Abstract
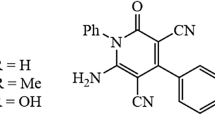
The inhibiting efficiency of three newly synthesized organic compounds:5-((4'-(dimethylamino)-[1,1'-biphenyl]-4-yl)methylene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (HM-1228), 5-((4'-(dimethylamino)-[1,1'-biphenyl]-4-yl)methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (HM-1227) and 5-((4'-(dimethylamino)-[1,1'-biphenyl]-4-yl)methylene)pyrimidine-2,4,6(1H,3H,5H)-trione (HM-1226) in oilfield produced water on the corrosion of carbon steel has been examined via electrochemical measurements; potentiodynamic polarization (PDP) and electrochemical impedance (EIS) techniques. The adsorption of these compounds on the surface of carbon steel followed Langmuir isotherm. In addition, the surface morphology of uninhibited and inhibited carbon steel was examined by Atomic Force Microscopy (AFM), observing surface improvement when carbon steel samples exposed to the inhibited corrosive solutions. The average surface roughness (Ra) in oilfield produced water solution in the presence of 0.5 mM of HM-1228 inhibitor was 138.28 nm compared to the uninhibited surface 571.62 nm. To explore the corrosion inhibition mechanism, quantum chemical calculations and Monte Carlo simulations were utilized. The HM-1228 inhibitor demonstrated the highest corrosion inhibition efficiency at 94.8% by PDP measurements. The higher corrosion inhibition of compound HM-1228 can be attributed to the presence of di-N-ethyl groups that enhance both electron donating ability and lipophilic properties.
Similar content being viewed by others
Introduction
Carbon steel is one of the most widely used construction materials in various industries, such as oil and gas transportation, chemical, and petrochemical. Its alloys are particularly prevalent due to their unique mechanical and machinery properties, including hardness, strength, and ductility. However, a major challenge associated with the use of carbon steel alloys is their lack of corrosion resistance, especially in moist conditions. This can be considered as a drawback as it can lead to significant industrial problems and jeopardize the safety of humans. Therefore, there has been a growing interest among researchers and industry specialists in finding solutions to mitigate corrosion, focusing on corrosion protection measures to control these undesired consequences and prolong the lifespan of structural materials1,2. Since the useful features of carbon steel can compromised by severe attack from the corrosive environment of various oilfield operations3,4,5. One of the most prevalent corrosive environments in the crude oil and gas industry is oil-well formation water, which occurs naturally in oil fields and contain a variety of dissolved inorganic and organic chemicals, dissolved salts, and dissolved gases6,7. One viable approach for industry experts to mitigate corrosion is the use of corrosion inhibitors which protect the steel surface via the formation of a protective film. The use of corrosion inhibitors, which is a highly effective industry technique, is commonly utilized to reduce metal loss8,9,10. Some organic compounds are synthesized and used as corrosion inhibitors due to their several features including the low price, high inhibition efficacy, and ease of synthesis11,12,13,14. However, the use of organic compounds as corrosion inhibitors is one of the most challenging tasks because most organic compounds are neither cheap nor effective in most cases. It is generally established that organic compounds including sulphur, oxygen, and nitrogen atoms protect the steel surface from the destructive media via adsorption15. In a previous work in literature, the thiobarbituric acid was tested for its inhibition efficiency for API X60 steel in NaCl solution saturated with CO2, which showed corrosion inhibition above 90%16. In recent years, ongoing efforts have been devoted to synthesize new, more efficient corrosion inhibitors, which is an important direction in metal corrosion prevention.
Even though previous studies on thiopyrimidine derivatives have been published as corrosion inhibitors, our aim in this study is to investigate the inhibition efficiency of novel biphenylidene-thiopyrimidine derivatives as corrosion inhibitors for carbon steel in oilfield produced water using a combination of experimental and computational chemistry approaches.
Experimental
Three novel biphenylidene-thiopyrimidine derivatives HM-1228, HM-1227, and HM-1226 were synthesized. The chemical structures and molecular formulas are presented in Table 1. The full details on the synthesis process and characterization are in the following experimental part.
Methodology for preparation of the studied inhibitors
Preparation of (N,N-dimethylamino)biphenylidene-thiopyrimidine derivatives 5a-c:
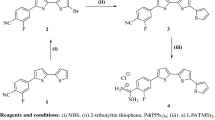
4\-(N,N-Dimethylamino)-[1,1\-biphenyl]-4-carbaldehyde (3): To a solution of 4-bromo-N,N-dimethylaniline (1) (1.5 g, 7.5 mmol), and Pd(PPh3)4 (230 mg) in toluene (15 mL) was added 7.5 mL of 2M Na2CO3 (aqueous), and methanolic solution of (4-formylphenyl)boronic acid 2 (1.35 g, 9 mmol). The reaction mixture was heated at 80 °C with stirring for ~ 12 h, where after the reaction mixture was extracted with ethyl acetate (200 mL, 3x). The organic layer was passed through celite to remove Pd, the organic extract was dried and then evaporated to dryness. The solid was filtered off and recrystallized from ethanol to furnish the anticipated 4\-(dimethylamino)-[1,1\-biphenyl]-4-carbaldehyde 3. Biphenylcarbaldehyde 3 was obtained in 82% yield as a yellow solid, m.p. = 189–191 °C, Lit.17 m.p. = 192–195 °C. IR (KBr) ν\/cm−1: 3035 (sp2 C–H, stretch), 2892 (sp3 C–H, stretch), 2805, 2713 (C–H of CHO, stretch), 1693 (C = O, stretch), 1594, 1541 (C = C, stretch). MS (EI) m/e (rel.int.) for C15H15NO (225.29); 225.39 (M+, 42.24), 77 (100).
