Abstract
We designed and developed two new types of hydrogen fuel cell (HFC) buses (motorcoach and minibus) with a mobile laboratory system. Feasibility studies have been performed for mobile laboratory testing, particularly for the laboratory performance of COVID-19 RT-PCR (PCR). We evaluated the driving range capability, PCR sample size capacity, turnaround time (TAT), and analytical performance for the detection of SARS-CoV-2. Saliva samples were used for the current study, and the analytical performance was compared with that of the reference PCR. The estimated driving range and sample size capacity of the HFC and HFC minibus were 432 km and 2847 samples, respectively, for the HFC motorcoach and 313 km and 1949 samples for the HFC minibus. For the TAT, the median time between sample submission and completion of PCR was 86 min for the motorcoach and 76 min for the minibus, and the median time between sample submission and electronic reporting of the result to each visitor was 182 min for the motorcoach and 194 min for the minibus. A secondary analysis of 1574 HFC mobile laboratory testing samples was conducted, and all negative samples were found to be negative by reference PCR. Furthermore, all samples were confirmed to be positive by reference PCR or other molecular examinations.
Similar content being viewed by others
Introduction
Automated laboratory testing offers a significant advantage for rapid, accurate, and high-throughput laboratory testing. However, the need for heavy medical devices and electronic power are barriers to their utilization at sample collection sites; thus, samples must be transported to centralized laboratories for laboratory testing. Consequently, it takes days for patients to obtain results. Point-of-care testing (POCT), including rapid antigen testing1,2 and POCT-type molecular testing3,4,5,6,7, overcomes these problems. Rapid antigen testing has been widely used, and its use has augmented the field of COVID-19 testing. However, the sensitivity of antigen testing is inferior to that of molecular testing2, and POCT-type molecular testing is not suitable for the analysis of many samples.
Mobile laboratory systems are alternative options for laboratory systems to manage a large volume of laboratory samples with rapid reporting8 and have been clinically implemented during the coronavirus disease (COVID-19) pandemic9,10,11,12. A hydrogen fuel cell (HFC) bus uses hydrogen as a power source. Water and heat are the only byproducts of HFC vehicles. An HFC bus has a large capacity for energy storage and electricity supply and has the feature of low vibration amplitude for the conversion of hydrogen energy to electric power, both of which are significant merits for mobile clinical laboratories.
In 2021, we designed and developed two types of HFC buses equipped with a laboratory system for disasterinfection control (Fig. 1): a motorcoach-type HFC bus (HFC motorcoach) and a minibus-type HFC bus (HFC minibus). The motorcoach was the first commercially available HFC bus in Japan, and was a remodeled SORA (Toyota Motor Corporation). The HFC minibus was fabricated as a prototype vehicle by Toyota Motor Corporation by retrofitting a fuel cell system on a diesel-powered minibus (COASTER; Toyota Motor Corporation), and was remodeled for the current project for use as a mobile laboratory system. Both HFC buses were equipped with automated laboratory systems with electronic reporting, and patients received their laboratory results via e-mail soon after the submission of clinical samples.
In the current study, we evaluated the performance of a mobile laboratory system on HFC buses by focusing on reverse transcription-polymerase chain reaction (RT-PCR) for COVID-19. The mobile range, laboratory capacity, turnaround time (TAT), and accuracy of the laboratory testing were evaluated.
Methods
This study was performed between October 2021 and March 2022. The evaluation was performed at Tsukuba City Office, Ibaraki Prefectural Office, public health centers, temporary PCR centers, and the University of Tsukuba Hospital. The mobile range was evaluated based on the hydrogen mass consumed during actual driving and RT-PCR testing. The TAT was measured between patient sample submission and the reporting of results via e-mail to each device. The accuracy of laboratory testing was evaluated in comparison with that of a reference RT-PCR assay in a centralized laboratory.
