Abstract
Lung adenocarcinoma (LUAD) is the most common and aggressive subtype of lung cancer, and coronavirus disease 2019 (COVID-19) has become a serious public health threat worldwide. Patients with LUAD and COVID-19 have a poor prognosis. Therefore, finding medications that can be used to treat COVID-19/LUAD patients is essential. Bioinformatics analysis was used to identify 20 possible metformin target genes for the treatment of COVID-19/LUAD. PTEN and mTOR may serve as hub target genes of metformin. Metformin may be able to cure COVID-19/LUAD comorbidity through energy metabolism, oxidoreductase NADH activity, FoxO signalling pathway, AMPK signalling system, and mTOR signalling pathway, among other pathways, according to the results of bioinformatic research. Metformin has ability to inhibit the proliferation of A549 cells, according to the results of colony formation and proliferation assays. In A549 cells, metformin increased glucose uptake and lactate generation, while decreasing ATP synthesis and the NAD+/NADH ratio. In summary, PTEN and mTOR may be potential targets of metformin for the treatment of COVID-19/LUAD. The mechanism by which metformin inhibits lung adenocarcinoma cell proliferation may be related to glucose metabolism regulated by PI3K/AKT signalling and mTOR signalling pathways. Our study provides a new theoretical basis for the treatment of COVID-19/LUAD.
Similar content being viewed by others
Introduction
Among malignant tumors, lung cancer is the leading cause of illness and death. The majority (80–85%) of lung cancers are classified as non-small cell lung cancer (NSCLC), which includes subtypes such as lung squamous cell carcinoma (LUSC), lung adenocarcinoma (LUAD), and large-cell carcinoma (LCC)1. LUAD, which is known for its high prevalence and aggressive nature, exhibits significant heterogeneity and invasiveness. The absence of early recognizable clinical symptoms and efficient diagnostic techniques often lead to the diagnosis of advanced stage LUAD in a majority of patients2, and do not satisfy the requirements for surgical resection, which is a major factor in the unfavorable prognosis of LUAD as a whole. Currently, the introduction of immunotherapy has introduced a new approach to the clinical management of lung cancer. Various immune checkpoint inhibitors (ICIs) have shown promising efficacy in clinical trials3. However, some patients do not benefit from immunotherapy4,5 or develop resistance to medication6. In addition, concerns regarding side effects and adverse reactions persist. Glucose deprivation can also lead to energy stress. Cancer cells die more selectively than normal cells. There is growing evidence that metabolic reprogramming plays an important role in tumor development. This metabolic variability and flexibility enable tumor cells to maintain the reduction–oxidation (redox) balance and generate ATP as an energy source biosynthesis process that is essential for cell survival, growth, and proliferation. Targeting the metabolic differences between tumor and normal cells is a promising novel anticancer strategy7. Therefore, it is crucial to investigate the mechanisms underlying LUAD pathogenesis, progression, and metastasis and to identify effective biomarkers and therapeutic targets that can significantly enhance the survival rate of patients with LUAD.
The global outbreak of Coronavirus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses a significant public health threat, with an increasing number of countries affected and millions of people at risk8. Recent studies have revealed that individuals with malignancies often experience chronic immunosuppression, rendering them more vulnerable to SARS-CoV-2 infection than healthy individuals. This susceptibility frequently leads to poorer prognoses9. Consequently, the COVID-19 pandemic presents substantial challenges for cancer diagnosis, treatment, and prognosis10. Peter et al. reported that patients with LUAD diagnosed with COVID-19 exhibited unfavorable prognoses due to frailty11. Furthermore, research has indicated that both immunotherapy and chemotherapy can induce chronic immunosuppression in patients with lung cancer, which may be exacerbated by COVID-19 infection12. COVID-19 is associated with endothelial dysfunction, inflammation, and hypercoagulability, the co-occurrence of which is associated with an increased rate of serious adverse events, including mortality13. Therefore, it is imperative to explore potential drugs that are suitable for treating patients with COVID-19/LUAD.