5-((4'-(Dimethylamino)-[1,1'-biphenyl]-4-yl)methylene)-1,3-diethyl-2-thioxodihydro-pyrimidine-4,6(1H,5H)-dione (5a)
To a solution of biphenylcarbaldehyde 3 (200 mg, 0.89 mmol), 1,3-diethyl-2-thiobarbituric acid (4a) (1.78 mmol) in a mixture of 30 mL methanol/acetic acid (2:1). The reaction mixture was heated at reflux for ~ 12 h and the precipitate was filtered off, washed with methanol and recrystallized from ethanol/Ethyl acetate to afford biphenylidene-thiobarbituric acid derivative 5a as a greenish-brown solid in 71% yield, m.p. = 216–218 °C. IR (KBr) ν\/cm−1: 2974, 2927 (sp3 C–H, stretch), 1697, 1664 (C = O, stretch), 1598, 1554 (C = C, stretch), 1396 (C = S, stretch). 1H-NMR (DMSO-d6); δ 1.19–1.22 (m, 6H, 2CH3 of diethyl groups-H’s), 2.97 (s, 6H, –N(CH3)2), 4.40–4.44 (m, 4H, 2CH2 of diethyl groups-H’s), 6.81 (d, J = 9 Hz, 2H, Ar–H’s of dimethylaniline ring), 7.71 (d, J = 9 Hz, 2H, Ar–H’s of phenyl ring), 7.78 (d, J = 9 Hz, 2H, Ar–H’s of phenyl ring), 8.29 (d, J = 9 Hz, 2H, Ar–H’s of dimethylaniline ring), 8.38 ppm (s, 1H, methine-H). MS (EI) m/e (rel.int.) for C23H25N3O2S (407.53); 407.41 (M+, 25.89), 150.56 (100).
5-((4'-(Dimethylamino)-[1,1'-biphenyl]-4-yl)methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (5b)
Compound 5b was prepared adopting the same methodology used for preparation of compound 5a, starting with 2-thiobarbituric acid (4b) instead of 1,3-diethyl-2-thiobarbituric acid (4a). Thiopyrimidine derivative 5b was obtained as a greenish-brown solid in 75% yield, m.p. > 300 °C. IR (KBr) ν\/cm−1: 3451, 3419 (N–H, stretch), 3158 (sp2 C–H, stretch), 2926 (sp3 C–H, stretch), 1697, 1652 (C = O), 1612, 1523 (C = C, stretch), 1395 (C = S, stretch). 1H-NMR (DMSO-d6); δ 2.97 (s, 6H, –N(CH3)2), 6.80 (d, J = 8.5 Hz, 2H, Ar–H’s of dimethylaniline ring), 7.71 (d, J = 8.0 Hz, 2H, Ar–H’s of phenyl ring), 7.77 (d, J = 8 Hz, 2H, Ar–H’s of phenyl ring), 8.27 (s, 1H, methine-H), 8.32 (d, J = 8.5 Hz, 2H, Ar–H’s of dimethylaniline ring), 12.33 (s, 1H, NH), 12.43 ppm (s, 1H, NH). 13CNMR; δ 39.5 (2C), 112.6 (2C), 117.1, 124.6, 127.7 (2C), 129.9 (2C), 130.2 (2C), 135.6, 145.0, 150.6, 155.6, 159.8, 162.1, 178.3. MS (EI) (m/e, %) for C19H17N3O2S (351.42); 351.53 (M+, 28.96), 93.34 (Base peak, 100).
5-((4'-(Dimethylamino)-[1,1'-biphenyl]-4-yl)methylene)pyrimidine-2,4,6(1H,3H,5H)-trione (5c)
Compound 5c was furnished adopting the same methodology used for preparation of 5a, starting with barbituric acid (4c) instead of 1,3-diethyl-2-thiobarbituric acid (4a). Compound 5c was obtained as a greenish-brown solid in 72% yield, m.p. = 250–252 °C. IR (KBr) ν\/cm−1: 3201 (N–H, stretch), 3060 (sp2 C–H, stretch), 2851 (sp3 C–H, stretch), 1753, 1701, 1658 (C = O, stretch), 1596, 1547 (C = C, stretch). 1H-NMR; δ 2.96 (s, 6H, –N(CH3)2), 6.80 (d, J = 8.5 Hz, 2H, Ar–H’s of dimethylaniline ring), 7.69 (d, J = 8.5 Hz, 2H, Ar–H’s of phenyl ring), 7.75 (d, J = 8.5 Hz, 2H, Ar–H’s of phenyl ring), 8.26–8.28 (m, 3H), 11.22 (s, 1H, NH), 11.35 ppm (s, 1H, NH). MS (EI) m/e (rel.int.) for C19H17N3O3 (335.36); 335.94 (M+, 38.79), 334.95 (M+ − 1, 100).
Corrosion measurements
Corrosion experiments were performed using API 5L X52 carbon steel specimens with weight percentage element composition shown as follows: C (0.114), Si (0.346), Mn (0.967), Ni (0.071), Cr (0.163), Al (0.043), Cu (0.124), Nb (0.053), Pb (0.034), Ti (0.04) and Fe (98.027). The aggressive media utilized in this study is oilfield produced water obtained from oil well of Badr El-Din Petroleum Company, Egypt. Physical properties and chemical composition of the oil field produced water are presented in Table 2. The equivalent weight of inhibitor was dissolved in 10 ml dimethyl-sulfoxide then the volume was completed with absolute ethanol up to 100 ml. The concentration ranges of the studied inhibitors were 0.001–0.5 mM. Electrochemical studies (PDP and EIS) were performed by a three-electrode electrochemical glass cell using Volta lab 80. API 5L X52 carbon steel with an uncovered surface area of 0.8 cm2 was utilized as the working electrode, Pt plate was served as the counter electrode, and Ag/AgCl/KClsat electrode was employed as reference electrode. Prior to measurements, the working electrode was polished to mirror in series on 360–2500 silicon carbide papers, then washed with deionized water and dimethyl ketone, and finally dried under warm air. EIS experiments were carried out in a frequency range between 105 Hz and 10─2 Hz at open circuit potential (EOCP), with a signal (AC) amount perturbation of 10 mV18,19. PDP tests were evaluated at ± 300 mV based on EOCP with a steady sweep rate of 1 m mV s−120,21. Each electrochemical test had been executed at least twice to confirm the accuracy of the data.
Surface morphology investigations
Surface analysis (AFM) of API 5L X52 carbon steel specimens, with dimensions 15 × 10 × 2.5 mm, was performed by Nano surf Flex AFM analysis without and with exposure to oil field produced water solution without and with 0.5 mM of the prepared inhibitor HM-1228 at 25 ± 1 °C. Carbon steel samples were stored, and then dried for AFM analysis10,22.
Computational calculations
The ground-state geometry optimizations were carried out with Density Function (DFT) method on the investigated biphenylidene-thiopyrimidine derivatives by Gaussian 09, Revision A0223,24. Prior to the geometrical optimization step, the most stable configuration of compounds is chosen and relaxed with MM2 force field tool. The DFT calculations were computed at basis-set 6-31G (d,p) and in absence of symmetry limitations and the Becke three parameter-hybrid-B325 with the L-Y-P (Lee–Yang–Parr) correction functional26 (B3LYP)27. For the representation of solvent effect, the Polarized continuum (PCM) model was adopted. The quantum chemical parameters such as EHOMO, ELUMO, ΔE were calculated by DFT and analyzed28. The theoretical formulation of the HSAB principle is an effective approach to obtain some quantum chemical descriptors29,30. This method is based on the first and second partial derivatives of energy with regards to electron number at constant external potential.