Specifications of the motorcoach-type and minibus-type HFC buses
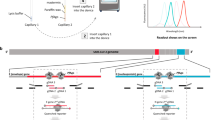
Both buses were designed to achieve a maximum electricity output of 9 kW (1.5 kW × 6 outlets, 100 V) for electricity in the laboratory (Fig. 1a) and meet the requirements of biohazard level 2 (P2) according to the World Health Organization (WHO) criteria. Both buses can supply and receive up to 9 kW of electricity (Fig. 1b) with a low noise level during operation (Supplementary Fig. 1). The quality of the power output was confirmed to meet the requirements of medical electrical equipment at ambient temperatures (30 °C/0 °C). Before the current study, both buses were certified to operate on public roads. Both buses permitted only one person as a driver, and no passenger could ride the bus while it was driven. Both the current HFC buses were designed for their equipment, including molecular identification systems, to be changed easily.
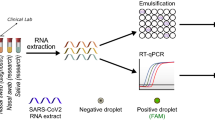
The general flow of the mobile laboratory system is illustrated in Fig. 2. The laboratory system included an electronic reservation system, a data management system, safety cabinets, refrigerators for samples and reagents, automated purification systems [magLEAD, Precision System Science Co., Ltd. (PSS), Chiba, Japan], automated PCR examination systems (GENECUBE, TOYOBO Co., Ltd., Osaka, Japan)13,14,15,16,17,18,19,20, and an electronic reporting system. The Reservations can be performed by the patient using an electronic device, and the laboratory results are sent to their registered e-mail address soon after the submission of the samples. The detailed layout of each bus for laboratory testing is described in Supplementary Fig. 2.
GENECUBE is a Qprobe-PCR-based automated rapid molecular identification system that can detect target genes in a short time and simultaneously analyze up to 24 samples and 4 assays in a single examination. The system automatically performs a direct molecular examination, including preparation of the reaction mixtures and amplification and detection of target genes, in 30 min. GENECUBE HQ SARS-CoV-2 (TOYOBO Co., Ltd.)13 has been approved as In-Vitro Diagnostics (IVD) product in Japan, and its use along with rapid purification methods with magLEAD14, were commercially available in clinical laboratories. Through the examination from preparation of samples to RT-PCR, the entire procedure was performed in the buses using plastic droppers. Positive and negative samples were set to each run in this study, and all positive samples were re-evaluated before reporting. The details of the workflow inside buses and the estimated TAT of each process along with a comparison with a conventional laboratory are summarized in Supplementary Table 1, Supplementary Figs. 3 and 4.
Hydrogen fuel consumption for driving and laboratory testing (COVID-19 RT-PCR)
Hydrogen fuel consumption by the motorcoach and minibus was measured during a one-way trip to several different testing sites from the University of Tsukuba Hospital, with RT-PCR examinations conducted in those places. Negative anonymized saliva samples and SARS-CoV-2 reference material, the AccuPlex™ SARS-CoV-2 Reference Material Kit (SeraCare; SeraCare Life Sciences, Inc., Milford, Massachusetts, the United States), were used for laboratory testing. The sample size was determined based on the PCR test capacity of each bus (Supplementary Fig. 4).
Comparison of the diagnostic accuracy of laboratory testing (COVID-19 RT-PCR) in the HFC bus and that of the reference RT-PCR assay in a centralized laboratory
Between February 1, 2022, and March 31, 2022, HFC buses were sent to two temporary COVID-19 PCR centers in Tsukuba at the request of the Ibaraki Prefectural Office (Supplementary Fig. 5). Asymptomatic workers at clinics, hospitals or social welfare facilities who were in close contact with COVID-19 patients in Ibaraki Prefecture used temporary PCR centers. Eligible individuals made an online reservation and submitted saliva samples by the drive-through method in the temporary PCR centers (Fig. 2c). Saliva samples were chosen for the sample of temporary COVID-19 PCR centers due to easier specimen collection compared with nasopharyngeal samples and anterior nasal samples and the nonnecessity of swabs and liquid medium; there are serious shortages of both due to high demand during the COVID-19 pandemic.