Metformin is one of the most commonly prescribed medications for type 2 diabetes mellitus. Its well-established therapeutic effect combined with its high safety profile and affordability have positioned metformin as a first-line drug option for the clinical management of type 2 diabetes14. Several studies have suggested that metformin improves the prognosis of many cancers and reduces cancer morbidity and mortality, including gastric15, liver16 and breast cancers17. Some studies have shown that metformin reduces mortality in patients infected with SARS-CoV-2 virus18,19,20. In patients hospitalized with COVID‑19, metformin can reduce the markers of inflammation, oxidative stress, and thrombosis21. Based on this premise, we hypothesized that metformin could be a potential treatment option for patients with COVID-19 and LUAD. We used network pharmacology and various bioinformatics techniques to explore the therapeutic targets and pharmacological mechanisms of metformin in COVID-19/LUAD. Our aim was to determine the potential therapeutic benefits associated with the use of metformin under these conditions.
Results
Identification results of COVID-19-related genes
In the GSE180226 dataset, we obtained data from 23 lung tissue samples (containing 20 COVID-19 positive patients and three healthy individuals). In the GSE211378 dataset, we obtained 264 whole-blood samples from 160 COVID-19 convalescent donors and 40 from healthy donors. The DEGs in the GEO dataset were analyzed using GEOR2. 6451 and 207 DEGs, obtained from GSE180226 dataset and GSE211378 dataset (Fig. 1A,B, Supplementary files 1). In addition, 5337, 9897, 104, 15, 1831, and 11 DEGs of COVID-19 were found in GeneCards, CTD, TTD, OMIM, DisGeNET, and pharmaGKB, respectively (Fig. 2A, Supplementary files 1). To ensure the accuracy of the prediction results, genes appearing in two or more databases were classified as those associated with COVID-19. Based on this criterion, 6321 COVID-19-related genes were screened (Fig. 2A, Supplementary files 1).
Screening for DEGs from GEO datasets of COVID-19 and LUAD. The red and blue dots represented genes with increased or decreased expression in COVID-19 patients or LUAD patients, respectively, while the black dots represented genes with no significant difference in expression between COVID-19 or LUAD patients. (A) Volcano plot of DEGs in the GSE180226 dataset of COVID-19. (B) Volcano plot of DEGs in the GSE211378 dataset of COVID-19. (C) Volcano plot of DEGs in the GSE31210 dataset of LUAD. (D) Volcano plot of DEGs in the GSE75037 dataset of LUAD.
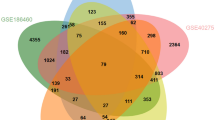
Screening the target genes of metformin for the treatment of COVID-19/LUAD. (A) Genes associated with COVID-19 from 2 GEO datasets and six databases. (B) Genes associated with LUAD from 2 GEO datasets, TCGA-LUAD dataset and five databases. (C) Target genes of metformin from five datasets. (D) The target genes of metformin for the treatment of LUAD/COVID-19.
Identification results of LUAD-related genes
In the GSE31210 dataset, we included sequencing data from 246 lung tissue samples (226 LUAD tissues and 20 normal lung tissues). In the GSE75037 dataset, we obtained sequencing data from 83 LUAD and 83 matched adjacent nonmalignant lung tissues. A total of 2699 and 9902 DEGs were obtained from the GSE31210 and GSE75037 datasets, respectively (Fig. 1C,D, Supplementary files 2). In addition, 9288, 37,986, 7, 139, and 4245 DEGs of LUAD were found in GeneCards, CTD, DisGeNET, pharmaGKB, and TCGA-LUAD, respectively (Fig. 2B, Supplementary files 2). In this study, we considered DEGs that appeared in at least two databases to be LUAD-related genes, resulting in 14,208 LUAD-related genes. (Fig. 2B, Supplementary files 2).
Screening the target genes of metformin for the treatment of COVID-19/LUAD
Metformin target genes were explored using five online databases related to drug targets. The results showed that 10, 37, 33, 10, and 885 target genes of metformin were screened from the TTD, DGlbd, Swiss Target Prediction, String, and CTD databases, respectively. To ensure the accuracy of the prediction results, genes appearing in two or more databases were classified as metformin target genes. Based on this criterion, 34 metformin target genes were screened (Fig. 2C, Supplementary files 3). The results of the Venn analysis showed a total of 20 potential target genes of metformin for the treatment of COVID-19/LUAD was obtained (Fig. 2D, Supplementary files 4).