Insightful data on the nature of chemical interactions and chemical reactivity of molecules can be studied, where some useful quantum chemical descriptors such as chemical hardness (η), chemical potential (μ), and electronegativity (χ) are evaluated. μ and η are calculated from the first derivation of the electronic energy and chemical potential as a function of electron number (N) at constant external potential, v(r), respectively31,32.
The HOMO and LUMO orbital energies correlates with the ionization energies (Eq. 3) and electron affinity of a molecule (Eq. 4)according to Koopman's Theorem33.
The hardness \(\eta\), electronegativity \(\chi\) and chemical potential (\(\mu\) are also calculated by applying the following equations.
Softness is the inverse of chemical hardness, and it can be used to indicate the polarizability of the molecule and it is given as the following equation:
The global electrophilicity index (by Parr) (ω)34 quantifies the propensity of chemical species to accept electrons. A nucleophile with higher reactivity is distinguished by lower values of µ and ω, whereas an electrophile with higher reactivity is characterized by a higher value of µ and ω. This novel reactivity index evaluates the energy stabilization that occurs when the system obtains an extra electronic charge ∆N from its surroundings.
The global electrophilicity index (ω), nucleophilicity (ε), the fraction of electrons transferred (ΔNFET), the back donation energy ΔEb-d, electronic charge accepting capability and the initial molecule–metal interaction energy Δψ, were calculated in terms of global hardness (η) and electronegativity (χ) are calculated as the following Eqs. 34.
Herein, \({\varphi }_{\mathrm{Fe}}\) represents the work function of a metal surface, which is recently used as a measure of its electronegativity, and the \({\eta }_{\mathrm{Fe}}\) is absolute hardness of iron. Whereas \({\chi }_{\mathrm{inh}}\), and \({\eta }_{\mathrm{inh}}\) denote to the absolute electronegativity and hardness of inhibitor molecule, respectively. In order to obtain the ΔN values, we use a theoretical value of \({\varphi }_{\mathrm{Fe}}\) = 4.82 eV and ηFe = 0 by assuming that for a metallic bulk I = A since they are softer than the neutral metal atoms35.
Molecular simulations
Molecular simulations by Monte Carlo method which is available in adsorption locator tool in Materials Studio 7.0 is utilized to explore the lowest configuration adsorption energy on simulated inhibited and uninhibited Fe-surface in aqueous solution36,37,38. A simulation box with dimension 24.18 × 24.18 × 42.65 cm was used in the simulation procedure. First, the Fe (110) was cleaved from pure Fe crystal, then the surface was enlarged to a super cell 8 × 8 to accommodate the inhibitors on Fe surface. A vacuum slap with thickness 30 cm is built over the surface and the top layer surface atoms are dimensionally constrained. COMPASS force field is used in the simulation of corrosion inhibition process, as it is a high quality forcefield that enables consolidating frameworks of inorganic and organic compounds37. The tolerance of energy convergence is 10−4 kcal/mol with maximum force 0.005 kcal/mol/Å, and displacement of 5 × 10−5 Å. All the adsorption simulations are carried out in fine quality conditions. The simulation box contains Fe -surface layer and 1 inhibitor molecule, and 100 water molecules. The results from simulation were analyzed and discussed.
Results and discussion
Synthesis and characterization of inhibitors
Preparation of the new biphenylidene-thiopyrimidine derivatives 5a-c began with a Suzuki coupling by treatment of 4-bromo-N,N-dimethylaniline (1) with (4-formylphenyl)boronic acid 2 in the presence of Pd(0) as a catalyst and 2M Na2CO3 (aqueous) as a base and heating in toluene at 80 °C to furnish the biphenylcarbaldehyde compound 3 which is subsequently condensed with thiobarbituric acid derivatives 4a–c to afford the target thiopyrimidine derivatives 5a–c in good yields (71–75%) Fig. 1.
The newly synthesized biphenylidene-thiopyrimidines 5a–c were assured based on their spectral data. Thus, IR spectrum of 5a revealed no N–H absorption band, whereas, 5b and 5c indicated the appearance of N–H absorption bands at 3451, 3419 cm−1 (5b), 3201 cm−1 (5c), in addition to, thione groups at 1396 cm−1 (5a), 1395 cm−1 (5b). Whereas, IR spectra for compounds 5a–c showed carbonyl groups in the range of 1652 to 1753 cm−1. 1H-NMR spectrum of compound 5a displayed the protons of diethyl groups as two multiplets at δ 1.19–1.22 (6H, 2 × CH3 of diethyl groups-H’s), 4.40–4.44 (4H, 2 × CH2 of diethyl groups-H’s), dimethylamino group (2 × N-Me) as a singlet signal at δ 2.97 integrated for six hydrogens, four aromatic hydrogens of dimethylaniline ring as two doublets at δ 6.81 (2H) and δ 8.29 (2H) with coupling constant J = 9 Hz, four aromatic hydrogens of phenyl ring as two doublets at δ 7.71 (2H) and δ 7.78 (2H), plus one singlet signal of methylidene at δ 8.38 (1H). Mass spectrum of 5a furnished m/e peak at 407.41 of its molecular ion peak (M+, 25.89). 1H-NMR spectrum of compound 5b displayed a singlet signal at δ 2.97 integrated for six hydrogens (2 × N-Me), four doublet signals; two of them referring to Ar–H’s of dimethylaniline ring at δ 6.80 (2H) and δ 8.32 (2H) with coupling constant J = 8.5 Hz, while other two doublets at δ 7.71 (2H) and δ 7.77 (2H) with coupling constant J = 8 Hz of Ar–H’s of phenyl ring, one singlet signal of methylidene at δ 8.27 (1H), plus two singlet signals integrated for one hydrogen each at δ 12.33 (NH) and 12.43 (NH). Mass spectrum of 5b furnished peak at m/e 351.53 of its M+. Once more, 1H-NMR spectrum of compound 5c displayed a singlet signal at δ 2.96 integrated for six hydrogens (2 × N-Me), one doublet signal at δ 6.80 integrated for two hydrogens (Ar–H’s of dimethylaniline ring), two doublet signals at δ 7.69 (2H) and δ 7.75 (2H) referring to Ar–H’s of phenyl ring, the other three aromatic protons appeared as multiplet at δ 8.26–8.28 ppm (methine-H’s and the remaining two Ar–H’s of dimethylaniline ring), plus two singlet signals integrated for one hydrogen each at δ 11.22 (NH), 11.35 (NH). Mass spectrum of 5c displayed a molecular ion peak m/e at 335.94 of its M+.