The samples were immediately analyzed in the buses, and the results were reported to visitors electronically through their registered email address. We analyzed the data and performed an analysis of the diagnostic accuracy of COVID-19 RT-PCR between February 1, 2022, and February 28, 2022, for the HFC motorcoach and between March 23, 2022, and March 28, 2022, for the HFC minibus. Residual saliva samples were anonymized and preserved at − 80 °C until evaluation.
For the analytical evaluation, the preserved frozen saliva samples were sent to LSI Medience Corporation for comparison, and then a reference real-time RT-PCR examination was performed using a Maxwell® RSC Viral Total Nucleic Acid Purification Kit for ribonucleic acid (RNA) extraction (Promega Corporation, Wisconsin, the United States) according to the manufacturer’s instructions and a Cobas® Z480 Real-Time RT-PCR System (Roche, Basel, Switzerland) using a method developed by the National Institute of Infectious Diseases (NIID), Japan, for SARS-CoV-221. A duplicate analysis for N2 genes was performed for the evaluation of SARS-CoV-2. The Ampdirect 2019 Novel Coronavirus Detection Kit (Shimadzu Corporation, Kyoto, Japan)22 and cobas 8800 system and cobas SARS-CoV-2 & Influenza A/B (cobas; Roche Molecular Systems, New Jersey, the United States)23 were used to evaluate discrepant samples. The differences in the limit of detection among molecular examinations for COVID-19 are shown in Supplementary Table 2, which was evaluated with SARS-CoV-2 reference material, anonymized nasopharyngeal samples, and saliva samples.
Ethical considerations
The ethics committee of the University of Tsukuba Hospital approved the protocol of the present study (approval number: R03-043). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from participants who provided saliva samples used for TAT analyses. For other analyses, we used residual samples as a retrospective study that were completely anonymous; therefore, the requirement for obtaining informed consent was waived by the ethics board of the University of Tsukuba Hospital.
Statistical analyses
The total concordance rate, positive concordance rate, and negative concordance rate were calculated with 95% confidence intervals (CIs). The correlation between the cycle threshold (Ct) value of the real-time RT-PCR assay (NIID method) and the Sp value of GENECUBE was evaluated using Spearman's rank correlation coefficient. P values of < 0.05 were considered to indicate statistical significance. All statistical analyses were conducted using the R 4.1.2 software program (The R Foundation, Vienna, Austria) with the "readxl", "tidyverse", and "epiR" packages.
Results
Evaluation of hydrogen fuel consumption in driving and laboratory testing (COVID-19 RT-PCR)
The evaluation of hydrogen fuel consumption in driving and laboratory testing is summarized in Fig. 3. For the motorcoach, the average fuel consumption in driving and laboratory testing (COVID-19 RT-PCR) was 18.0 km/kg (range 15.7–20.1 km/kg) and 118.6 samples/kg (range 78.6–137.5 samples/kg), respectively. For the minibus, the average fuel consumption in driving and laboratory testing (COVID-19 RT-PCR) was 32.3 km/kg (range 25.8–41.2 km/kg) and 200.9 samples/kg (range 129.6–259.2 samples/kg), respectively.
Hydrogen fuel consumption for driving and laboratory testing (COVID-19 RT-PCR). Points a–g in the figure indicate laboratory test sites. a: Tsuchiura Public Health Center, b: Ryugasaki Public Health Center, c: Chikusei Public Health Center, d: Itako Public Health Center, e: Ibaraki Prefectural Office, f: Hitachinaka Public Health Center, g: Hitachi Public Health Center. The maps were created by the authors using Adobe Illustrator ver.28.0. (https://www.adobe.com/). The estimated maximum power of the driving distance and the laboratory testing throughput of COVID-19 RT-PCR of the hydrogen fuel cell bus were calculated according to the hydrogen storage of each bus (motorcoach, 24 kg; minibus, 9.7 kg) and average hydrogen consumption. The difference in driving distance between each bus is due to the driving route of each bus from the University of Tsukuba to the destination. The driving distance was calculated by Google Maps for the motorcoach and by the onboard distance calculation system for the minibus. The data on the average temperature of each site on the examination date were cited from the data on the website of the Japan Meteorological Agency; https://www.jma.go.jp/jma/indexe.html. The temperature inside the buses during the examination was not recorded. RT-PCR, reverse transcription-polymerase chain reaction.