Gene network analysis and PPI network construction
To identify the potential target genes of metformin for the treatment of COVID-19/LUAD, we utilized the GeneMANIA database, which is a comprehensive platform based on multiple transcriptomic and proteomic databases. This platform collects vast amounts of datasets and interactions to discover genes with similar functions and build interaction networks. Our analysis revealed that NADH oxidoreductase activity, phosphatidylinositol-mediated signalling, and protein kinase signalling may be involved in the mechanism of action of metformin in treating COVID-19/LUAD (Fig. 3A). Furthermore, Cytoscape software was used to visualize the degree of each gene in the PPI network, and PTEN, mTOR, and PPARA were identified as core targets for metformin treatment against COVID-19/LUAD due to their high degree within the network (Fig. 3B).
Construction of PPI networks for target genes of metformin for the treatment of COVID-19/LUAD. (A) The PPI network was derived from the GeneMANIA database. The different relationships between nodes were indicated by the different colored connecting lines. Nodes were enriched in different functions indicated by the colors of the nodes. (B) PPI networks constructed from nodal degree values by Cytoscape software. The degree of the node was proportional to the depth of the node color.
Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis of 20 potential target genes of metformin for the treatment of COVID-19/LUAD
To investigate the pharmacological mechanism of metformin in the treatment of COVID-19/LUAD, we performed a bioinformatics analysis of 20 potential target genes. The results of GO analysis showed that the BP enrichment results were mainly related to hypoxia, regulation of oxygen levels, and small molecule and energy metabolism (Fig. 4A). The MF enrichment results were mainly related to oxidoreductase NADH activity, phosphatidylinositol activity, and protein kinase activity (Fig. 4C). CC analysis showed that these genes mainly constitute the membrane structure (Fig. 4E). In addition, KEGG enrichment results showed that these genes were mainly involved in the FoxO signalling pathway, AMPK signalling pathway, mTOR signalling pathway and other insulin-related pathways (Fig. 4G). The results of the correlation analysis of BP, CC, MF, and KEGG term enrichment analysis are shown in Fig. 4B,D,F,H. The results of the top ten BP, CC, MF, and KEGG enrichment analyses and the corresponding genes are presented using circus plots (Fig. 5A–D, Supplementary files 5).
Metformin inhibits the proliferation of LUAD by affecting its energy metabolism
First, we examined the effects of metformin on the expression of these three target genes. The results showed that the expression level of PTEN was significantly increased and the expression level of mTOR was significantly decreased in A549 cells treated with metformin, whereas PPARA did not show any significant change (Fig. 6A). Based on the results of the bioinformatic analysis of 20 potential target genes, we suggest that metformin might have effect on energy metabolism in LUAD. The results of the proliferation assay showed that the viability of A549 cells treated with 10 mM and 20 mM metformin was significantly lower than that of the untreated group (Fig. 6B). In addition, the colony formation assay showed similar results, with the number of clones of A549 cells treated with 10 mM and 20 mM metformin being significantly lower than that of the untreated group. (Fig. 6C). To further investigate the mechanism by which metformin inhibited A549 cells, we examined the effects of metformin on glucose consumption and lactate production in A549 cells. As expected, glucose consumption and lactate production of A549 cells were significantly higher compared than in the controls (Fig. 6D,E). Furthermore, in A549 cells treated with 10 mM and 20 mM metformin, ATP production was significantly lower than that in the untreated group (Fig. 6F). As NADH dehydrogenation is an essential part of ATP production, we investigated the effect of metformin on the levels of NAD+ and NADH in A549 cells. The results showed that NAD+ levels were significantly lower in the metformin group than in the untreated group (Fig. 6G). In addition, NADH in A549 cells treated with 10 mM and 20 mM metformin was higher than that in the untreated group (Fig. 6H). In addition, NAD+/NADH levels were significantly lower than those in the untreated group (Fig. 6I). These results indicate that metformin inhibited the production of ATP from the tricarboxylic acid cycle in A549 cells with the aid of depressing NADH dehydrogenation and promoted glycolysis, thereby inhibiting the proliferation of A549 cells.