Potentiodynamic polarization measurements
The polarization curves for API 5L X52 carbon steel in oilfield produced water without and with various doses of inhibitors are shown in Fig. 2 at 25 °C. Electrochemical parameters obtained from PDP curves namely, corrosion current density (icorr), corrosion potential (Ecorr), cathodic and anodic Tafel slopes (βa and βc), corrosion rate (CR) and inhibition effectiveness (\({\eta }_{PDP}\%\)) were estimated and recorded in Table 3. It is obvious from Table 3 that the corrosion current density considerably decreased in the existence of these investigated inhibitors compared to the blank solution, indicating that all three corrosion inhibitors retarded the corrosion of API 5L X52 carbon steel in oilfield produced water39,40.
The obtained findings in Table 3 show that the existence of inhibitors in deep oil wells formation water solution led to small changes of corrosion potential (Ecorr) less than ± 85 mV compared to the inhibitor-free solution. These findings suggest that these inhibitors behave as mixed control-type inhibitors41,42. Moreover, the anodic Tafel slopes and the cathodic Tafel slopes were approximately constant and small altered by the dose of inhibitors, which indicated that the prepared inhibitors do effect on the corrosion rate in oilfield produced water and the corrosion mechanism does not change in formation water solution after adding the studied inhibitors. Also, the inhibitor controls both the dissolution of the anodic steel and the cathodic hydrogen evolution reactions by blocking the active reaction sites on metal surface. This could be ascribed to the interaction mechanism between the lone pairs of sulfur, oxygen and nitrogen atoms present in the inhibitors and the empty d-orbital of metal surface via coordination bonds43,44,45.
The inhibition effectiveness (IE%) and surface coverage degree (ϴ) from this method was computed by the following expression46:
where \({i}_{corr}^{0}\) and \({i}_{corr}\) denote the corrosion current density for API X52 carbon steel in oil field produced water without and with inhibitors, respectively. The data in Table 3 exhibited that the inhibition effectiveness obtained using the Icorr values rise with the increasing of the inhibitor concentrations and the order of inhibition effectiveness of the inhibitors in oilfield produced water solutions follows the order of: HM-1228˃ HM-1227˃ HM-1226.
EIS studies
The corrosion inhibition of carbon steel in oilfield produced water without and with several doses of inhibitors was performed using EIS method. The electrical equivalent circuit as displayed in Fig. 3 is precisely proposed to fit the EIS measurements (by Z-View Software) consisting of the solution resistance (Rs), the charge transfer resistance(Rct), double-layer capacity (Cdl), film resistance (Rf) and film capacitance (Cf). Nyquist and bode plots for inhibitors were represented in Figs. 4 and 5, respectively. EIS curves exposed that the impedance response of metal in oilfield produced water was considerably altered after adding the inhibitor molecules. It can be shown that the inhibition efficiency (IE%) and charge transfer resistance (Rct) increase with increasing concentration of the inhibitor. The concentration of the inhibitors does not differ in the form of the EIS figures, suggesting that these inhibitors control the corrosion intereaction activity rather than alter the corrosion inhibition mechanism47,48. Moreover, the diameter of impedance improves with the doses of compounds, indicating the adsorption barrier layers of these inhibitors formed on metal in oil field produced water, preventing the dissolution of iron in oilfield produced water4,22.
The impedance parameters were recorded in Table 4. The data in Table 4 revealed that Rct values were enhanced with increasing the concentration of the investigated inhibitors, showing improvement in the inhibition performance of these compounds. The value of Cdl was determined from frequency fmax , where the imaginary component of the impedance is maximal (− Zmax) by the next Eq. 49:
The inhibition efficency (ƞ%) and surface coaverge (\(\theta\)) of the inhibitors were calculated by the following expression50:
where Rct and Rct(inh) are the values of the charge transfer resistance in the absence and presence of these inhibitors, respectively. It is evident from Table 4 that the inhibitor dose values increase the inhibition efficency and the Rct value, and decrease Cdl value, indicating that the thickness of the ptoective layer developed at the metal/solution interface increases. The tested compounds act as adsorption inhibitors because the Rct values for the sample exposed to inhibitors are consistently greater than the Rct values without the inhibitor and the Cdl values with the inhibitor are consistently lower than their respective values without the inhibitor. The defensive layer formed on carbon steel surface provides a hindrance in corrosive medium and this barrier increases the inactive surface area that declines the oxidation of the steel surface51. As a result, Cdl value was reduced upon increasing the dose of inhibitors. This can be described by replacing the water molecules with adsorption of the studied inhibitor compounds that form a protective layer on the metal surface electrode and blocks corrosion reaction sites on metal surface52,53,54.
The data showed that the Rf values improve with the increase in the doses of the inhibitors, while the (Cf) values decrease. These results confirm the establishment of a protective layer of organic inhibitors on metal surface.
The higher inhibition efficacy could be due to the existence of S, O, N atoms, and an aromatic moieties and the use of a compound that provides strong adsorption centres and increases the layer thickness10. The order of inhibition effectiveness of the inhibitors in oilfield produced water solutions follows the order of: HM-1228˃ HM-1227˃ HM-1226, which was consistent with the obtained PDP data.
Adsorption consideration
Adsorption isotherms was followed for understanding the adsorption mode of the tested organic inhibitors on metallic surfaces55. The adsorption isotherms can give good insights on the anti-corrosion performance between the carbon steel surface and the organic compounds56.
The type of adsorption was discriped by plotting, Ci/θ versus Ci for inhibitors, these curves are represented as shown in Fig. 6. The empirical data revealed that the adsorption behaviour of these inhibitors could best be described by Langmuir adsorption isotherm following the below equation:
where ϴ is the degree of surface coverage obtained from polarization data, Kads is the standard adsorption equilibrium constant and Ci is the molar inhibitor concentration.
The correlation coefficients of these curves and slope values are close to unity, indicating that the inhibition capacity of these investigated organic inhibitors is owing to the ability of these compounds to adsorp on carbon steel surface and the adsorption of these compounds obey the Langmuir adsorption isotherm57,58.
