)—a partnership between PHAC, Health Canada, CPHLN, CIHR, healthcare facilities, and academic researchers, established by Genome Canada in April 2020—was funded through the VOC Strategy to (i) provide pan-Canadian coordinated cross-agency viral and human host sequencing to track viral origin, spread, and evolution, to inform time-sensitive decision-making relevant to Canada’s health authorities; and (ii) contribute to building national data infrastructure to address future infectious disease outbreaks and emergencies (Genome Canada, 2021b; Genome Canada Canadian COVID-19 Genomics Network and Canadian Public Health Laboratory Network CanCOGeN Working Group, 2021; Public Health Agency of Canada, 2021k). At the time of writing, CanCOGen was leading two viral sequencing projects to understand COVID-19 spread and host responses—VirusSeq (sequencing of viral samples from people testing positive for COVID-19) and HostSeq (sequencing of genomes of people diagnosed with COVID-19) (Genome Canada, 2021c; Public Health Agency of Canada, 2021b).
The Coronavirus Variants Rapid Response Network (CoVaRR-Net)—a network of interdisciplinary researchers chaired by the Deputy Minister of Health—was also funded through the federal VOC Strategy in March 2021 to develop mechanisms (e.g., a national biobank, wastewater monitoring, and resource-sharing agreements) to facilitate rapid research on VOCs from the immunology, laboratory science, genomics and sequencing, modeling and computational biology, and public health and social systems perspectives (CanCOVID, 2021c; CoVaRR-Net, 2021). CoVaRR-Net collaborates with the NML at PHAC, CanCOGeN, and PT public health labs, with plans to also establish an Indigenous Network for VOCs (CoVaRR-Net, 2021). In the long-term, CoVaRR-Net intends to morph into a Pandemic Preparedness Network with established channels to rapidly mobilize findings to Canadian and international decision-makers (CanCOVID, 2021c; CoVaRR-Net, 2021).
Lastly, in April 2020, the Health Portfolio funded the COVID-19 Immunity Task Force (CITF) to support, fund, and harmonize research on COVID-19 seroprevalence and vaccine effectiveness, safety, and immunogenicity, to develop population immunity models that can guide FPT pandemic decisions (COVID-19 Immunity Task Force, 2021a). To conduct its work, the CITF has developed partnerships with FPT governments, public health agencies, academic institutions, and community organizations, and the CITF Secretariat is housed at McGill University in Canada (COVID-19 Immunity Task Force, 2021a). In its first year, the CITF funded the SeroTracker—a seroprevalence dashboard using Canadian and international serosurvey data (COVID-19 Immunity Task Force, 2021b). Studies on vaccine safety and effectiveness are overseen by the Vaccine Surveillance Reference Group—an expert group reporting to the President of PHAC, NACI, and other FPT actors that is independent from the CITF, but is supported by its Secretariat (COVID-19 Immunity Task Force, 2021c).
Evidence syntheses
The mounting demand for evidence reviews across federal departments and PT authorities, caused by the rapid pace and low methodological quality of much of the early COVID-19 research, exceeded the capacity of PHAC departments (e.g., the Emerging Science Group) to perform this work internally. Arm’s-length actors, such as the NCCs and the CCA, were leveraged to build surge capacity for evidence review, synthesis, and dissemination, as these are the core functions of these organizations. However, while these channels were well-equipped for long-term projects, there remained a need for rapid short-term evidence reviews on focused research questions.
Two initiatives were developed to meet this demand: CanCOVID and the COVID-19 Evidence Network to Support Decision-making (COVID-END) (Public Health Agency of Canada, 2021k). CanCOVID—an invitation-based network of verified members of the COVID-19 research and response community (both within Canada and internationally)—was created by the OCSA in April 2020 with ISED funding, following the recommendations of the Expert Panel on COVID-19, the DSA Network, and the U15 Group of Canadian Research Universities (a collective of Canada’s most research-intensive universities) (CanCOVID, 2021a; Office of the Chief Science Advisor, 2020a; Public Health Agency of Canada, 2021k). The mission of CanCOVID is to foster collaborations and rapid knowledge sharing within the scientific community, and to provide a line of communication between scientists and decision-makers (CanCOVID, 2021a). The CanCOVID platform consists of moderated subject-based channels hosting rapid evidence reviews and other resources on over 20 topics, such as COVID-19 in schools, testing and tracing, mental health and wellbeing, and health equity (CanCOVID, 2021a; Office of the Chief Science Advisor, 2020a).
COVID-END is a CIHR-funded time-limited network of over 50 Canadian and international research groups specializing in evidence synthesis, health technology assessment, and guideline development. COVID-END was created in April 2020 and is partnered with the NCC for Methods and Tools, which is housed at McMaster University in Canada (COVID-END, 2021; Grimshaw et al., 2020; Office of the Chief Science Advisor, 2020a). The COVID-END network performs evidence syntheses (including ‘living’ reviews) and environmental scans on various COVID-19-related topics, such as public health measures, clinical therapeutics, health system resource management, and economic and social responses (COVID-END, 2021; Office of the Chief Science Advisor, 2020a). COVID-END also aims to reduce duplication of research projects by providing a platform for researcher collaboration and coordination (COVID-END, 2021).
Discussion
In this paper, we described Canada’s science advice ecosystem prior to the COVID-19 pandemic and detailed the new science advisory bodies formed by the federal Health and Science Portfolios throughout the first two years of the COVID-19 pandemic. The COVID-19 pandemic has yielded a significantly more complex federal science advice ecosystem than any previous public health emergencies in Canada. For comparison, one ad-hoc group of volunteer experts (including physicians, infection control practitioners, and administrators) self-organized into a scientific advisory committee during the SARS outbreak in 2003, focusing on quarantine guidelines, as well as hospital isolation precautions, employee screening, and patient transfers (Naylor, 2003). The response to the H1N1 pandemic in 2009 was led by the federal Health Portfolio, with time-limited task groups established to support key actors in the FPT governance structure, such as the PHN Council (Public Health Agency of Canada, 2011). Below, we reflect on our findings and discuss directions for future research to strengthen the federal science advice ecosystem for public health emergencies in Canada.
Interpretation and implications for science advice for public health emergencies
Canada’s seemingly ad-hoc approach to science advice during the first two years of the COVID-19 pandemic, involving the formation of advisory bodies with unclear coordination and time-limited mandates, presents a concern not only for ongoing pandemic management, but also—as seen in previous outbreaks—sustained preparedness for future public health emergencies. For instance, while the H1N1 pandemic marked significant engagement of mathematical modeling experts to support decisions related to antivirals, vaccines, and other public health measures (Moghadas et al., 2011), new distinct external modeling expert groups were established by both the Health and Science Portfolios during the COVID-19 pandemic (Office of the Chief Science Advisor, 2020c; Public Health Agency of Canada, 2021f, 2022b). An Office of Audit and Evaluation (2020) review of Canada’s response to the first COVID-19 wave further documented that the Health Portfolio lacked a senior lead responsible for modeling and that the coordination between the Health Portfolio and other federal departments involved in modeling was poor (Office of Audit and Evaluation, Health Canada and Public Health Agency of Canada, 2020), potentially resulting in duplicated efforts. Another notable example involves the vaccine-focused advisory groups. Although the federal government has had a longstanding process for obtaining medical and scientific advice on vaccine safety and efficacy through NACI, guidance regarding vaccine distribution was largely the responsibility of PT governments (Ismail et al., 2010). The establishment of the COVID-19 Vaccine Task Force by the Science Portfolio, however, suggests that a logistics-oriented advisory body was needed at the federal level to advise on vaccine procurement, development, and manufacturing (Government of Canada, 2020a, 2020b).