Effect of metformin on energy metabolism in LUAD. (A) The expression of PTEN, mTOR and PPARA in A549 cells treated with metformin in GSE146982 datasets. (B) Proliferation curves of A549 cells treated with 0 mM, 10 mM, 20 mM metformin, n = 3. (C) Clone formation of A549 cells treated with 0 mM, 10 mM, 20 mM metformin. (D) Glucose consumption and (E) lactate production of A549 cells treated with 0 mM, 2 mM, 4 mM, 10 mM metformin, n = 3. (F) ATP, (G) NAD+, (H) NADH and (I) NAD+/NADH of A549 cells treated with 0 mM, 10 mM, 20 mM metformin, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
A growing body of evidence shows that metformin is effective in the treatment and prognosis of lung cancer22,23,24. Some studies have demonstrated that metformin can reduce inflammatory responses in diabetes patients with SARS-CoV-2 infection and has better clinical outcomes21,25. Chronically compromised immune systems make patients with LUAD more susceptible to SARS-CoV-2 infection than healthy individuals. Available data confirm that patients with cancer may have a higher risk of SARS-CoV-2 infection and, more importantly, a higher risk of serious complications26. Given the antiviral and anticancer properties of metformin, we investigated its target genes and mechanisms in the treatment of COVID-19/LUAD.
We identified PTEN, mTOR, and PPARA as possible core target genes for metformin in the treatment of COVID-19/LUAD by screening genes based on their expression levels. The results of bioinformatics analysis showed that the mechanism of metformin in COVID-19/LUAD might be related to energy metabolism, NADH oxidoreductase activity, FoxO signalling pathway, AMPK signalling pathway, and mTOR signalling pathway. PTEN is a tumor suppressor involved in the regulation of several signalling pathways, including the PI3K/AKT, FoxO, AMPK, and mTOR pathways27. Through the regulation of these signalling pathways, PTEN is involved in the regulation of tumor metabolism. Consistent with these results, our findings indicate that by treating A549 cells with metformin, PTEN expression was elevated, while mTOR expression, an oncogene that is frequently upregulated in human cancer28, was decreased. In addition, metformin suppressed the proliferation of A549 cells, based on the results of the proliferation and colony formation assays. In a previous study, metformin was found to inhibit the growth of hepatitis C virus-infected cells via mTOR by increasing PTEN and autophagy29. These results demonstrated that metformin may inhibit lung adenocarcinoma cell proliferation by altering PTEN and mTOR expression, but we were unable to explore metformin potentially inhibit tumor angiogenesis, metastasis, and chemotherapy resistance. In previous study, Ndembe et al. demonstrated metformin can prevent cisplatin resistance in an inactive liver kinase B1 in vivo model of LUAD30. And metformin-repressed NSCLC growth and metastasis by miR-381-YAP-snail axis activity disrupts31. Moreover, metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer32. These studies demonstrated metformin involves multiple signalling pathways and related mechanisms in the treatment of tumors. Because our study was limited to the basic theoretical level and lacked relevant clinical studies, the dosage and side effects of metformin in the treatment of COVID-19/LUAD are unclear. Meanwhile the responsiveness of patients with different stages of LUAD to metformin has not been explored.
As PTEN and mTOR are involved in cellular energy metabolism, combined with the results of our bioinformatics analyses, we conjectured that metformin may exert its cancer-suppressing effects by regulating glucose metabolism. Meanwhile some studies found targeting glucose metabolism could treat COVID-1933,34. As expected, metformin promoted glucose consumption and lactate production in A549 cells and decreased ATP production and the NAD+/NADH ratio. NAD+ is involved in many redox reactions involved in energy generation, glycolysis, tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS), fatty acid oxidation, and serine biosynthesis35. A higher NAD+/NADH ratio is found in cancer cells than in non-cancerous cells, suggesting that NAD+ plays an important role in this metabolic conversion36. Our results demonstrate that metformin inhibits lung adenocarcinoma cell proliferation by regulating glucose metabolism. Therefore metformin for the treatment of COVID-19/LUAD may work by modulating glucose metabolism. Since PI3K/AKT/mTOR pathway and glucose metabolism are associated with tumor metastasis37,38 and tumor angiogenesis39, metformin may perhaps be able to treat LUAD by modulating PI3K/AKT/mTOR pathway and glucose metabolism. Further experiments are needed to investigate this.