The values of Kads were determined from intercepts of the straight lines on the Ci/θ axis and also related to the standard free energy of adsorption \((\Delta G_{ads}^{^\circ } )\) as shown in the following Eq. 18,59:
where R is the universal gas constant, T is the absolute temperature (K), and the value 55.5 is the molar concentration of water in solution. The \(\Delta G_{ads}^{^\circ }\) and Kads values of organic inhibitors are recorded in Table 5. The data of Kads data reveal that the strong adsorption of these inhibitors on metal surface in oilfield produced water and the -ve of \(\Delta G_{ads}^{^\circ }\) revealed that the adosption process of inhibitors on carbon steel surface is spontaneous60. The ∆G◦ads values revealed that the adsorption of these organic inhibitors on the surface of carbon steel in oilfield produced water is a combination of physical and chemical adsorption61.
Quantum chemical calculations
DFT calculations were computed to explore the chemical and physical properties for the investigated biphenylidene-thiopyrimidine derivatives. The structures of these compounds were first geometry-optimized at ground state in aqueous phase at DFT level. The optimized structures of the studied inhibitors are given in Fig. 7. The frontier molecular orbital (FMO) theory states that the (EHOMO) and (ELUMO) are important chemical reactivity indices for investigating the reactivity of chemical species in some chemical reactions. The majority of chemical interactions especially processes where adsorption of compounds occur such as lubrication and corrosion inhibitive properties, could be explained by exploring the donor–acceptor interaction between the adsorbed molecules and frontier Molecular orbitals (FMOs) of adsorbent atoms62. The increase in the values of EHOMO is often associated with higher capacity of a molecule to donate electrons to an appropriate acceptor molecule which have vacant molecular orbitals63,64,65. In contrast, the lower the ELUMO, the more likely that the reacting compound possesses higher capacity to accept the electrons. Thus, when the value of ELUMO is lower, the molecule has higher tendency to gain electrons in particular chemical interactions. The EHOMO can be considered a measure of the ionization potential and the tendency of a species to undergo electrophilic attack. On the contrary, the energy of the LUMO is a measure of the tendency of the molecules to undergo nucleophilic attack. Hence, an enhancement in the tribological, and corrosion inhibition properties of the additives is anticipated with an increasing trend in EHOMO, as with the decreasing trend in ELUMO. This enhancement in the adsorption of inhibitors on metallic surface is associated with the formation of chemisorbed film. Furthermore, the gap between ELUMO and EHOMO i.e., (ΔE) is an important stability index that was shown to have a correlation with corrosion inhibition potentials in corrosive and tribological systems62. The larger ΔE value indicates high molecular stability in a chemical reaction. Furthermore, the ΔE has also been associated with the hardness and softness. When the energy gap (ΔE) is minimum between HOMO and LUMO orbitals of the interacting molecules, it is evident of the soft nature of compounds as ease of polarization is expected. On the other hand, when the energy gap is large, this gives rise to the hard nature of chemical compound. Therefore, the increase in EHOMO and decrease in ELUMO and ΔE is accompanied by an increase in that corrosion inhibition efficiency of compounds66. The output of quantum chemical calculations including, EHOMO, ELUMO and orbital energy gap (ΔE), dipole moment (μ) is listed in Table 6. Exploring the values of EHOMO, ELUMO and ΔE for the studied compounds, the order of inhibition can be anticipated as follows: HM-1228 > HM-1227 >HM-1226. We found that the corrosion inhibition actions of the three studied inhibitors is increasingly consistent with the decreasing order in gap energy, and ELUMO, and the higher values µ and ω which characterizes higher reactivity for electrophiles and the higher energy stabilization that occurs when the system obtains an extra electronic charge ∆N from its surroundings. Unlike the trend of some quantum parameters were found insignificantly correlated with the experimental %I.Es. The presence of di-N-ethyl groups in HM-1228 increases its electron donating ability as well as enhance lipophilic properties compared to HM-1227 (contain two N–H hydrophilic groups). It is evident that the existence of sulfur atom increases the compound capacity to donate electron through lone pair sharing. On the other side, the donating ability is less in HM-1226 than in HM-1227, due to the lower effect of oxygen atom in donating electrons.
Molecular simulations
In the present study, the interaction of biphenylidene-thiopyrimidine derivatives in aqueous media is explored by Monte Carlo simulation. This method is considered as a powerful tool to provide insights on the equilibrium position of inhibitors on the Fe surface. The most stable optimized structure of inhibitors was introduced in the simulation studies. In order to model part of the corrosive acid environment, the effect of water and corrosive species were also considered in Monte Carlo simulation. Figure 8 displays a top and side view of investigated inhibitor species adsorped on the top surface of the attacked metal in vacuum conditions during simulation process. While the output equilibrium optimized structure of inhibitor compounds on Fe (110) in aqueous media is presented in Fig. 9. It is obvious from both Figs. 8 and 9 that the position of inhibitors is close and in parallel to the metal surface. This gives rise to the adsorption of the inhibitors on corroding metal, implying a potency to form a protective layer, hence protect the metal surface by shielding the metal from the attack of aggressive ions. In tables 7 and 8, the calculated molecular simulation parameters are listed. It can be seen that the adsorption energies of the tested inhibitors are all negative values, suggesting strong interaction occurs between investigated inhibitor compounds with metal surface. The increasing order of adsorption energy of compounds in vacuum conditions was found in agreement with experimental methods HM-1228 > HM-1227 > HM-1226. MC simulations provide useful insights into the performance trend of these inhibitors on the Fe (110) surface. The Inhibitor HM-1228 was the highest in terms of corrosion inhibition due to the presence of two N-substituted –C2H5 groups on the thiopyrimidine moiety; the existence of electron donating groups is significant in increasing the corrosion inhibition of compounds.
Surface analysis (AFM)
AFM was used to investigate the surface morphology of carbon steel in contact with oilfield-produced water. AFM 3D images of API 5L X52 carbon steel specimens before and after 40 days of immersion in oilfield-produced water without and with 0.5 mM inhibitor (HM-1228) at 25 °C are depicted in Fig. 10a,b,c. AFM image Fig. 10a showed that the morphology of API 5L X52 carbon steel was a very smooth and homogeneous surface10. However, API 5L X52 carbon steel form (topography) had severe damage and a rough surface after immersion in oilfield-produced water for 40 days without inhibitor HM-1228 as shown in Fig. 10b. In contrast, the shape of API 5L X52 carbon steel was improved by introducing HM-1228 inhibitor as shown in Fig. 10c and the corrosion degree of carbon steel sample was greatly mitigated. This can be due to the formation of an inhibitive layer/coherent film on API 5L 52X from HM-1228 inhibitor molecules7,22. The AFM image revealed that the presence of tested inhibitor (HM-1228) at concentration 0.5 mM in the corrosive solution has an improvement effect on of carbon steel surface, whereas the surface was experienced severe damage when exposed to the corrosive medium without the inhibitor. This explains the maximum inhibition effectiveness of the HM-1228 inhibitor at this concentration.