The COVID-19 pandemic can be characterized as a “complex intergovernmental problem”, requiring well-coordinated intergovernmental collaboration (at times through novel avenues) (Paquet and Schertzer, 2020). Wilson et al. (2004) proposed that intergovernmental relationships in public health in Canada can be broadly described by considering vertical (i.e., across the FPT orders of government) and horizontal (i.e., across sectors within each order of government) relationships. Currently, there are no formal horizontal coordination mechanisms for science advice in public health at the federal level, and a 2019 OCSA simulation exercise of science advice in a complex national emergency revealed that science advice was generally fragmented across Canada’s federal departments (Office of the Chief Science Advisor, 2020a). The PHN governance structure and pandemic response plans largely focus on vertical coordination between FPT actors within the health sector. Indeed, the latest (2017) internal operational review of the PHN identified a need to increase the visibility of the PHN among federal departments outside of the Health Portfolio (Dyke, 2017). While the newly established DSA Network is the intended mechanism for horizontal coordination of science advice across federal departments (e.g., through ex-officio membership), it remains to be seen whether this approach has translated to routinized interdepartmental engagement. Notably, among its short- to mid-term goals, the 2021 interim FPT Public Health Response Plan for Ongoing Management of COVID-19 stated that the Health Portfolio intended to work with the OCSA to support the COVID-19 response (Public Health Agency of Canada, 2021f). Explicating this relationship between the Health and Science Portfolios in science advice procurement should be prioritized in Canada’s revised pandemic plans to improve the coordination of science advice across sectors. As recommended by the Senate Committee review of Canada’s H1N1 response, pandemic plans should also be responsive to “[real-time] observations on the ground, rather than a potential worst-case scenario” (Eggleton et al., 2010).
As a stress test of the current systems and processes, the COVID-19 pandemic opens a policy window of opportunity to better institutionalize the federal science advice ecosystem (Di Ruggiero et al., 2022; Kuchenmüller et al., 2022; Paquet and Schertzer, 2020; Quirion et al., 2016). Institutionalization of science advice can be understood as the process and outcome of maintaining and reinforcing norms and practices that, based on collective meaning and endowment of resources, allow expertise and evidence to become legitimized and routinized in health policy-making (Kuchenmüller et al., 2022). Kuchenmüller et al. (2022) emphasize that strengthening the governance of science advice—that is, establishing formalized structures that span the boundaries and promote interaction between science and policy—is one of the foundational domains of institutionalization that protects the science advice ecosystem from contextual and political changes. Resourcing and institutionalizing science advice has also been identified as an important step towards strengthening the governance of public health functions at the federal level overall and achieving learning public health systems in Canada (Di Ruggiero et al., 2022).
Several approaches to institutionalizing science advice for public health emergencies have been proposed in Canada within the past two decades. After SARS, a CIHR-led document review and stakeholder consultation recommended developing a centre of population and public health research evidence, which would function in concert with the then newly established PHAC and NCCs to synthesize, appraise, and disseminate population and public health research and identify evidence gaps (Kiefer et al., 2005). The Fundamental Science Review (2017) recommended legislating an independent National Advisory Council on Research and Innovation, with formalized relationships with the Prime Minister’s Office, the OCSA, and the Ministers of ISED and Health, to oversee the federal science ecosystem, including extramural research fundingFootnote 10 (Canada’s Fundamental Science Review and Naylor, 2017). Recently, some scholars have called for creating a federal agency for science advice for health emergenciesFootnote 11, solely focused on centralizing and coordinating science advice across federal departments to set the standard for how science advice is procured and used during and outside of health emergencies (Tuohy, 2021).
The need for better institutionalization of well-coordinated intergovernmental collaboration on science advice for public health emergencies is mirrored globally. An International Network for Government Science Advice review of 22 countries’ early COVID-19 responses noted that the predominance of newly struck inter-ministerial committees suggested that countries both recognized a need for better horizontal collaboration and lacked pre-existing mechanisms to implement it (Allen et al., 2020). Another review of 28 countries’ early COVID-19 responses showed that while the formation of multidisciplinary committees was a feature of high-performing responses, poor coordination among such committees to rapidly inform policy decisions was a feature of low-performing responses (Haldane et al., 2021). Recent case studies of the UK, Sweden, Philippines, and North Carolina (US) COVID-19 responses concluded that a standing independent national authority dedicated to science advice for health security and health emergencies was necessary to ensure that pandemic decisions are evidence-informed and expert-driven (Brusselaers et al., 2022; Di Ruggiero et al., 2022; Vallejo and Ong, 2022; Weinkle, 2022). Comparative research is needed to elucidate the optimal institutional arrangement for science advice for public health emergencies in Canada. In particular, we highlight two such research considerations: the independence of science advisory bodies and the role of subnational advisory bodies.
Directions for future research on science advice in Canada
Independence of science advisory bodies
Effective science advice must be, and must be perceived to be, independent from individuals or groups that may have a vested interest in the outcome of the decision for which the advice was requested, such as the decision-makers using the advice (Groux et al., 2018; Royal Society of Canada, 2015). For instance, while the CPHO has a mandate to directly and independently communicate with the Cabinet and the public, some legal scholars have argued that this model of the CPHO role is inherently vulnerable to generating tensions between the (private) advisory role and the (public) communicator role (Fafard et al., 2018). Specifically, the CPHO’s authority to communicate on public health matters is not coupled with security of tenure, which may challenge the CPHO’s ability to openly advocate on issues that run counter to the Cabinet’s position (Fafard et al., 2018; Fierlbeck and Lahey, 2013; MacAulay et al., 2022; Wilson and Keelan, 2008). Similar tensions could be inferred about time-limited expert advisory groups, as, while members of these groups are external to the federal government, the federal departments procuring advice are involved in member selection, establishing the terms of reference, collecting materials to be evaluated by the advisory groups, and terminating participation of individual members or the advisory group overall (Health Canada, 2011; Public Health Agency of Canada, 2021g). Questions have also been raised about the independence of certain arm’s-length advisory bodies, like the CCA. Although the CCA maintains relative autonomy in expert selection, deliberation, and production of their reports, its continued operation depends on federal funding (Groux et al., 2018; Quirion et al., 2016).
Concerns about the independence of science advisory bodies from those requesting advice do not immediately invalidate the advice—indeed, as acknowledged by Bijker et al. (2009), independence and institutionalization of science advisory bodies is a balance, whereby “science that is too detached from the policy domain can barely contribute to the decision process…but science that manifests itself as having a pronounced political message quickly risks becoming a part of political fighting”. Others have similarly argued that proximity of advisory roles, such as the CPHO, to federal actors may give advisors greater influence over the decision-makers’ agenda and enhance the actionability of advice in the context of decision-makers’ priorities (Fafard and Forest, 2016; Groux et al., 2018; MacAulay et al., 2022). Evaluations of the degree of independence of advisory actors (including their means of expert appointment; the flow of information between the advisory groups and decision-makers; and the roles of experts, decision-makers, and commissioners in issue selection and advice generation) (Groux et al., 2018) are needed to inform a nuanced understanding of how advisory group independence shapes science advice in public health emergencies.
Independence of science advisory bodies can also be interpreted as protection of science advice from the influence of commercial or political interests (Groux et al., 2018). The federal policies on external advisory committees state that “a person’s affiliations and interests do not necessarily prevent him or her from being a member of an advisory body, since his or her input could nevertheless be valuable to the advisory body’s mandate” (Health Canada, 2011; Public Health Agency of Canada, 2021g). ISED thus made a deliberate decision to include individuals from the biotechnology sector with potential conflicts of interest (COI) in the COVID-19 Therapeutics (Innovation, Science and Economic Development Canada, 2021d) and Vaccine (Government of Canada, 2020b) Task Forces. Although advisory body members must disclose their interests prior to each meeting and recuse themselves from commenting on issues where they may have real or perceived COIs (Health Canada, 2011; Public Health Agency of Canada, 2021g), the registries of these disclosures only began to be published in September 2020, in response to public pressure (and in breaking from the standard ISED policy for volunteer external advisory groups, whose COIs are not overseen by the federal ethics commissioner and thus, are not listed in the commissioner’s public registry) (Connolly, 2020a, 2020b; Innovation, Science and Economic Development Canada, 2021c; Lexchin, 2022; National Research Council of Canada, 2021a, 2021b). Furthermore, as documented by Lexchin (2022) and Grundy (2022), the nature and severity of these COIs was not publicized and could not be inferred, as the content of Task Force meetings is not publicly available and the primary interest served by the Task Forces is not clearly identified in their mandates. Given the considerable engagement of industry representatives in COVID-19 advisory bodies, it is imperative to evaluate how the present COI mitigation policies have shaped the quality of science advice.