Analysis of the diagnostic accuracy of COVID-19 RT-PCR in the two HFC buses
During the evaluation period, 18 days of examination data for the motorcoach (n = 1395) and 3 days of examination data (n = 179) for the minibus were evaluated. The median hydrogen consumption during the COVID-19 RT-PCR was 57.2 samples/kg [IQR (interquartile range) 35.2–90.4] for the motorcoach and 135.0 samples/kg (IQR 111.8–177.4) for the minibus (Table 1). For the TAT, the median time between the sample submission and the completion of COVID-19 RT-PCR was 86 min (IQR 73–96 min) for the motorcoach and 76 min (IQR 71–84 min) for the minibus, and the median time between sample submission and electronic reporting of the result to each visitor was 182 min (IQR 134–272 min) for the motorcoach and 194 min (IQR 167–227 min) for the minibus (Table 1). Detailed information is described in Supplementary Table 3.
The results of the comparison between the molecular examination in buses and a reference RT-PCR assay in the laboratory are shown in Table 2 (n = 1574). The total concordance rate, positive concordance rate and negative concordance rate were 98.4% (95% CI 97.7–99.0%), 100% (95% CI 95.1–100%) and 98.3% (95% CI 97.6–98.9%), respectively. Of the 25 bus-positive and reference RT-PCR-negative cases, 23 were positive by an Ampdirect 2019 Novel Coronavirus Detection Kit. The 2 samples were further evaluated with a cobas 8800 system and cobas SARS-CoV-2 & influenza A/B, and both samples were found to be positive.
The correlation of the Sp value of GENECUBE in buses and the Ct value of the reference RT-PCR assay is shown in Supplementary Fig. 6.
Discussion
To date, this is the first report on the clinical implementation of HFC buses as mobile laboratories. The current evaluation showed that both types of buses had sufficient hydrogen storage for driving and laboratory testing (COVID-19 RT-PCR). With the implementation of the newly developed mobile laboratory systems in COVID-19 PCR centers, visitors could receive the RT-PCR results within a few hours online after sample submission. The high diagnostic accuracy for the detection of SARS-CoV-2 RNA in saliva samples was confirmed by comparison with a reference RT-PCR assay.
The clinical implementation of mobile laboratories has dramatically progressed since the onset of the COVID-19 pandemic. In Japan, the clinical use of mobile laboratories was tentatively approved—as a special case—the COVID-19 pandemic in early February 202224. This approval allowed the current HFC mobile laboratory system to be introduced into clinical practice. Touron et al. reported the utility of the MobilDNA lab for COVID-1911 in France. This transportable laboratory system has been available since 2015. The system used was an Applied™ 7500 real-time PCR system operated by highly skilled medical technologists, and it was utilized in the COVID-19 pandemic as a high-throughput mobile laboratory, which reported results in one day. Ballard also reported the rapid reporting of molecular examinations using a mobile laboratory system with an automated molecular identification system (GeneXpert) in Australia9. They showed that the median TAT from sample collection to reporting of results was approximately 2 h, which was significantly faster than that of a conventional laboratory; however, it was limited in the number of samples, and the TAT was more than 4 h in some cases.