Furthermore, the average surface roughness (Ra) obtained for the polished carbon steel surface was 84.35 nm, while the average surface roughness (Ra) in oilfield produced water solution decreased from 571.62 nm (uninhibited surface) to 138.28 nm by introducing 0.5 mM of HM-1228 inhibitor (Inhibited surface), respectively. These results indicated the inhibitory nature of the film formed on the surface of API 5L X52 carbon steel by HM-1228 inhibitor compounds10,67.
Mechanism of corrosion inhibition
The biphenylidene-thiopyrimidine derivatives exhibited outstanding corrosion inhibition due to the adsorption of the molecules of these derivatives on the surface of carbon steel using mutual physical and chemical adsorptions in oil wells formation water as explained in Fig. 11. Thiopyrimidine moiety, biphenyl rings, and dimethylamino group are the active centers for adsorption mechanism in the prepared inhibitors. Biphenylidene-thiopyrimidine derivatives can adsorb physically and inhibit the passage of other corrosive ions towards the internal carbon steel surface which impede the corrosion activity. Moreover, these derivatives can be adsorbed chemically on the carbon steel surface through their high electron density cloud, due to structural factors emerging from their possession of S, O, N heteroatoms, π-electrons of aromatic rings, that helped to extend the double bond conjugation throughout the whole structure causing better electron distribution and a more planar conformation on the substrate surface6. Therefore, the chemical adsorption was possible via the coordinating bonds between the lone electron pairs located on heteroatoms (N, O, S) of inhibitor molecules and the empty d-orbitals of Fe on API 5L X70 type carbon steel surface18,22. The previous discussion was consistent with the experimental values of \({\Delta G}_{ads}^{0}\). Accordingly, these derivatives can form a protective adsorbed layer onto the surface of carbon steel via physical and chemical reactions, isolating the carbon steel from further dissolution and corrosion process.
Conclusions
In conclusion, the research paper presents the synthesis, characterization, and investigation of three novel biphenylidene-thiopyrimidine derivatives as corrosion inhibitors for carbon steel in oilfield produced water. The results demonstrate that these inhibitors exhibit excellent inhibition efficiency, with HM-1228 being the most effective followed by HM-1227 and HM-1226. The polarization curves indicate a decrease in corrosion current density with increasing inhibitor doses, suggesting that these compounds act as mixed type inhibitors. EIS data further support this finding, showing a decline in Cdl values and an increase in both Rct and IE% compared to the blank solution. The adsorption of these inhibitors on the carbon steel surface follows Langmuir adsorption isotherm. Additionally, DFT calculation and MC simulations provide insights into the adsorption sites in the inhibitor's molecules, which align well with experimental observations. AFM surface analysis reveals that the inhibitor molecules form a protective layer on the carbon steel surface, effectively insulating it from destructive media. Overall, this research highlights the potential of these biphenylidene-thiopyrimidine derivatives as effective corrosion inhibitors for carbon steel in oilfield produced water Supplemantary Figures S1–S3.
Data availability
The published article and its supplementary information files contain all the data generated or analyzed in this study.
References
Jafari, Y., Ghoreishi, S. M. & Shabani-Nooshabadi, M. Electrosynthesis, characterization and corrosion inhibition study of dbsa-doped polyaniline coating on 310 stainless steel. Iran. J. Chem. Chem. Eng. 36, 23–32 (2017).
Shabani-Nooshabadi, M. & Kazemi-Darafshani, M. Root and shoot extracts of Ajuga chamaecistus subsp. scoparia as natural inhibitors for 304 stainless steel corrosion in strong acidic medium. Surf. Eng. Appl. Electrochem. 53, 560–569 (2017).
Zhu, S. D. et al. Corrosion of N80 carbon steel in oil field formation water containing CO2 in the absence and presence of acetic acid. Corros. Sci. 53, 3156–3165 (2011).
Migahed, M. A., Shaban, M. M., Fadda, A. A., Ali, T. A. & Negm, N. A. Synthesis of some quaternary ammonium gemini surfactants and evaluation of their performance as corrosion inhibitors for carbon steel in oil well formation water containing sulfide ions. RSC Adv. 5, 104480–104492 (2015).
Zhang, G. A. & Cheng, Y. F. Electrochemical characterization and computational fluid dynamics simulation of flow-accelerated corrosion of X65 steel in a CO2-saturated oilfield formation water. Corros. Sci. 52, 2716–2724 (2010).
Shaban, M. M. et al. Anti-corrosion, antiscalant and anti-microbial performance of some synthesized trimeric cationic imidazolium salts in oilfield applications. J. Mol. Liq. 351, 118610 (2022).
Zhang, Q. H. et al. Dextran derivatives as highly efficient green corrosion inhibitors for carbon steel in CO2-saturated oilfield produced water: Experimental and theoretical approaches. Chem. Eng. J. 424, 130519 (2021).
Farhadian, A. et al. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci. 175, 108871 (2020).
Yassin, A. Y., Abdelghany, A. M., Shaban, M. M. & Abdallah, Y. M. Synthesis, characterization and electrochemical behavior for API 5L X70 carbon steel in 5% sulfamic acid medium using PVVH/PEMA blend filled with gold nanoparticles. Coll. Surf. A Physicochem. Eng. Asp. 635, 128115 (2022).
Zhang, Q. H. et al. Two amino acid derivatives as high efficient green inhibitors for the corrosion of carbon steel in CO2-saturated formation water. Corros. Sci. 189, 109596 (2021).
Zheng, Z. et al. Mercaptopropionic acid-modified oleic imidazoline as a highly efficient corrosion inhibitor for carbon steel in CO2-saturated formation water. Corros. Sci. 194, 109930 (2022).
Ismail, M. A. et al. Novel cationic aryl bithiophene/terthiophene derivatives as corrosion inhibitors by chemical, electrochemical and surface investigations. Sci. Rep. 12, 3192 (2022).