Role of subnational authorities in the science advice ecosystem
The presence of PT public health authorities and science advisory structures adds another layer of complexity to the national science advisory milieu. Similar to the role of the CPHO at the federal level, there are inherent contradictions between the advisory and spokesperson functions of CMOHs at the PT level (Fafard et al., 2018; MacAulay et al., 2022). These tensions have become especially salient during the COVID-19 pandemic, as the CMOH scope of work and public visibility have been stretched arguably beyond that which was originally intended (MacAulay et al., 2022). Across Canada’s PTs, this has reignited the debate on the amount of authority CMOHs should have in their advisory, managerial, and public communicator roles, as well as the extent of independence from decision-makers CMOHs should be able to exercise in carrying out these roles (Attaran and Hardcastle, 2020; Bellefontaine, 2020; Weber, 2020). Empirical evaluations of how the CMOH role (and its public and political perception) has evolved throughout the pandemic across different PT institutional and political contexts are ongoing (MacAulay et al., 2022).
Outside the health sector, there were no PT-level science advisory structures analogous to the federal OCSA before the COVID-19 pandemic (Council of Canadian Academies, 2017). The sole exception is the province of Quebec, where the position of the Chief Scientist of Québec was created in 2011 to advise the Minister of the Economy and Innovation and to oversee the three Québec research funds (Gouvernement du Québec, 2016; Quirion et al., 2016). In the first year of the COVID-19 pandemic, the Chief Scientist’s advisory role was extended to supporting the provincial Premier and the Minister of Health and Social Services (Gouvernement du Québec, 2020). New science advisory groups were also created across the other PTs throughout the COVID-19 pandemic. For example, the Ontario COVID-19 Science Advisory TableFootnote 12 is a group of volunteer scientific experts and health system leaders that provides weekly evidence summaries to the COVID-19 Health Coordination Table of the Province of Ontario (Ontario COVID-19 Science Advisory Table, 2022). In British Columbia, the COVID-19 Modeling GroupFootnote 13 is a group of experts in epidemiology, mathematics, and data analysis from the province’s three major universities and the private sector, working on rapid response modeling of the COVID-19 pandemic (BC COVID-19 Modeling Group, 2022). Although both groups were largely untethered to the PT governments and formal advisory structures and could be considered “shadow” science advisory actorsFootnote 14, their influence on FPT decision-making and the public, and their ability to exercise their independence despite political pressures should be evaluated. It also remains unclear to what extent federal advice was considered in the advice and decisions made at the PT or local level.
Limitations
A strength of this jurisdictional review is the provision of a comprehensive account of Canada’s federal science advisory ecosystem for public health from SARS to the first two years of the COVID-19 pandemic, with recommendations for policy and research. This complements reviews from other scholars of Canada’s early federal science advice mechanisms not specific to public health (Quirion et al., 2016) and Canada’s federal public health governance structure (Di Ruggiero et al., 2022). Descriptive country-level case studies also serve a critical step for future analyses of cross-country comparisons seeking to understand how contextual differences may have shaped policy outcomes (Marmor, 2017).
Some limitations should also be acknowledged. First, we relied on publicly available literature. As such, while we attempted to achieve a comprehensive search, we are only able to describe the most salient science advice actors and mechanisms documented in the retrieved sources. Relatedly, we did not consider informal pathways to procuring and providing science advice or international exchanges of expertise and evidence. This may have resulted in “false saturation”, whereby certain advisory bodies may have been missed due to their informal status or short lifespan (“survivorship” bias). Second, although most public health measures for COVID-19 are within the remit of subnational authorities in Canada, our focus on science advice at the federal level limits us from commenting on how science advice was mobilized in these subnational structures. Third, the COVID-19 pandemic is still ongoing at the time of writing; thus, our goal was to provide a descriptive account of Canada’s evolving federal science advisory landscape throughout the first two years of the pandemic, rather than to make evaluative inferences about its effectiveness (e.g., in terms of translation of science advice into policy decisions and public guidance, application of science advice in the context of the precautionary principle, or the perceptions of science advisory bodies and their advice by decision-makers and the public). Furthermore, a recent independent assessment of 100 Government of Canada policies found that inferring whether policies were informed by scientific evidence from publicly available information is difficult (Qaiser et al., 2022). This suggests a need for high-quality qualitative research with primary data collection. Finally, while we captured the release of select reports on COVID-19 in certain populations (e.g., long-term care residents, children), given the disproportionate and inequitable impact of the pandemic on elderly, low income, racialized, and migrant groups, an in-depth assessment of the nature and content of the advice provided is needed.
Conclusion
This detailed review of the first two years of the COVID-19 pandemic in Canada has highlighted the challenges faced by the federal government in procuring science advice to support decision-making during a public health emergency. In its response to COVID-19, the federal government appeared to follow the existing playbook, which was established after the SARS and H1N1 outbreaks and was largely focused on the health sector. However, the COVID-19 pandemic also fueled a rapid proliferation of time-limited science advisory bodies within both the federal Health and Science Portfolios, with limited and unclear mechanisms for horizontal coordination and collaboration. Although the engagement of federal departments outside the health sector is promising, the COVID-19 experience in Canada supports the need to institutionalize science advisory bodies for public health to improve pandemic preparedness and ensure rapid mobilization of well-coordinated and independent advice in future emergencies. This review also identified pressing areas for further inquiry to strengthen science advice for public health in Canada, such as on COI mitigation strategies and the complex interplay between the federal and subnational science advisory bodies.
Data availability
Datasets were not generated and/or analyzed for this study. This work analyzed published literature sources that are cited in the manuscript and are accessible either publicly or with academic institutional credentials.
Notes
In this paper, when referring to “subnational” governments, we primarily mean PT authorities. However, it is relevant to note that within some PTs, public health governance is further decentralized through regional or municipal health authorities, helmed by locally elected or appointed boards that are led by Medical Officers of Health (Bana et al., 2018; Di Ruggiero et al., 2022). During the COVID-19 pandemic, some of these authorities implemented protective public health measures (e.g., indoor masking) through local bylaws either in conflict with or prior to official provincial guidance.
Although largely beyond the scope of the present paper, it should be noted that the three most populous provinces in Canada also have arm’s-length province-wide agencies responsible for public health surveillance, laboratories, and provision of technical advice and guidance—the British Columbia Centre for Disease Control, Public Health Ontario, and l’Institut national de santé publique du Québec (Bana et al., 2018; Di Ruggiero et al., 2022).
In Canada, deputy ministers are appointed senior civil servants acting as deputy heads and managerial/administrative heads of FPT governmental departments or ministries. Deputy ministers are primarily responsible for the coordination of policy, program advice, and technical analysis to the minister (the department head—an elected member of Parliament) and for the management of the department and program implementation.
The Canadian Ministry—commonly referred to as the Cabinet—consists of the Prime Minister of Canada and 38 federal Ministers. The Cabinet is the central decision-making forum in government that brings all the Ministers together to set the federal government’s policies and priorities. The day-to-day work of the Cabinet is carried out by Cabinet committees, whose mandates and membership are set out by the Prime Minister (with the exception of the Treasury Board, whose mandate and membership are established in law).
As outlined in the FPT Public Health Response Plan for Biological Events (Pan-Canadian Public Health Network, 2018b), public health emergency response in Canada is classified into four levels: normal (level 1), heightened (level 2), escalated (level 3), and emergency (level 4). Level 1 or 2 responses typically involve ongoing monitoring and assessment and do not require a coordinated FPT response. Events qualifying for a level 3 or 4 response typically affect multiple jurisdictions and require federal surge capacity or centralized planning; bulk purchasing and consistent use of limited resources (e.g., medical countermeasures); new or revised technical guidance documents and recommendations; collated national epidemiological data; and consistent approaches to border screening, contact identification and follow-up, and public and professional communications (Pan-Canadian Public Health Network, 2018b). Level 3 or 4 events thus trigger the implementation of a coordinated FPT response and activation of the SAC and the SAC Secretariat (Pan-Canadian Public Health Network, 2018b).
In May 2021, the unanimous adoption of Motion M-38 marked the creation of a new Standing Committee on Science and Research in the House of Commons, effective in the next Parliament (Dufour, 2021). The Standing Committee was created to affirm the government’s commitment to evidence-informed decision-making (Evidence for Democracy, 2021) and its mandate includes “all matters relating to science and research, including any reports of the Chief Science Advisor” (House of Commons Canada, 2021).