In our current HFC bus-based mobile laboratory system, laboratory examinations were completed in approximately one hour, most of the results were reported within 3 h after the samples were obtained, and the TAT was improved to 2 h (Supplementary Table 3a) after adjusting for the workflow in late February, which increased the frequency of reporting RT-PCR results from once a day to twice a day for the motorcoach. TAT can be shortened by increasing the frequency of reporting because there is an approximately 100-min interval in both HFC buses between the completion of the molecular examination and the reporting of the results. The test capacity for fuel consumption was clearly lower in the COVID-19 PCR centers (Table 1) than in the initial evaluation (Fig. 3). According to the current evaluation, the capacity was influenced by the average temperature at the test sites and the duration for which the laboratory staff stayed in the HFC buses, both of which are important factors for the consumption of hydrogen for air conditioning. Additionally, the number of tests per run in COVID-19 PCR centers was lower than that in the initial evaluations due to frequent molecular examinations, which shortened the TAT in a real-world clinical setting. Despite these influencing factors, the HFC bus could process 100 samples in temporary COVID-19 PCR centers without a delay in TAT or a hydrogen energy shortage.
In addition, the current system exhibits sufficient analytical performance for the detection of SARS-CoV-2 RNA. Additionally, laboratory processing was automated, including reservation and reporting. All the processes were easy to handle without pipetting during laboratory examination, and the current system can be managed by inexperienced technicians in case of emergency use. The current systems require large amounts of electricity for automated laboratory systems and air conditioning (nearly 9 kW) for the appropriate working environment of technicians, and the HFC bus is essential to supply electricity in addition to the merit of low vibration amplitude.
The current evaluation clarified the characteristics of both HFC buses in a mobile laboratory. The motorcoach has a superior driving range and test capacity and has sufficient workspace for laboratory staff in the bus, whereas the minibus clearly has more efficient hydrogen consumption during driving and laboratory testing. In addition, the minibus can use highway roads and be driven without a special driver’s license. For clinical use, minibus is superior to motorcoaches for rapid response to urgent requirements, such as contact tracing of hospital outbreaks or rapid molecular examination for new emerging infectious diseases at or near airports. In the real-world setting, the minibus was used as a temporary COVID-19 PCR center (14,654 patients) and for contact tracing of COVID-19 outbreaks at 7 healthcare facilities in fiscal year 2022. Meanwhile, the motorcoach will be of use if we need to evaluate a large number of residents per day. Both HFC buses will have significant utility for rapid responses in cases of novel emerging diseases, or for case tracing if highly virulent COVID-19 variants emerge.
Note that the system is not only capable of stand-alone, self-sustaining testing in an HFC bus, but also has the ability to be connected to other energy resources. Future design implications of such mobile laboratories include the ability to supply power to external facilities and receive power from an external source, including mobile sources such as EV or fuel cell vehicles (FCV). In this experiment, when weather conditions made it difficult to travel to the hydrogen station, power could be supplied from the FCV during the inspection, and the inspection could then be performed successfully. It is also important to supply electricity to the surrounding environment, such as a small workspace for sample collection and lighting facilities during the night. We believe that we can connect the developed mobile laboratory to medical institutions and public facilities with safe, robust, and stable mobile testing.
The present study has several limitations. First, the hydrogen fuel consumption during driving and laboratory testing was measured just after the development of the systems, and inexperience may have affected the data. Additionally, the current data were mainly collected in the winter season, which requires heating inside the buses; this requirement differs in other seasons. Second, the current evaluation, including the preceding limit of detection study (Supplementary Table 2), showed sufficient analytical performance of molecular examination for the detection of SARS-CoV-2 RNA; however, we could not use fresh positive samples for comparison with the reference RT-PCR assay. Storage processes involving freezing and thawing have been reported to affect viral load in samples25. Third, we used hydrogen energy; however, hydrogen supply and the number of hydrogen fueling stations are clearly insufficient in most countries by 2023, which complicates the universal clinical application of the results of the current research. Third, in the current study, HFC mobile laboratory systems were only evaluated for SARS-CoV-2 by RT-PCR in saliva samples. Current molecular examination platforms can evaluate other respiratory samples and various pathogens15,16,17,18,19,20, and the test platforms of both HFC buses can easily be changed upon request. The feasibility of using other samples, pathogen targets, or other molecular identification platforms for HFC mobile laboratories needs to be evaluated.