Migahed, M. A. et al. Synthesis of a new family of Schiff base nonionic surfactants and evaluation of their corrosion inhibition effect on X-65 type tubing steel in deep oil wells formation water. Mater. Chem. Phys. 125, 125–135 (2011).
Chauhan, D. S., Mazumder, M. A. J., Quraishi, M. A., Ansari, K. R. & Suleiman, R. K. Microwave-assisted synthesis of a new piperonal-chitosan schiff base as a bio-inspired corrosion inhibitor for oil-well acidizing. Int. J. Biol. Macromol. 158, 231–243 (2020).
Farag, A. A., Eid, A. M., Shaban, M. M., Mohamed, E. A. & Raju, G. Integrated modeling, surface, electrochemical, and biocidal investigations of novel benzothiazoles as corrosion inhibitors for shale formation well stimulation. J. Mol. Liq. 336, 116315 (2021).
Usman, B. J., Gasem, Z. M., Umoren, S. A. & Solomon, M. M. Eco-friendly 2-Thiobarbituric acid as a corrosion inhibitor for API 5L X60 steel in simulated sweet oilfield environment: Electrochemical and surface analysis studies. Sci. Rep. 9, 830 (2019).
Sasaki, S., Niko, Y., Klymchenko, A. S. & Konishi, G. Design of donor–acceptor geometry for tuning excited-state polarization: Fluorescence solvatochromism of push–pull biphenyls with various torsional restrictions on their aryl–aryl bonds. Tetrahedron 70, 7551–7559 (2014).
Shaban, M. M. et al. Novel trimeric cationic pyrdinium surfactants as bi-functional corrosion inhibitors and antiscalants for API 5L X70 carbon steel against oilfield formation water. J. Mol. Liq. 305, 112817 (2020).
Hegazy, M. A., Badawi, A. M., Abd El Rehim, S. S. & Kamel, W. M. Corrosion inhibition of carbon steel using novel N-(2-(2-mercaptoacetoxy) ethyl)-N, N-dimethyl dodecan-1-aminium bromide during acid pickling. Corros. Sci. 69, 110–122 (2013).
Negm, N. A. et al. High performance corrosion inhibition of novel tricationic surfactants on carbon steel in formation water: Electrochemical and computational evaluations. J. Mol. Liq. 262, 363–375 (2018).
Melian, R. et al. Anticorrosion properties of 5, 5′-dithiobis-(2-nitrobenzoic acid) and sodium sulfite compounds for aluminum alloy 2024–T3 in saline solution: Electrochemical, characterization and theoretical investigations. J. Mol. Liq. 331, 115661 (2021).
El-Monem, A., Shaban, M., Migahed, M. A. & Khalil, M. M. Synthesis, characterization, and computational chemical study of aliphatic tricationic surfactants as corrosion inhibitors for metallic equipment in oil fields. ACS Omega 5(41), 26626–26639 (2020).
Frisch, M. J. et al. Gaussian 09, revision A02 (Gaussian Inc, 2009).
Frisch, M. J. et al. Gaussian. Inc., Wallingford CT at (2009).
Becke, A. D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 96(3), 2155–2160 (1992).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. N. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998).
Parr, R. G. & Pearson, R. G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Liu, S.-B. Conceptual density functional theory and some recent developments. Acta Phys. Chim. Sin. 25, 590–600 (2009).
Frau, J. & Glossman-Mitnik, D. Conceptual DFT descriptors of amino acids with potential corrosion inhibition properties calculated with the latest minnesota density functionals. Front. Chem. 5, 16 (2017).
Bellafont, N. P., Illas, F. & Bagus, P. S. Validation of Koopmans’ theorem for density functional theory binding energies. Phys. Chem. Chem. Phys. 17, 4015–4019 (2015).
Parr, R. G., Szentpaly, L. & Liu, S. Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924 (1999).
Shahraki, M., Dehdab, M. & Elmi, S. Theoretical studies on the corrosion inhibition performance of three amine derivatives on carbon steel: Molecular dynamics simulation and density functional theory approaches. J. Taiwan Inst. Chem. Eng. 62, 313–321 (2016).
ElBelghiti, M. et al. Experimental, quantum chemical and Monte Carlo simulation studies of 3, 5-disubstituted-4-amino-1, 2, 4-triazoles as corrosion inhibitors on mild steel in acidic medium. J. Mol. Liq. 218, 281–293 (2016).
Sun, H., Ren, P. & Fried, J. R. The compass force field: Parameterization and validation for phosphazenes. Comput. Theor. Polym. Sci. 8, 229–246 (1998).
Bunte, S. W. & Sun, H. Molecular modeling of energetic materials: the parameterization and validation of nitrate esters in the COMPASS force field. J. Phys. Chem. B 104, 2477–2489 (2000).
Singh, A. et al. Corrosion inhibition performance of imidazolidine derivatives for J55 pipeline steel in acidic oilfield formation water: Electrochemical, surface and theoretical studies. J. Taiwan Inst. Chem. Eng. 95, 341–356 (2019).
Berdimurodov, E. et al. Novel glycoluril pharmaceutically active compound as a green corrosion inhibitor for the oil and gas industry. J. Electroanal. Chem. 907, 116055 (2022).
Shivakumar, S. S. & Mohana, K. N. Corrosion behavior and adsorption thermodynamics of some Schiff bases on mild steel corrosion in industrial water medium. Int. J. Corros. 2013, 45698523 (2013).
Hussien, B. M., Migahed, M. A., Shaban, M. M., Negm, N. A. & Moawad, Z. Synthesis and inhibition performance of diquaternary ammonium gemini surfactants on carbon steel pipelines corrosion in gas field. In Offshore Mediterranean Conference and Exhibition (OnePetro, 2017).
Umoren, S. A. Polypropylene glycol: A novel corrosion inhibitor for× 60 pipeline steel in 15% HCl solution. J. Mol. Liq. 219, 946–958 (2016).
Obot, I. B., Obi-Egbedi, N. O. & Eseola, A. O. Anticorrosion potential of 2-mesityl-1H-imidazo [4, 5-f][1, 10] phenanthroline on mild steel in sulfuric acid solution: experimental and theoretical study. Ind. Eng. Chem. Res. 50, 2098–2110 (2011).
Li, X., Deng, S., Lin, T., Xie, X. & Du, G. 2-Mercaptopyrimidine as an effective inhibitor for the corrosion of cold rolled steel in HNO3 solution. Corros. Sci. 118, 202–216 (2017).