We defined 20 potential target genes of metformin for the treatment of COVID-19/LUAD as gene set 1 by analyzing data from open databases. Functional analysis of gene set 1 using the GeneMANIA database showed that the mechanism of metformin in the treatment of COVID-19/LUAD may be related to the activity of oxidoreductase NADH, phosphatidylinositol-mediated signalling pathway, and protein kinase B signalling pathway. A growing body of evidence has shown that NAD+ mediates both antiviral and anti-inflammatory mechanisms. Increased NAD+ levels have been reported to play a role in the prevention and treatment of severe COVID-19 and other viral infections40. Moreover, higher NAD+/NADH and NADP+/NADPH ratios contribute to tumor progression, development, and survival36. The phosphatidylinositol-mediated signalling pathway is a key pathway that enhances nutrient uptake, macromolecule synthesis, and cell survival. It is activated in a wide range of cancers41. Studies have confirmed that the protein kinase B signalling pathway may promote glycolysis, migration, and invasion of NSCLC cells42. In addition, the phosphatidylinositol-mediated signalling pathway and protein kinase B pathway play important roles in the process of viral infection, and the intervention of these signalling pathways contributes to the treatment of viral infection43,44,45,46. Metformin inhibited the migration and invasion of cancer cells by downregulating the protein kinase B signalling pathway and NAD+/NADH ratio47,48. These results revealed a potential mechanism of metformin in the treatment of COVID-19/LUAD.
Conclusions
In this study, we found that PTEN and mTOR could be potential targets for metformin in the treat COVID-19/LUAD. Glucose metabolism regulated by the PI3K/AKT signalling pathway and mTOR signalling pathways may be the mechanisms by which metformin inhibits LUAD cell proliferation. However, our study has several shortcomings and deficiencies. The data used in this study were obtained from a public database, and there is no basic experiment on COVID-19 for verification. In addition, there is a lack of clinical data to support the use of metformin for the treatment of COVID-19/LUAD due to limited funding. Taken together, our study provides a new theoretical basis for treating COVID-19/LUAD.
Materials and methods
Flow of research
As shown in Fig. 7, our study followed a specific flow. First, we conducted a comprehensive search across various databases to identify the genes associated with Metformin, COVID-19, and LUAD. Next, we performed a Venn diagram analysis to determine the potential target genes of metformin for treating COVID-19/LUAD. The identified genes were then subjected to Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Finally, basic experiments were performed to validate the effects of metformin on glucose metabolism in LUAD.
Screening target genes of metformin
To accurately identify target genes influenced by metformin treatment, we used five reputable online databases related to drug targets: CTD (http://ctdbase.org/), Swiss Target Prediction (http://www.swisstargetprediction.ch/), DGIdb (https://www.dgidb.org/), STITCH (http://stitch.embl.de/), and TTD (http://db.idrblab.net/). By cross-referencing the results of these databases, candidate target genes were selected if they appeared in two or more sources.
Screening genes of associated with COVID-19
The GSE180226 dataset was obtained from the GEO database specifically related to COVID-19 research, and the differentially expressed genes (DEGs) were analyzed by GEOR2 with filtering criteria (p < 0.01 and absolute value of log fold change > 1). The DEGs of GSE211378 datasets were analyzed using GEOR2 with filtering criteria (p < 0.01 and absolute value of log fold change > 0.1). In addition, the genes associated with COVID-19 were explored using six online databases: GeneCards (https://www.genecards.org), CTD (http://ctdbase.org/), TTD (http://db.idrblab.net/), OMIM (https://omim.org/), DisGeNET (https://www.disgenet.org/), and pharmaGKB (https://www.pharmgkb.org/). To ensure the accuracy of the prediction results, genes appearing in two or more databases were classified as those associated with COVID-19.
Screening genes of related to LUAD
Two LUAD datasets (GSE31210 and GSE75037) were obtained from the GEO database, and the differentially expressed genes (DEGs) were analyzed using GEOR2 with filtering criteria (p < 0.01 and absolute value of log fold change > 1). Furthermore, five online databases, GeneCards (https://www.genecards.org), CTD (http://ctdbase.org/), TTD (http://db.idrblab.net/), DisGeNET (https://www.disgenet.org/), and pharmaGKB (https://www.pharmgkb.org/) were used to explore the genes associated with LUAD. In addition, the GEPIA (http://gepia.cancer-pku.cn) database was used to explore the DEGs of TCGA-LUAD using filtering criteria (p < 0.01, absolute value of log fold change > 1). To ensure the accuracy of the prediction results, genes appearing in two or more databases were classified as those related to LUAD.
Construction of PPI network and analysis of hub genes
The GeneMANIA database (http://genemania.org/) was used to construct the protein–protein interaction (PPI) network for metformin target genes in individuals with COVID-19/LUAD comorbidity. To identify the hub genes involved in metformin treatment for COVID-19/LUAD, the Cytoscape software was used to visualize the degree of target genes.