As we focus on describing the emergence of new science advisory bodies, a detailed discussion of the federal whole-of-government incident management decision-making structure is largely beyond the scope of this paper. Nonetheless, the apparent need for a federal decision-making committee outside of the existing incident response mechanism is notable. The Sub-Committee on the Federal Response to COVID-19 is situated within the Cabinet Committee on Safety, Security and Emergencies, is chaired by the Minister of Intergovernmental Affairs, Infrastructure and Communities, and has representation from 11 federal ministers (Prime Minister of Canada Office, 2021c). The Cabinet Committee on Safety, Security and Emergencies, chaired by the Minister of Emergency Preparedness, considers national safety and security threats, manages ongoing emergencies, and ensures strategic and integrated emergency management across the federal government (Prime Minister of Canada Office, 2021a).
The Public Health Working Group on Remote and Isolated Communities is a new group established during the COVID-19 pandemic in response to the recommendations of the 2017 internal operational review of the PHN, which identified a need for better engagement of remote and Indigenous communities in the PHN structure (Di Ruggiero et al., 2022; Dyke, 2017). The Surveillance Expert Working Group and the Canadian Pandemic Influenza Plan Task Group are existing PHN task groups, named in the 2018 FPT Public Health Response Plan for Biological Events (Pan-Canadian Public Health Network, 2018b).
The overall objective for Canada’s pandemic preparedness and response—“first, to minimize serious illness and overall deaths, and second to minimize societal disruption among Canadians as a result of an influenza pandemic”—has remained unchanged from the first release of the CPIP in 2004 (Pan-Canadian Public Health Network, 2018a).
Progress on this recommendation is ongoing, with ISED announcing the Council on Science and Innovation in January 2019; however, the status of the Council is not publicly known as of December 2021 (Canada’s Fundamental Science Review & Naylor, 2017; Innovation, Science and Economic Development Canada, 2019).
Some scholars have also called for turning the new Cabinet Sub-Committee on the Federal Response to COVID-19 into a standing committee on emergencies to ensure that emergency planning is an ongoing function between acute crises (Cappe, 2020). Formalizing the relationship between the science advice ecosystem with this decision-making body is an important consideration for institutionalizing science advice for public health in Canada.
At the time of writing, the Ontario COVID-19 Science Table was housed at the Dalla Lana School of Public Health at the University of Toronto and its Scientific Director and Secretariat were funded by the Dalla Lana School of Public Health and Public Health Ontario (Ontario COVID-19 Science Advisory Table, 2022). The Science Table’s outputs (e.g., briefings, modeling projections, and other reports) were publicly available.
At the time of writing, the COVID-19 Modeling Group was sponsored by the participating provincial universities and the Pacific Institute for the Mathematical Sciences (a mathematics research consortium of select universities from Canada and the United States) (BC COVID-19 Modeling Group, 2022). The Modeling Group’s outputs were publicly available.
Shadow science advice can be defined as “formal or informal mechanisms of advice established outside of governmental science advisory processes to provide a counter or opposition body of legitimate, authoritative, and credible guidance to policy makers” (Pielke, 2021). Several shadow advisory groups have emerged during the COVID-19 pandemic internationally, including the Independent SAGE in the UK, the Science Forum COVID-19 in Sweden, the Red Team in the Netherlands, and the OCTA Research Group in the Philippines (Brusselaers et al., 2022; Pielke, 2021; Vallejo & Ong, 2022). Many such groups have gained legitimacy in the public eye due to their direct communication with the media and the public; however, the ethical and appropriate practices for shadow science advice remain to be established (Pielke, 2021). Interestingly, according to a March 31, 2022 announcement, the Ontario COVID-19 Science Table (a volunteer group of experts previously untethered to the provincial government) has begun to be folded into Public Health Ontario—an arm’s-length governmental agency (Wallace, 2022).
References
Allen K, Buklijas T, Chen A, Simon-Kumar N, Cowen L, Wilsdon J, Gluckman P (2020) Tracking global evidence-to-policy pathways in the coronavirus crisis: a preliminary report. International Network for Government Science Advice. https://www.ingsa.org/wp-content/uploads/2020/09/INGSA-Evidence-to-Policy-Tracker_Report-1_FINAL_17Sept.pdf
Allin S, Fitzpatrick T, Marchildon GP, Quesnel-Vallée A (2021) The federal government and Canada’s COVID-19 responses: from ‘we’re ready, we’re prepared’ to ‘fires are burning.’ Health Econ Policy Law, 1–19. https://doi.org/10.1017/S1744133121000220
Attaran A, Hardcastle L (2020, November 13). Politicians are failing. Canada’s chief medical officers need to step in. Maclean’s. https://www.macleans.ca/opinion/politicians-are-failing-canadas-chief-medical-officers-need-to-step-in/
Bana R, Cram J, Hu J, Pawa J, Waddell K (2018). Public Health Systems in Canada. Public Health Physicians of Canada. https://www.phpc-mspc.ca/resources/Documents/PHSC-24Jul20.pdf
BC COVID-19 Modelling Group (2022) About. BC COVID-19 Modelling Group. https://bccovid-19group.ca/
Bellefontaine M (2020, September 30) UCP votes against independent role for chief medical officer of health Social Sharing. CBC News. https://www.cbc.ca/news/canada/edmonton/alberta-chief-medical-officer-health-committee-1.5745437
Bhatia D, Vaga K, Roerig M, Allin S, Pawa J, Marchildon G (2020) COVID-19 Case and Contact Management Strategies in Canada (Rapid Review No. 27). North American Observatory on Health Systems and Policies, Toronto. https://ihpme.utoronto.ca/wp-content/uploads/2020/11/NAO-Rapid-Review-27_EN.pdf
Bijker WE, Bal R, Hendriks R (2009) The work and the product of scientific advising. In: The Paradox of Scientific Authority. The MIT Press. https://doi.org/10.7551/mitpress/7934.003.0008
Braën A (2004) 1. Health and the distribution of powers in Canada. In: Forest P-G, Marchildon G, McIntosh T (eds.). The Governance of Health Care in Canada. University of Toronto Press. https://doi.org/10.3138/9781442681392-004
Brideau I, Brosseau L (2019) The distribution of legislative powers: an overview (Background Paper No. 2019-35-E; p. 20). Legal and Social Affairs Division, Parliamentary Information and Research Service, Library of Parliament. https://bdp.parl.ca/sites/PublicWebsite/default/en_CA/ResearchPublications/201935E
Brusselaers N, Steadson D, Bjorklund K, Breland S, Stilhoff Sörensen J, Ewing A, Bergmann S, Steineck G (2022) Evaluation of science advice during the COVID-19 pandemic in Sweden. Humanit Soc Sci Commun 9(1):91. https://doi.org/10.1057/s41599-022-01097-5
Cameron D, Simeon R (2002) Intergovernmental relations in Canada: the emergence of collaborative federalism. Publius J Federalism 32(2):49–72. https://doi.org/10.1093/oxfordjournals.pubjof.a004947
Canada & Privy Council Office (2004) Securing an open society: Canada’s national security policy. Privy Council Office. https://www.deslibris.ca/ID/200507
Canada Research Coordinating Committee. (2020, February 26). About the Canada Research Coordinating Committee. Government of Canada. https://www.canada.ca/en/research-coordinating-committee/corporate/about-canada-research-coordinating-committee.html
Canada’s Fundamental Science Review, & Naylor, CD (2017) Investing in Canada’s Future–Strengthening the Foundations of Canadian Research (p. 25). Government of Canada. http://www.sciencereview.ca/eic/site/059.nsf/eng/home
Canadian Institute for Health Information (2020) Pandemic experience in the long-term care sector: how does Canada compare with other countries? CIHI, 9, Ottawa, ON: CIHI, 9
Canadian Institutes of Health Research (2018, December 20). About Us. Government of Canada. https://cihr-irsc.gc.ca/e/37792.html
Canadian Public Health Association (2021) Review of Canada’s Initial Response to the COVID-19 Pandemic. Canadian Public Health Association. https://www.cpha.ca/review-canadas-initial-response-covid-19-pandemic