In conclusion, the evaluation of the newly developed mobile laboratory system confirmed that HFC buses have sufficient energy storage for driving and laboratory testing (COVID-19 RT-PCR), and that they are feasible for the rapid reporting of highly accurate COVID-19 RT-PCR results.
Data availability
The data include sensitive information about the health of human research subjects and thus cannot be directly deposited openly. However, anonymized individual-level data that enable full replication of the study results are available from the corresponding author.
References
Dinnes, J. et al. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 7, CD013705 (2022).
Hanson, K. E. et al. The infectious diseases Society of America guidelines on the diagnosis of COVID-19: Antigen testing. Clin. Infect. Dis. 23, 557 (2021).
Akashi, Y. et al. Clinical performance of the cobas Liat SARS-CoV-2 & influenza A/B assay in nasal samples. Mol. Diagn. Ther. 26, 323–331 (2022).
Akashi, Y. et al. Analytical and clinical evaluation of a point-of-care molecular diagnostic system and its influenza A/B assay for rapid molecular detection of the influenza virus. J. Infect. Chemother. 25, 578–583 (2019).
Dinnes, J. et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 3, CD013705 (2021).
Kiyasu, Y. et al. Clinical evaluation of the rapid nucleic acid amplification point-of-care test (Smart Gene SARS-CoV-2) in the analysis of nasopharyngeal and anterior nasal samples. J. Infect. Chemother. 28, 543–547 (2022).
Moore, N. M., Li, H., Schejbal, D., Lindsley, J. & Hayden, M. K. Comparison of two commercial molecular tests and a laboratory-developed modification of the CDC 2019-nCoV reverse transcriptase PCR assay for the detection of SARS-CoV-2. J. Clin. Microbiol. 58, e00938-e1020 (2020).
World Health Organization. Guidance for Rapid Response Mobile Laboratory (RRML) Classification. https://www.who.int/europe/publications/i/item/9789289054928 (2021).
Ballard, S. A. et al. Lab-in-a-van: Rapid SARS-CoV-2 testing response with a mobile laboratory. EBioMedicine 79, 103983 (2022).
Roh, K. H. et al. Guidelines for mobile laboratories for molecular diagnostic testing of COVID-19. Ann. Lab. Med. 42, 507–514 (2022).
Touron, P. et al. A mobile DNA laboratory for forensic science adapted to coronavirus SARS-CoV-2 diagnosis. Eur. J. Clin. Microbiol. Infect. Dis. 40, 197–200 (2021).
Xing, W. et al. A highly automated mobile laboratory for on-site molecular diagnostics in the COVID-19 pandemic. Clin. Chem. 67, 672–683 (2021).
Kiyasu, Y. et al. A prospective evaluation of the analytical performance of GENECUBE((R)) HQ SARS-CoV-2 and GENECUBE((R)) FLU A/B. Mol. Diagn. Ther. 25, 495–504 (2021).
Naito, A. et al. The evaluation of the utility of the GENECUBE HQ SARS-CoV-2 for anterior nasal samples and saliva samples with a new rapid examination protocol. PLoS ONE 16, e0262159 (2021).
Hida, Y. et al. Rapid detection of the Mycobacterium tuberculosis complex by use of quenching probe PCR (geneCube). J. Clin. Microbiol. 50, 3604–3608 (2012).
Miyazaki, N. et al. Evaluation of rapid measurement of Chlamydia trachomatis and Neisseria gonorrhoeae by using automatic gene analyzer “GENECUBE”. Jpn. J. Antibiot. 69, 291–298 (2016).
Ito, Y. et al. Rapid detection of the macrolide sensitivity of pneumonia-causing Mycoplasma pneumoniae using quenching probe polymerase chain reaction (GENECUBE((R))). Mol. Diagn. Ther. 22, 737–747 (2018).