Gene ontology (GO) term and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis
To gain insights into the biological functions and pathways associated with metformin treatment for COVID-19/LUAD, we performed KEGG Pathway Enrichment Analysis and GO analysis, including Biological Process (BP), Cellular Component (CC), and Molecular Function (MF) using the DAVID online analytical tool available at https://david.ncifcrf.gov/summary.jsp. The enrichment analysis results were visualized using online tools provided by http://www.bioinformatics.com.cn.
Cell culture
The human lung adenocarcinoma cell line A549 was obtained from the American Academy of Sciences and cultured in 1640 medium containing 10% calf serum at 37 °C in an incubator with 5% CO2. The medium was changed every alternate day. Experiments were performed when the cells had grown to logarithmic phase.
Cell proliferation assay
A549 cells (5000 cells/well) treated with metformin (Sigma-Aldrich; Merck KGaA) 0 mM, 10 mM and 20 mM were seeded in 96-well plates and incubated with complete medium for 0 h, 24 h, 48 h. Next, 20 μL of 0.5 mg/mL MTS reagent (Promega) was added to each well on days 0, 1, 2 respectively, and incubate for 1 h. Finally, the absorbance was measured at 490 nm using a μQuant Universal Microplate Spectrophotometer (Bio-Tek Instruments, Winooski, USA). The absorbance was normalized to day 0 data, and proliferation curves were plotted.
Colony formation assay
Five hundred of A549 cells (500 µL) were plated in triplicate in 6-well plates in RPMI 1640 medium. The medium was replaced every 2 days. Colonies were counted after 6 days.
Glucose consumption and lactate production
A549 cells were grown to logarithmic phase in 6-well plates and treated with 0 mM, 2 mM, 4 mM and 10 mM metformin for 24 h. The glucose and lactate contents of the fresh medium and the medium after incubating the cells for 24 h were measured using an Automatic Analyzer H7180ID. Glucose consumption and lactate production were then calculated and normalized to the respective cell numbers.
ATP assay
ATP was measured using the Luminescent ATP Detection Assay Kit (Abcam Co., Ltd.) according to the manufacturer's instructions. Briefly, 1 × 106 cells were resuspended in 100 µL ATP assay buffer. Add 50 µL of the reaction mixture to the standard and sample wells. Then, 50 µL of background reaction mix was added to the sample background control wells. Incubate the plate at RT for 30 min, protected from light. The plate was measured at OD 570 nm for the colorimetric assay and at Ex/Em = 535/587 nm for the fluorometric assay.
NAD+/NADH assay
NAD+/NADH ratio was measured using the NAD+/NADH Assay Kit (Abcam Co., Ltd.) according to the manufacturer's instructions. Briefly, 2 × 106 cells were homogenized in 200 µL of extraction buffer for NAD+ or NADH and neutralized. The concentrations of NAD+ and NADH in homogenates were measured using a microplate reader. NAD+/NADH levels were normalized to the cell numbers.
Statistical analysis
Student's t-test was used to compare the differences between the two groups, and data are reported as mean values ± SD. The p-value < 0.05. Statistical analyses were performed using GraphPad Prism version 9.
Ethics approval and consent to participate
This study did not require ethical board approval because it did not include human or animal trials.
Data availability
The datasets presented in this study can be found in the online repositories, including CTD (http://ctdbase.org/), Swiss Target Prediction (http://www.swisstargetprediction.ch/), DGIdb (https://www.dgidb.org/), STITCH (http://stitch.embl.de/), and TTD (http://db.idrblab.net/), GeneCards (https://www.genecards.org), OMIM (https://omim.org/), DisGeNET (https://www.disgenet.org/), GEPIA (http://gepia.cancer-pku.cn), GeneMANIA database (http://genemania.org/) and pharmaGKB (https://www.pharmgkb.org/).
Abbreviations
- LUAD:
-
Lung adenocarcinoma
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Acute Respiratory Syndrome Coronavirus 2
- DEGs:
-
Differentially Expressed Genes
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
References
Hu, H. et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 39, 1531–1547 (2021).
Musika, W., Kamsa-Ard, S., Jirapornkul, C., Santong, C. & Phunmanee, A. Lung cancer survival with current therapies and new targeted treatments: A comprehensive update from the Srinagarind Hospital-based cancer registry from (2013 to 2017). Asian Pac. J. Cancer Prev. 22, 2501–2507 (2021).