Canadian Public Health Laboratory Network. (2011) Core Functions of Canadian Public Health Laboratories. National Collaborating Centre for Infectious Diseases. https://nccid.ca/wp-content/uploads/sites/2/2020/03/CPHLN-Core-Functions.pdf
CanCOVID (2021a) About CanCOVID. CanCOVID. https://cancovid.ca/about/
CanCOVID (2021b, January 28) Canada’s COVID-19 Task Forces Progress Update [Webinar]. CanCOVID. https://cancovid.ca/2021/01/covid-19-task-forces-progress-update/
CanCOVID (2021c, April 27) CoVaRR Net–Canada’s latest research network on the pandemic response [Webinar]. CanCOVID. https://cancovid.ca/event/covarr-net-canadas-latest-research-network-on-the-pandemic-response/
Cappe M (2020, August 24) Governance: institutions, processes and people. Centre for International Governance Innovation. https://www.cigionline.org/articles/governance-institutions-processes-and-people/
CBC News (2008, January 25) Scientists lament closing of key advisory office. CBC News. https://www.cbc.ca/news/science/scientists-lament-closing-of-key-advisory-office-1.756700
Connolly A (2020a, September 5) COVID-19 vaccine task force members have declared 18 conflicts of interests so far. Global News. https://globalnews.ca/news/7314231/coronavirus-vaccine-task-force-canada/
Connolly A (2020b, September 22) Canadians can now see conflicts of interest declared by COVID-19 vaccine task force. Global News. https://globalnews.ca/news/7351016/covid-19-vaccine-task-force-conflicts-of-interest-disclosures/
Council of Canadian Academies (2017) Science Policy: Considerations for Subnational Governments. Alberta WaterSMART Solutions. http://deslibris.ca/ID/10092128
Council of Canadian Academies (2021) About the Council of Canadian Academies. Council of Canadian Academies. https://cca-reports.ca/about/
CoVaRR-Net (2021) Our Mandate. CoVaRR-Net. https://covarrnet.ca/our-mandate/
COVID-19 Immunity Task Force (2021a) Mandate & Strategy. COVID-19 Immunity Task Force. https://www.covid19immunitytaskforce.ca/mandate-strategy/
COVID-19 Immunity Task Force (2021b) SeroTracker. COVID-19 Immunity Task Force. https://www.covid19immunitytaskforce.ca/serotracker/
COVID-19 Immunity Task Force (2021c) Vaccine surveillance reference group (VSRG). COVID-19 Immunity Task Force. https://www.covid19immunitytaskforce.ca/vaccine-surveillance-reference-group-vsrg/
COVID-END (2021) About us. McMaster Health Forum. https://www.mcmasterforum.org/networks/covid-end/about-covid-end
Delacourt S (2021, February 21) ‘The nudge unit’: Ottawa’s behavioural-science team investigates how Canadians feel about vaccines, public health and who to trust. Toronto Star. https://www.thestar.com/politics/political-opinion/2021/02/21/the-nudge-unit-ottawas-behavioural-science-team-investigates-how-canadians-feel-about-vaccines-public-health-and-who-to-trust.html
Di Ruggiero E, Bhatia D, Umar I, Arpin E, Champagne C, Clavier C, Denis J-L, Hunter D (2022) Governing for the public’s health: Governance options for a strengthened and renewed public health system in Canada. National Collaborating Centre for Healthy Public Policy, National Collaborating Centres for Public Health. https://nccph.ca/projects/reports-to-accompany-the-chief-public-health-officer-of-canadas-report-2021/governing-for-the-publics-health-governance-options-for-a-strengthened
Dufour P (2021, June 1). Parliament and science: towards a more effective dialogue. evidence for democracy. https://evidencefordemocracy.ca/fr/content/parliament-and-science-towards-more-effective-dialogue
Dyke E (2017) Public health network operational review findings and recommendations: final report [Internal Report]. Pan-Canadian Public Health Network. Unpublished
Eggleton A, Ogilvie KK, Standing Senate Committee on Social Affairs, Science and Technology (2010) Canada’s response to the 2009 H1N1 influenza pandemic. Senate, Government of Canada. https://sencanada.ca/content/sen/Committee/403/soci/rep/rep15dec10-e.pdf
Evidence for Democracy (2019) The fundamental science review: where are we at? Evidence for democracy. https://evidencefordemocracy.ca/en/content/fundamental-science-review-where-are-we
Evidence for Democracy (2021, December 16) Overheard at the Standing Committee on Science and Research. Evidence foR Democracy. Overheard at the Standing Committee on Science and Research
Fafard P, Forest P-G (2016) The loss of that which never was: evaluating changes to the senior management of the Public Health Agency of Canada: CHANGES TO THE PUBLIC HEALTH AGENCY OF CANADA. Can Public Adm 59(3):448–466. https://doi.org/10.1111/capa.12174
Fafard P, McNena B, Suszek A, Hoffman SJ (2018) Contested roles of Canada’s Chief Medical Officers of Health. Can J Public Health 109(4):585–589. https://doi.org/10.17269/s41997-018-0080-3
Fierlbeck K (2010) Public health and collaborative governance. Can Public Adm 53(1):1–19. https://doi.org/10.1111/j.1754-7121.2010.00110.x
Fierlbeck K, Lahey W (2013) Health Care Federalism in Canada: Critical Junctures and Critical Perspectives. McGill-Queen’s Press-MQUP
Genome Canada (2021a) About. Genome Canada. https://www.genomecanada.ca/en/about
Genome Canada (2021b) Genomics is on a mission to respond to COVID-19. Genome Canada. https://www.genomecanada.ca/en/cancogen
Genome Canada (2021c, April 27) Genome Canada goes live with national Data Portal to track COVID-19 in real time. Genome Canada. https://www.genomecanada.ca/en/news/genome-canada-goes-live-national-data-portal-track-covid-19-real-time
Genome Canada Canadian COVID-19 Genomics Network (CanCOGeN) & Canadian Public Health Laboratory Network CanCOGeN Working Group (2021) Canadian national COVID-19 genomics surveillance priorities for existing and emerging variants of concern Canada. Commun Dis Rep 47(3):139–141. https://doi.org/10.14745/ccdr.v47i03a03
Gouvernement du Québec (2016, April) The Chief Scientist—Mandates. Gouvernement Du Québec. https://www.scientifique-en-chef.gouv.qc.ca/en/le-scientifique-en-chef/mandats/
Gouvernement du Québec (2020) Chief scientist of Québec’s Report 2019-2020. Gouvernement du Québec. https://www.scientifique-en-chef.gouv.qc.ca/wp-content/uploads/Rapport_SciChefQC_2019-2020_VA.pdf
Government of Canada (1985a) National Research Council Act. Justice Laws Website. https://laws-lois.justice.gc.ca/eng/acts/N-15/page-1.html#h-363424
Government of Canada (1985b) Statistics Act. Justice Laws Website. https://laws.justice.gc.ca/eng/acts/S-19/FullText.html
Government of Canada (2006, December 12) Public Health Agency of Canada Act. Justice Laws Website. https://lois-laws.justice.gc.ca/eng/acts/P-29.5/page-1.html#h-401111
Government of Canada (2007) Emergency Management Act (S.C. 2007, c. 15). Justice Laws Website. https://laws-lois.justice.gc.ca/eng/acts/e-4.56/
Government of Canada (2014, February 27) About Health Canada. Government of Canada. https://www.canada.ca/en/health-canada/corporate/about-health-canada.html
Government of Canada (2016, August 2) The role of the chief public health officer. Government of Canada. https://www.canada.ca/en/public-health/corporate/organizational-structure/canada-chief-public-health-officer/role-chief-public-health-officer.html
Government of Canada (2017, August 8). Health Portfolio. Government of Canada. https://www.canada.ca/en/health-canada/corporate/health-portfolio.html
Government of Canada (2019) Canada’s health care system. Government of Canada, https://www.canada.ca/en/health-canada/services/health-care-system/reports-publications/health-care-system/canada.html#a4
Government of Canada (2020a) Government of Canada announces major steps in treating and preventing COVID-19 through vaccines and therapies [Press release]. Government of Canada. https://www.canada.ca/en/innovation-science-economic-development/news/2020/08/government-of-canada-announces-major-steps-in-treating-and-preventing-covid-19-through-vaccines-and-therapies.html
Government of Canada (2020b) COVID-19 vaccine task force. Government of Canada. https://nrc.canada.ca/en/corporate/covid-19-vaccine-task-force
Government of Canada (2020c) Ad-hoc COVID-19 Clinical Pharmacology Task Group. Government of Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/covid-19-clinical-pharmacology-task-group.html
Government of Canada (2020d) Ad-hoc COVID-19 Clinical Pharmacology Task Group: terms of reference. Government of Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/covid-19-clinical-pharmacology-task-group/terms-reference.html#a31