Hida, Y. et al. Evaluation of performance of the GENECUBE assay for rapid molecular identification of Staphylococcus aureus and methicillin resistance in positive blood culture medium. PLoS ONE 14, e0219819 (2019).
Hara, T. et al. Clinical evaluation of a non-purified direct molecular assay for the detection of Clostridioides difficile toxin genes in stool specimens. PLoS ONE 15, e0234119 (2020).
Morinaga, Y. et al. Evaluation of GENECUBE Mycoplasma for the detection of macrolide-resistant Mycoplasma pneumoniae. J. Med. Microbiol. 69, 1346–1350 (2020).
Shirato, K. et al. Development of genetic diagnostic methods for detection for novel coronavirus 2019(nCoV-2019) in Japan. Jpn. J. Infect. Dis. 73, 304–307 (2020).
Mizoguchi, M. et al. Comparative performance and cycle threshold values of 10 nucleic acid amplification tests for SARS-CoV-2 on clinical samples. PLoS ONE 16, e0252757 (2021).
Craney, A. R. et al. Comparison of two high-throughput reverse transcription-PCR systems for the detection of severe acute respiratory syndrome coronavirus 2. J. Clin. Microbiol. 58, e00890-e920 (2020).
Ministry of Health, Labor and Welfare. Administrative Notice. https://www.mhlw.go.jp/content/000896379.pdf (2022).
Dzung, A. et al. Prolonged unfrozen storage and repeated freeze-thawing of SARS-CoV-2 patient samples have minor effects on SARS-CoV-2 detectability by RT-PCR. J. Mol. Diagn. 23, 691–697 (2021).
Acknowledgements
This research was funded by the Cross-ministerial Strategic Innovation Promotion Program (SIP, Cabinet Office). We thank Dr. Koki Kaku for his instruction and advice as a sub-program director of the current SIP project. We also thank Mr. Shimpei Miura, Mr. Katsutoshi Tanei, and their colleagues (Toyota Motor Corporation) for their technical support and assistance in the development of mobile laboratory systems for hydrogen fuel cell buses; Mr. Miyazaki's colleagues (Izumi Motor Car Co., Ltd.) for remodeling the hydrogen fuel cell buses to support the laboratory function; staff of Kanto Railway Co., Ltd. for operating the hydrogen fuel cell buses; city officials of Tsukuba City and the Prefectural Office staff of Ibaraki Prefecture for supporting examination; staff of ENEOS Corporation for support regarding hydrogen filling; Dr. Toru Nanmoku, Ms. Rikako Kano, the staff of the University of Tsukuba Hospital, the staff of the LSI Medience Corporation, and the staff of the National Research Institute for Earth Science and Disaster Resilience for their support of this study. All photographs in this manuscript were taken by the authors.
Author information
Authors and Affiliations
Contributions
M.O. performed laboratory testing and drafted the manuscript. K.S. drafted the manuscript, created the mobile laboratory system, designed the study and supervised the project. A.E. provided technical support for the hydrogen fuel cell buses and drafted the manuscript. A.N., T.K., and J.K. performed laboratory testing. Y.A. performed statistical analysis and revised the manuscript. S.N. collected the samples and Y.K. and Y.K. revised the manuscript. M.T. performed the laboratory testing and supervised the project. T.S. created the mobile laboratory system and designed the study. Y.K. supervised this project. H.S. drafted the manuscript, created the mobile laboratory system, designed the study and supervised the project. All authors contributed to the writing of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
TOYOBO Co., Ltd., provided lecture fees for Hiromichi Suzuki and advisory fees for Hiromichi Suzuki. Hiromichi Suzuki received advisory fees from PSS. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okude, M., Suzuki, K., Naito, A. et al. Development of a mobile laboratory system in hydrogen fuel cell buses and evaluation of the performance for COVID-19 RT-PCR testing. Sci Rep 13, 17546 (2023). https://doi.org/10.1038/s41598-023-44925-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44925-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.