She, L. et al. Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with PD-L1 tumor proportion score 1% or greater. Lung Cancer 138, 88–94 (2019).
Scott, S. C. & Hann, C. L. Immunotherapy for small cell lung cancer: Established applications and novel approaches. Clin. Adv. Hematol. Oncol. 19, 654–663 (2021).
Zhou, F. & Zhou, C. C. Immunotherapy in non-small cell lung cancer: Advancements and challenges. Chin. Med. J. (Engl.) 134, 1135–1137 (2021).
Boyero, L. et al. Primary and acquired resistance to immunotherapy in lung cancer: Unveiling the mechanisms underlying of immune checkpoint blockade therapy. Cancers (Basel) 12, 3729 (2020).
Ghantous, A., Hernandez-Vargas, H., Byrnes, G., Dwyer, T. & Herceg, Z. Characterising the epigenome as a key component of the fetal exposome in evaluating in utero exposures and childhood cancer risk. Mutagenesis 30, 733–742 (2015).
Majumder, J. & Minko, T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 23, 14 (2021).
Liang, W. et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 21, 335–337 (2020).
Moubarak, S. et al. COVID-19 and lung cancer: Update on the latest screening, diagnosis, management and challenges. J. Int. Med. Res. 50, 665781321 (2022).
Strang, P. & Schultz, T. Dying with cancer and COVID-19, with special reference to lung cancer: Frailty as a risk factor. Cancers (Basel) 14, 6002 (2022).
Elkrief, A. et al. Learning through a pandemic: The current state of knowledge on COVID-19 and cancer. Cancer Discov. 12, 303–330 (2022).
Roncon, L., Zuin, M., Rigatelli, G. & Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 127, 104354 (2020).
Kim, Y. S. et al. Metformin use reduced the overall risk of cancer in diabetic patients: A study based on the Korean NHIS-HEALS cohort. Nutr. Metab. Cardiovasc. Dis. 30, 1714–1722 (2020).
Cheung, K. S. et al. Metformin use and gastric cancer risk in diabetic patients after Helicobacter pylori eradication. J. Natl. Cancer Inst. 111, 484–489 (2019).
Ma, S., Zheng, Y., Xiao, Y., Zhou, P. & Tan, H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore) 96, e6888 (2017).
El-Benhawy, S. A. & El-Sheredy, H. G. Metformin and survival in diabetic patients with breast cancer. J. Egypt Public Health Assoc. 89, 148–153 (2014).
Scheen, A. J. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes Metab. 46, 423–426 (2020).
Li, Y. et al. Metformin in patients with COVID-19: A systematic review and meta-analysis. Front. Med. (Lausanne) 8, 704666 (2021).
Saygili, E. S., Karakilic, E., Mert, E., Sener, A. & Mirci, A. Preadmission usage of metformin and mortality in COVID-19 patients including the post-discharge period. Ir. J. Med. Sci. 191, 569–575 (2022).
Usman, A. et al. Metformin use in patients hospitalized with COVID-19: Lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes. J. Thromb. Thrombolysis 53, 363–371 (2022).
Lu, T. et al. Metformin inhibits human non-small cell lung cancer by regulating AMPK-CEBPB-PDL1 signaling pathway. Cancer Immunol. Immunother. 71, 1733–1746 (2022).
Brancher, S. et al. Metformin use and lung cancer survival: A population-based study in Norway. Br. J. Cancer 124, 1018–1025 (2021).
Kang, J. et al. The associations of aspirin, statins, and metformin with lung cancer risk and related mortality: A time-dependent analysis of population-based nationally representative data. J. Thorac. Oncol. 16, 76–88 (2021).
Ma, Z., Patel, N., Vemparala, P. & Krishnamurthy, M. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci. Rep. 12, 5553 (2022).
Bakouny, Z. et al. COVID-19 and cancer: Current challenges and perspectives. Cancer Cell 38, 629–646 (2020).
Chen, C. Y., Chen, J., He, L. & Stiles, B. L. PTEN: Tumor suppressor and metabolic regulator. Front. Endocrinol. (Lausanne) 9, 338 (2018).
Hua, H. et al. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 12, 71 (2019).
Del, C. J. et al. Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy. PLoS One 13, e191805 (2018).