Government of Canada (2021) COVID-19 testing and screening expert advisory panel: reports and summaries. Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/testing-screening-advisory-panel/reports-summaries.html
Government of Canada (2022, February 11) COVID-19 vaccination in Canada. Government of Canada. https://health-infobase.canada.ca/covid-19/vaccination-coverage/
Grimshaw JM, Tovey DI, Lavis JN, on behalf of COVID-END (2020) COVID-END: an international network to better co-ordinate and maximize the impact of the global evidence synthesis and guidance response to COVID-19. In: Collaborating in response to COVID-19: Editorial and methods initiatives across Cochrane. The Cochrane Collaboration, John Wiley & Sons, Ltd. https://doi.org/10.1002/14651858.CD202002
Groux GMN, Hoffman SJ, Ottersen T (2018) A typology of scientific advisory committees. Glob Challenges 2(9):1800004. https://doi.org/10.1002/gch2.201800004
Grundy Q (2022) Commentary–from transparency to accountability: finding ways to make expert advice trustworthy. Healthcare Policy|Politiques de Santé 17(3):28–33. https://doi.org/10.12927/hcpol.2022.26731
Guttmann A, Gandhi S, Wanigaratne S, Lu H, Ferreira-Legere L, Paul J, Gozdyra P, Campbell T, Chung H, Fung K, Chen B, Kwong J, Rosella L, Shah B, Saunders N, Paterson J, Bronskill S, Azimaee M, Vermeulen M, Schull M (2020) COVID-19 in Immigrants, Refugees and Other Newcomers in Ontario: Characteristics of Those Tested and Those Confirmed Positive, as of June 13, 2020. ICES, Toronto, ON. https://www.ices.on.ca/Publications/Atlases-and-Reports/2020/COVID-19-in-Immigrants-Refugees-and-Other-Newcomers-in-Ontario
Haldane V, Jung A-S, Neill R, Singh S, Wu S, Jamieson M, Verma M, Tan M, De Foo C, Abdalla SM, Shrestha P, Chua AQ, Nordström A, Legido-Quigley H (2021) From response to transformation: How countries can strengthen national pandemic preparedness and response systems. BMJ, e067507. https://doi.org/10.1136/bmj-2021-067507
Health Canada (2011, November 7) Health Canada Policy on External Advisory Bodies (2011). Government of Canada. https://www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/resource-centre/policy-external-advisory-bodies-health-canada-2011.html#deab
Health Canada (2021a, January 15) COVID-19 testing and screening expert advisory panel: overview. Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/testing-screening-advisory-panel.html
Health Canada (2021b, February 10) COVID-19 testing and screening expert advisory panel: members. Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/testing-screening-advisory-panel/members.html
Health Canada (2021c, February 17). Industry advisory roundtable on COVID-19 testing, screening, tracing and data management: overview. Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/testing-outreach-collaboration/industry-advisory-roundtable.html
House of Commons Canada (2021, November 22) Standing committee on science and research. Parliament of Canada. https://www.ourcommons.ca/Committees/en/SRSR
Impact & Innovation Unit (2018, May 22) What we do. Government of Canada. https://www.canada.ca/en/innovation-hub/services/what-we-do.html
Impact & Innovation Unit (2020, October 14). Annual report 2019-2020 (abridged). Government of Canada. https://www.canada.ca/en/innovation-hub/services/reports-resources/annual-report-2019-2020.html
Impact Canada Initiative (2022a) About impact Canada. Government of Canada. https://impact.canada.ca/en/about
Impact Canada Initiative (2022b) Applying BeSci to the COVID-19 Response. Government of Canada. https://impact.canada.ca/en/behavioural-science/timeline
Impact Canada Initiative (2022c) Campaigns. Impact Canada Initiative. https://impact.canada.ca/en/challenges/covid-communications/campaigns
Industry Canada (2008, June 21) [ARCHIVED]—Office of the National Science Advisor. Government of Canada. https://web.archive.org/web/20080621143954/http://strategis.ic.gc.ca/epic/site/onsa-bcns.nsf/en/Home
Innovation, Science and Economic Development Canada. (2018, August 29). Mandate. https://www.ic.gc.ca/eic/site/icgc.nsf/eng/h_00018.html
Innovation, Science and Economic Development Canada. (2019, January 8) Ministerial Appointment Opportunities (Anticipatory). Government of Canada. https://www.ic.gc.ca/eic/site/careers-carrieres.nsf/eng/00961.html
Innovation, Science and Economic Development Canada. (2020, July 31). External advisory bodies for Innovation, Science and Economic Development Canada. Government of Canada. https://www.ic.gc.ca/eic/site/icgc.nsf/eng/h_07689.html
Innovation, Science and Economic Development Canada. (2021a, January 12). Industry Strategy Council. Government of Canada. https://www.ic.gc.ca/eic/site/062.nsf/eng/h_00117.html
Innovation, Science and Economic Development Canada. (2021b, March 2). Archived—COVID-19 Therapeutics Task Force. Government of Canada. https://www.ic.gc.ca/eic/site/lsg-pdsv.nsf/eng/hn01780.html
Innovation, Science and Economic Development Canada. (2021c, March 2). Archived—COVID-19 Therapeutics Task Force Registry of Interests. Government of Canada. https://www.ic.gc.ca/eic/site/lsg-pdsv.nsf/eng/hn01783.html
Innovation, Science and Economic Development Canada. (2021d, March 2). Archived—Declaration of Interests Protocol for the COVID-19 Therapeutics Task Force. Government of Canada. https://www.ic.gc.ca/eic/site/lsg-pdsv.nsf/eng/hn01782.html
Innovation, Science and Economic Development Canada. (2021e, August 12). COVID-19 Exposure Notification App Advisory Council. Government of Canada. https://www.ic.gc.ca/eic/site/icgc.nsf/eng/h_07687.html
Innovation, Science and Economic Development Canada. (2021f, November 5). Innovation, Science and Economic Development portfolio. Government of Canada. https://www.ic.gc.ca/eic/site/icgc.nsf/eng/h_00022.html
Ismail SJ, Langley JM, Harris TM, Warshawsky BF, Desai S, FarhangMehr M (2010) Canada’s National Advisory Committee on Immunization (NACI): evidence-based decision-making on vaccines and immunization. Vaccine 28:A58–A63. https://doi.org/10.1016/j.vaccine.2010.02.035
Kiefer L, Frank J, Di Ruggiero E, Dobbins M, Manuel D, Gully PR, Mowat D (2005) Fostering evidence-based decision-making in Canada: examining the need for a Canadian Population and Public Health Evidence Centre and Research Network. Can J Public Health/Revue Canadienne de Santé Publique 96(3):I1–I19
Kuchenmüller T, Boeira L, Oliver S, Moat K, El-Jardali F, Barreto J, Lavis J (2022) Domains and processes for institutionalizing evidence-informed health policy-making: a critical interpretive synthesis. Health Res Policy Syst 20(1):27. https://doi.org/10.1186/s12961-022-00820-7
Lexchin J (2022) COVID-19 vaccine task force and conflicts of interest. Healthcare Policy|Politiques de Santé 17(3):20–27. https://doi.org/10.12927/hcpol.2022.26732
MacAulay M, Macintyre AK, Yashadhana A, Cassola A, Harris P, Woodward C, Smith K, de Leeuw E, Palkovits M, Hoffman SJ, Fafard P (2022) Under the spotlight: understanding the role of the chief medical officer in a pandemic. J Epidemiol Commun Health 76(1):100–104. https://doi.org/10.1136/jech-2021-216850
Marchildon GP, Allin S, Merkur S (2020) Canada: Health System Review (3rd ed., Vol. 22). North American Observatory on Health Systems and Policies & European Observatory on Health Systems and Policies, WHO Regional Office for Europe
Marmor TR (2017) Comparative studies and the drawing of policy lessons: describing, explaining, evaluating, and predicting. J Comp Policy Anal Res Pract 19(4):313–326. https://doi.org/10.1080/13876988.2017.1279439
Martin D, Miller AP, Quesnel-Vallée A, Caron NR, Vissandjée B, Marchildon GP (2018) Canada’s universal health-care system: Achieving its potential. Lancet 391(10131):1718–1735. https://doi.org/10.1016/S0140-6736(18)30181-8
McCoy LG, Smith J, Anchuri K, Berry I, Pineda J, Harish V, Lam AT, Yi SE, Hu S, Rosella L, Fine B (2020) Characterizing early Canadian federal, provincial, territorial and municipal nonpharmaceutical interventions in response to COVID-19: a descriptive analysis. CMAJ Open 8(3):E545–E553. https://doi.org/10.9778/cmajo.20200100
Moghadas SM, Pizzi NJ, Wu J, Tamblyn SE, Fisman DN (2011) Canada in the face of the 2009 H1N1 pandemic: Canada in the face of the 2009 H1N1 pandemic. Influenza Other Respir Viruses 5(2):83–88. https://doi.org/10.1111/j.1750-2659.2010.00184.x