Hussien, B. M., Al-Sabagh, A. M., Migahed, M. A., Shaban, M. M. & Moawad, Z. Corrosion control of X-60 type carbon steel in petroleum formation water under high pressure of CO2 at high temperature. In Offshore Mediterranean Conference and Exhibition (OnePetro, 2017).
Mobin, M., Aslam, R., Salim, R. & Kaya, S. An investigation on the synthesis, characterization and anti-corrosion properties of choline based ionic liquids as novel and environmentally friendly inhibitors for mild steel corrosion in 5% HCl. J. Colloid Interface Sci. 620, 293–312 (2022).
Solomon, M. M., Umoren, S. A., Quraishi, M. A. & Salman, M. Myristic acid based imidazoline derivative as effective corrosion inhibitor for steel in 15% HCl medium. J. Colloid Interface Sci. 551, 47–60 (2019).
Boukazoula, S., Haffar, D., Bourzami, R., Toukal, L. & Dorcet, V. Synthesis, characterizations, crystal structure, inhibition effects and theoretical study of novel Schiff base on the corrosion of carbon steel in 1 M HCl. J. Mol. Struct. 1261, 132852 (2022).
Murthy, R., Gupta, P. & Sundaresan, C. N. Theoretical and electrochemical evaluation of 2-thioureidobenzheteroazoles as potent corrosion inhibitors for mild steel in 2 M HCl solution. J. Mol. Liq. 319, 114081 (2020).
Migahed, M. A., Zaki, E. G. & Shaban, M. M. Corrosion control in the tubing steel of oil wells during matrix acidizing operations. RSC Adv. 6, 71384–71396 (2016).
Rezaeivala, M., Karimi, S., Tuzun, B. & Sayin, K. Anti-corrosion behavior of 2-((3-(2-morpholino ethylamino)-N3-((pyridine-2-yl) methyl) propylimino) methyl) pyridine and its reduced form on carbon steel in hydrochloric acid solution: Experimental and theoretical studies. Thin Solid Films 741, 139036 (2022).
Benahmed, M., Selatnia, I., Djeddi, N., Akkal, S. & Laouer, H. Adsorption and corrosion inhibition properties of butanolic extract of Elaeoselinum thapsioides and its synergistic effect with Reutera lutea (Desf.) Maires (Apiaceae) on A283 carbon steel in hydrochloric acid solution. Chem. Africa 3, 251–261 (2020).
Khamaysa, O. M. A. et al. Hydrazone-based green corrosion inhibitors for API grade carbon steel in HCl: Insights from electrochemical, XPS, and computational studies. Colloids Surf. A Physicochem. Eng. Asp. 626, 127047 (2021).
El Faydy, M. et al. Experimental investigation on the corrosion inhibition of carbon steel by 5-(chloromethyl)-8-quinolinol hydrochloride in hydrochloric acid solution. J. Mol. Liq. 219, 396–404 (2016).
Li, M. et al. Inhibition performances of imidazole derivatives with increasing fluorine atom contents in anions against carbon steel corrosion in 1 M HCl. J. Mol. Liq. 322, 114535 (2021).
Satpati, S. et al. Amino acid and cinnamaldehyde conjugated Schiff bases as proficient corrosion inhibitors for mild steel in 1 M HCl at higher temperature and prolonged exposure: Detailed electrochemical, adsorption and theoretical study. J. Mol. Liq. 324, 115077 (2021).
Parthipan, P., Cheng, L. & Rajasekar, A. Glycyrrhiza glabra extract as an eco-friendly inhibitor for microbiologically influenced corrosion of API 5LX carbon steel in oil well produced water environments. J. Mol. Liq. 333, 115952 (2021).
Mashuga, M. E., Olasunkanmi, L. O., Lgaz, H., Sherif, E.-S.M. & Ebenso, E. E. Aminomethylpyridazine isomers as corrosion inhibitors for mild steel in 1 M HCl: Electrochemical, DFT and Monte Carlo simulation studies. J. Mol. Liq. 344, 117882 (2021).
Zhang, X. et al. Bis-Mannich bases as effective corrosion inhibitors for N80 steel in 15% HCl medium. J. Mol. Liq. 347, 117957 (2022).
Moschona, A. et al. Homologous alkyl side-chain diphosphonate inhibitors for the corrosion protection of carbon steels. Chem. Eng. J. 405, 126864 (2021).
Galai, M. et al. Chemically functionalized of 8-hydroxyquinoline derivatives as efficient corrosion inhibition for steel in 1.0 M HCl solution: Experimental and theoretical studies. Surf. Interfaces 21, 100695 (2020).
Ouakki, M. et al. Electrochemical, thermodynamic and theoretical studies of some imidazole derivatives compounds as acid corrosion inhibitors for mild steel. J. Mol. Liq. 319, 114063 (2020).
Fouda, A.E.-A.S., Abd El-Maksoud, S. A., El-Sayed, E. H., Elbaz, H. A. & Abousalem, A. S. Effectiveness of some novel heterocyclic compounds as corrosion inhibitors for carbon steel in 1 M HCl using practical and theoretical methods. RSC Adv. 11, 19294–19309 (2021).
Rbaa, M. et al. Sample synthesis, characterization, experimental and theoretical study of the inhibitory power of new 8-hydroxyquinoline derivatives for mild steel in 1.0 M HCl. J. Mol. Struct. 1213, 128155 (2020).
Fang, J. & Li, J. Quantum chemistry study on the relationship between molecular structure and corrosion inhibition efficiency of amides. J. Mol. Struct. Theochem 593, 179–185 (2002).
Sarkar, T. K., Yadav, M. & Obot, I. B. Mechanistic evaluation of adsorption and corrosion inhibition capabilities of novel indoline compounds for oil well/tubing steel in 15% HCl. Chem. Eng. J. 431, 133481 (2022).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.A.I. provided the concept, supervised the preparation of the new compounds, H.A.A conducted the experiments related to the preparation, and characterization of new compounds, and M.M.S did the electrochemical experiments, calculations, and drafting of the electrochemical study part. A.S.F supervise and review the electrochemical part. A.S.A reviewed the theoretical studies. E.A.G supervised experiments, characterization of the new compounds. M.A.I. and A.S.A wrote the paper. All authors reviewed the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, H.A., Shaban, M.M., Abousalem, A.S. et al. Novel biphenylidene-thiopyrimidine derivatives as corrosion inhibitors for carbon-steel in oilfield produced water. Sci Rep 13, 16388 (2023). https://doi.org/10.1038/s41598-023-43312-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43312-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.