Ndembe, G. et al. Caloric restriction and metformin selectively improved LKB1-mutated NSCLC tumor response to chemo- and chemo-immunotherapy. J. Exp. Clin. Cancer Res. 43, 6 (2024).
Jin, D. et al. Metformin-repressed miR-381-YAP-snail axis activity disrupts NSCLC growth and metastasis. J. Exp. Clin. Cancer Res. 39, 6 (2020).
Qian, W. et al. Metformin suppresses tumor angiogenesis and enhances the chemosensitivity of gemcitabine in a genetically engineered mouse model of pancreatic cancer. Life Sci. 208, 253–261 (2018).
Ardestani, A. & Azizi, Z. Targeting glucose metabolism for treatment of COVID-19. Signal Transduct. Target. Ther. 6, 112 (2021).
Hosp, J. A. et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 144, 1263–1276 (2021).
Navas, L. E. & Carnero, A. NAD(+) metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 6, 2 (2021).
Moreira, J. D. et al. The redox status of cancer cells supports mechanisms behind the Warburg effect. Metabolites 6, 33 (2016).
Biray, A. C., Sezgin, B., Goker, B. B., Karci, H. B. & Gode, S. PI3K/AKT/mTOR pathway and autophagy regulator genes in paranasal squamous cell carcinoma metastasis. Mol. Biol. Rep. 47, 3641–3651 (2020).
Liu, J. & Cao, X. Glucose metabolism of TAMs in tumor chemoresistance and metastasis. Trends Cell Biol. 33, 967–978 (2023).
Hu, Y. et al. Flavokawain C inhibits glucose metabolism and tumor angiogenesis in nasopharyngeal carcinoma by targeting the HSP90B1/STAT3/HK2 signaling axis. Cancer Cell Int. 24, 158 (2024).
Zheng, M., Schultz, M. B. & Sinclair, D. A. NAD(+) in COVID-19 and viral infections. Trends Immunol. 43, 283–295 (2022).
Goncalves, M. D., Hopkins, B. D. & Cantley, L. C. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med. 379, 2052–2062 (2018).
Wang, S., Zhang, H., Du, B., Li, X. & Li, Y. Fuzzy planar cell polarity gene (FUZ) promtes cell glycolysis, migration, and invasion in non-small cell lung cancer via the phosphoinositide 3-kinase/protein kinase B pathway. J. Cancer 13, 2419–2429 (2022).
Deinhardt-Emmer, S. et al. Inhibition of phosphatidylinositol 3-kinase by pictilisib blocks influenza virus propagation in cells and in lungs of infected mice. Biomolecules 11, 808 (2021).
Blanco, J., Cameirao, C., Lopez, M. C. & Munoz-Barroso, I. Phosphatidylinositol-3-kinase-Akt pathway in negative-stranded RNA virus infection: A minireview. Arch. Virol. 165, 2165–2176 (2020).
Dunn, E. F. & Connor, J. H. Dominant inhibition of Akt/protein kinase B signaling by the matrix protein of a negative-strand RNA virus. J. Virol. 85, 422–431 (2011).
Che, L. et al. Intracellular antibody targeting HBx suppresses invasion and metastasis in hepatitis B virus-related hepatocarcinogenesis via protein phosphatase 2A–B56gamma-mediated dephosphorylation of protein kinase B. Cell Prolif. 55, e13304 (2022).
He, Y. et al. Metformin inhibits the migration and invasion of esophageal squamous cell carcinoma cells by downregulating the protein kinase B signaling pathway. Oncol. Lett. 15, 2939–2945 (2018).
Benjamin, D. et al. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 25, 3047–3058 (2018).
Funding
This study was supported by Hebei Health Commission Scientific Research Foundation Project (20220596).
Author information
Authors and Affiliations
Contributions
Bin Zhang: Conceptualization. Baoli Xiang: Methodology, Software. Yongwang Hou: Data curation, Writing—Original draft preparation, funding acquisition. Jiangmin Liu: Visualization, Investigation. Lina Geng and Yuhuan Xu: Supervision. Dandan Xu and Minghua Zhan: Software, Validation. Zhicong Yang: Writing—Reviewing and Editing. All authors have reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, Y., Yang, Z., Xiang, B. et al. Metformin is a potential therapeutic for COVID-19/LUAD by regulating glucose metabolism. Sci Rep 14, 12406 (2024). https://doi.org/10.1038/s41598-024-63081-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63081-0
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.