National Collaborating Centre for Infectious Diseases (2021a) PHAC: Wastewater Surveillance Program for COVID-19. National Collaborating Centre for Infectious Diseases. https://nccid.ca/wastewater-surveillance-for-covid-19/
National Collaborating Centre for Infectious Diseases (2021b) The Canadian Public Health Laboratory Network. National Collaborating Centre for Infectious Diseases. https://nccid.ca/cphln/
National Collaborating Centre for Infectious Diseases. (2022, February 24). The COVID-19 Public Health Agency of Canada (PHAC) Modelling Group. National Collaborating Centre for Infectious Diseases. https://nccid.ca/covid-19-phac-modelling-group/#subMenuSection1
National Collaborating Centres for Public Health (2021) Strengthening public health across Canada: the influence of the NCCS. National Collaborating Centres for Public Health. https://nccph.ca/projects/strengthening-public-health-across-canada-the-influence-of-the-nccs
National Research Council of Canada (2019, March 4) About the NRC. Government of Canada. https://nrc.canada.ca/en/corporate/about-nrc
National Research Council of Canada. (2021a, May 18). COVID-19 Joint Biomanufacturing Subcommittee Registry of Interests. Government of Canada. https://nrc.canada.ca/en/corporate/covid-19-joint-biomanufacturing-subcommittee
National Research Council of Canada. (2021b, November 18). COVID-19 Vaccine Task Force Registry of Interests. Government of Canada. https://nrc.canada.ca/en/corporate/covid-19-vaccine-task-force-registry-interests
Naylor CD (2003) Learning from SARS: renewal of public health in Canada: a report of the National Advisory Committee on SARS and Public Health. National Advisory Committee on SARS and Public Health. https://www.canada.ca/en/public-health/services/reports-publications/learning-sars-renewal-public-health-canada.html
Office of Audit and Evaluation, Health Canada and Public Health Agency of Canada (2020) Lessons Learned from the Public Health Agency of Canada COVID-19 Response (Phase One). Government of Canada. https://ourcommons.azureedge.net/data/Motion2020-10-26/2021-01-20-SPDP-8550-432-1-01/HESA_PHAC-ASPC_0000186_E.pdf
Office of the Chief Science Advisor (2018, August 27). Mandate. Government of Canada. https://www.ic.gc.ca/eic/site/063.nsf/eng/h_97667.html
Office of the Chief Science Advisor (2019) Chief Science Advisor Annual Report 2018-19. Government of Canada. https://www.ic.gc.ca/eic/site/063.nsf/eng/h_97756.html
Office of the Chief Science Advisor (2020a) Chief Science Advisor Annual Report 2019-20. Government of Canada. https://www.ic.gc.ca/eic/site/063.nsf/eng/h_98146.html
Office of the Chief Science Advisor (2020b, April 30) Expert Group on Health Systems. https://www.ic.gc.ca/eic/site/063.nsf/eng/h_98017.html
Office of the Chief Science Advisor (2020c, May 1) Expert Group on Modelling Approaches. https://www.ic.gc.ca/eic/site/063.nsf/eng/h_98036.html
Office of the Chief Science Advisor (2021a, January 14) COVID-19 Expert Panel. Government of Canada. https://www.ic.gc.ca/eic/site/063.nsf/eng/h_98013.html
Office of the Chief Science Advisor (2021b, November 12) Departmental Science Advisors Network. Government of Canada. https://science.gc.ca/eic/site/063.nsf/eng/h_98245.html
Office of the Chief Science Advisor (2022) Chief Science Advisor Annual Report 2020–21 [Preliminary version]. Government of Canada. https://science.gc.ca/eic/site/063.nsf/eng/h_98370.html
Ontario COVID-19 Science Advisory Table (2022) About the Science Table. Ontario COVID-19 Science Advisory Table. https://covid19-sciencetable.ca/about/
Our World in Data (2022, January 4) Daily new confirmed COVID-19 deaths per million people. University of Oxford. https://ourworldindata.org/covid-deaths
Pan-Canadian Public Health Network (2016, March 21) Overview [Government Website]. Pan-Canadian Public Health Network. http://www.phn-rsp.ca/
Pan-Canadian Public Health Network (2018a) Canadian pandemic influenza preparedness: planning guidance for the health sector. Public Health Agency of Canada. http://epe.lac-bac.gc.ca/100/201/301/weekly_acquisitions_list-ef/2019/19-06/publications.gc.ca/collections/collection_2019/aspc-phac/HP40-144-2018-eng.pdf
Pan-Canadian Public Health Network (2018b) Federal/Provincial/Territorial Public Health Response Plan for Biological Events. Public Health Agency of Canada. https://www.deslibris.ca/ID/10097284
Pan-Canadian Public Health Network (2020a, May 20) About the Pan-Canadian Public Health Network [Government Website]. Pan-Canadian Public Health Network. http://www.phn-rsp.ca/network-eng.php
Pan-Canadian Public Health Network (2020b, September 11) Pan-Canadian Public Health Network: Overview Presentation
Pan-Canadian Public Health Network (2021, March 5) Special Advisory Committee on the Epidemic of Opioid Overdoses [Government Website]. Pan-Canadian Public Health Network. http://www.phn-rsp.ca/sac-opioid-gcs-opioides/index-eng.php
Pan-Canadian Public Health Network (2022, February 15) Special Advisory Committee on COVID-19. Pan-Canadian Public Health Network. http://www.phn-rsp.ca/sac-covid-ccs/index-eng.php
Paquet M, Schertzer R (2020) COVID-19 as a complex intergovernmental problem. Can J Polit Sci 53(2):343–347. https://doi.org/10.1017/S0008423920000281
Pielke R (2021, May 30) Shadow Science Advice. The Honest Broker. https://rogerpielkejr.substack.com/p/shadow-science-advice?s=w
Prime Minister of Canada Office (2021a, December 3) Cabinet Committee on Safety, Security and Emergencies. Government of Canada. https://pm.gc.ca/en/cabinet-committee-mandate-and-membership#security
Prime Minister of Canada Office (2021b, December 3) Incident Response Group. Government of Canada. https://pm.gc.ca/en/cabinet-committee-mandate-and-membership#incident-response
Prime Minister of Canada Office. (2021c, December 3) Sub-Committee on the federal response to the Coronavirus disease (COVID-19). Government of Canada. https://pm.gc.ca/en/cabinet-committee-mandate-and-membership#covid-19
Public Health Agency of Canada (2006) Overview of emergency preparedness and response: Protecting the health and safety of Canadians. Public Health Agency of Canada
Public Health Agency of Canada (2011) Lessons learned review: Public Health Agency of Canada and Health Canada response to the 2009 H1N1 pandemic. Public Health Agency of Canada. https://www.deslibris.ca/ID/226211
Public Health Agency of Canada (2019, December 10) Centre for Immunization and Respiratory Infectious Diseases (CIRID). Government of Canada. https://www.canada.ca/en/public-health/services/infectious-diseases/centre-immunization-respiratory-infectious-diseases-cirid.html
Public Health Agency of Canada (2020a) Federal/provincial/territorial public health response plan for ongoing management of COVID-19. Government of Canada
Public Health Agency of Canada (2020b) Coronavirus disease (COVID-19): Outbreak update. Public Health Agency of Canada, Government of Canada. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection.html
Public Health Agency of Canada (2021a) Priority strategies to optimize testing and screening for COVID-19 in Canada: Report of the COVID-19 Testing and Screening Expert Advisory Panel (No. 1). Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/testing-screening-advisory-panel/reports-summaries/priority-strategies.html#a2
Public Health Agency of Canada (2021b, February 12) Government of Canada invests $53 million to address COVID-19 virus variants of concern. Government of Canada. https://www.canada.ca/en/public-health/news/2021/02/government-of-canada-invests-53-million-to-address-covid-19-virus-variants-of-concern.html
Public Health Agency of Canada (2021c, February 15) Points to consider: Public disclosure of outbreaks and cases of infectious diseases. Government of Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/public-health-ethics-consultative-group/points-consider-public-disclosure-outbreaks-cases-infectious-diseases.html
Public Health Agency of Canada (2021d, February 16) Public health ethics framework: A guide for use in response to the COVID-19 pandemic in Canada. Government of Canada. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/canadas-reponse/ethics-framework-guide-use-response-covid-19-pandemic.html
Public Health Agency of Canada (2021e, March 25) The pan-Canadian Health Data Strategy: Expert Advisory Group Terms of Reference. Government of Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/pan-canadian-health-data-strategy-terms-reference.html
Public Health Agency of Canada (2021f) Federal/provincial/territorial public health response plan for ongoing management of COVID-19. Government of Canada. https://www.canada.ca/content/dam/phac-aspc/documents/services/diseases/2019-novel-coronavirus-infection/federal-provincial-territorial-public-health-response-plan-ongoing-management-covid-19/fpt-response-plan-en.pdf
Public Health Agency of Canada (2021g, May 17) Public Health Agency of Canada’s Policy on External Advisory Bodies (2011) Summary. Health Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/policy.html
Public Health Agency of Canada (2021h, May 20) Harnessing the power of wastewater testing to detect COVID-19 outbreaks. Government of Canada. https://science.gc.ca/eic/site/063.nsf/eng/98235.html
Public Health Agency of Canada (2021i, June 7) Statement from the Chief Public Health Officer of Canada on June 7, 2021. Government of Canada. https://www.canada.ca/en/public-health/news/2021/06/statement-from-the-chief-public-health-officer-of-canada-on-june-7-2021.html
Public Health Agency of Canada (2021j, June 11) Public Health Agency of Canada’s Public Health Ethics Consultative Group (PHECG). Government of Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/public-health-ethics-consultative-group.html
Public Health Agency of Canada (2021k) A vision to transform Canada’s public health system: The Chief Public Health Officer of Canada’s Report on the State of Public Health in Canada 2021. Public Health Agency of Canada. https://www.canada.ca/en/public-health/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/state-public-health-canada-2021.html
Public Health Agency of Canada. (2021l, December 14). National Microbiology Laboratory. Government of Canada. https://www.canada.ca/en/public-health/programs/national-microbiology-laboratory.html#response
Public Health Agency of Canada (2021m, December 22) The pan-Canadian Health Data Strategy: Expert Advisory Group Overview. Government of Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/pan-canadian-health-data-strategy-overview.html
Public Health Agency of Canada (2021n, December 23) List of external advisory bodies for the Public Health Agency of Canada. Government of Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list.html
Public Health Agency of Canada (2022a, February 17) National Advisory Committee on Immunization (NACI): Statements and publications Government of Canada. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci.html
Public Health Agency of Canada (2022b, February 18). Mathematical modelling and COVID-19. Government of Canada. https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/epidemiological-economic-research-data/mathematical-modelling.html
Public Health Agency of Canada (2022c, March 11) National Microbiology Laboratory’s role in the COVID-19 response. Government of Canada. https://www.canada.ca/en/public-health/programs/national-microbiology-laboratory/role-covid-19-response.html
Public Health Agency of Canada (2022d, March 17) The Pan-Canadian Health Data Strategy: Expert Advisory Group Reports and summaries. Government of Canada. https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/pan-canadian-health-data-strategy-reports-summaries.html
Public Health Agency of Canada, Boyd R, Marshall H (2008) The Chief Public Health Officer’s report on the state of public health in Canada, 2008. Chief Public Health Officer. https://www.deslibris.ca/ID/214634
Public Safety Canada (2009) Federal Policy for Emergency Management. Government of Canada. https://www.publicsafety.gc.ca/cnt/rsrcs/pblctns/plc-mrgnc-mngmnt/index-en.aspx
Public Safety Canada (2011, January) Federal Emergency Response Plan January 2011. Government of Canada. https://www.publicsafety.gc.ca/cnt/rsrcs/pblctns/mrgnc-rspns-pln/index-en.aspx#a10
Qaiser F, Heer T, Azdajic I, Maxwell R (2022). An assessment of the transparency of evidence usage in the government of Canada (II; Eyes on Evidence, p. 29). Evidence for democracy. https://evidencefordemocracy.ca/en/research/reports/eyes-evidence-ii
Quirion R, Carty A, Dufour P, Jabr R (2016) Reflections on science advisory systems in Canada. Palgrave Commun 2(1):16048. https://doi.org/10.1057/palcomms.2016.48
Royal Society of Canada (2015) Royal Society of Canada Position Paper: strengthening government by strengthening scientific advice. Royal Society of Canada. https://rsc-src.ca/en/rsc-position-paper-strengthening-government-by-strengthening-scientific-advice
Statistics Canada (2020, October 21) About us. Government of Canada. https://www.statcan.gc.ca/en/about/about?MM=as
Tuohy C (2021, December 7) Institutionalizing science advising in Canada: improving the logic of expectations [Webinar]. Friesen roundtable-pandemic preparedness: science informing policy, virtual meeting. https://www.youtube.com/watch?v=fyys8kbMwsM&ab_channel=DallaLanaSchoolofPublicHealth
Unruh L, Allin S, Marchildon G, Burke S, Barry S, Siersbaek R, Thomas S, Rajan S, Koval A, Alexander M, Merkur S, Webb E, Williams GA (2021) A comparison of 2020 health policy responses to the COVID-19 pandemic in Canada, Ireland, the United Kingdom and the United States of America. Health Policy, S016885102100169X. https://doi.org/10.1016/j.healthpol.2021.06.012
Vallejo BM, Ong RAC (2022) OCTA as an independent science advice provider for COVID-19 in the Philippines. Humanit Soc Sci Commun 9(1):104. https://doi.org/10.1057/s41599-022-01112-9
Wallace K (2022, March 31). Province’s COVID-19 science advisory table to be folded into Public Health Ontario. Toronto Star. https://www.proquest.com/canadiannews/docview/2645989857/DC2868B18A1C4DC7PQ/1?accountid=14771
Weber B (2020, December 1) Public health must balance science and society: Former top doctor. CBC News. https://www.cbc.ca/news/canada/edmonton/covid-public-health-1.5823286
Weinkle J (2022) An evaluation of North Carolina science advice on COVID-19 pandemic response. Humanit Soc Sci Commun 9(1):352. https://doi.org/10.1057/s41599-022-01344-9
Wilson K, Keelan J (2008) Learning from Listeria: the autonomy of the Public Health Agency of Canada. Can Med Assoc J 179(9):877–879. https://doi.org/10.1503/cmaj.081441
Wilson K, McCrea-Logie J, Lazar H (2004) Understanding the impact of intergovernmental relations on public health: lessons from reform initiatives in the blood system and health surveillance. Can Public Policy/Analyse de Politiques 30(2):177. https://doi.org/10.2307/3552391
World Health Organization (2020) WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. World Health Organization. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
Acknowledgements
We gratefully acknowledge the local experts for generously lending their time to verify our findings. We also thank the Evaluation of Science Advice in a Pandemic Emergency (EScAPE—an International Network for Governmental Science Advice and University of Colorado Boulder-led international case series) investigators for their helpful input.
Author information
Authors and Affiliations
Contributions
All authors were involved in conceptualizing, designing, investigating, and interpreting this work. DB wrote the first draft of the manuscript and SA and EDR critically revised it for important intellectual content. All authors have reviewed and approved the final manuscript version for publication. SA and EDR are senior co-authors that jointly supervised this work. The authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors attest that no other individuals meeting the authorship criteria have been omitted.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
This article does not contain any studies with human participants performed by any of the authors. Local experts were consulted in their professional capacity to verify the accuracy of the information collected, rather than to provide additional data. The information collected from the local experts was not recorded verbatim, identified, or analyzed as a separate data source. As such, these consultations were exempt from ethics approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhatia, D., Allin, S. & Di Ruggiero, E. Mobilization of science advice by the Canadian federal government to support the COVID-19 pandemic response. Humanit Soc Sci Commun 10, 19 (2023). https://doi.org/10.1057/s41599-023-01501-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1057/s41599-023-01501-8