Abstract
One in six Americans suffers from hearing loss. While treatment with amplification is possible for many, the acceptance rate of hearing aids is low. Poor device fitting is one of the reasons. The hearing aid fitting starts with a detailed hearing assessment by a trained audiologist in a sound-controlled environment, using standard equipment. The hearing aid is adjusted step-by-step, following well-described procedures based on the audiogram. However, for many patients in rural settings, considerable travel time to a hearing center discourages them from receiving a hearing test and treatment. We hypothesize that hearing assessment with the patient’s hearing aid can reliably substitute the hearing test in the clinic. Over-the-counter hearing aids could be programmed from a distance and fine-tuned by the hearing aid wearer. This study shows that a patient-controlled hearing assessment via a hearing aid in a non-clinical setting is not statistically different from an audiologist-controlled hearing assessment in a clinical setting. The differences in hearing obtained with our device and the Gaussian Process are within 3 dB of the standard audiogram. At 250 Hz, the sound delivery with the hearing aid used in this study added an additional reduction of sound level, which was not compensated.
Similar content being viewed by others
Introduction
Hearing loss is the third most common chronic medical condition in the United States, affecting over 30 million Americans, including half of the adults between 70 and 79 years old1,2,3. Estimates show that less than 20% of these individuals wear hearing aids4,5,6. Several factors lead to the high incidence of untreated hearing loss, including the high cost of devices and limited access to professional services7, 8. Previous research has shown that untreated hearing loss correlates with accelerated cognitive decline, resulting in dementia, anxiety, and depression9,10,11,12,13,14,15,16. To better treat deaf or hard-of-hearing individuals, the government has removed some regulations for distributing hearing aids17. This regulatory change will ease patient access to hearing aids. However, it will also create challenges for hearing aid providers because the devices should be programmable outside of a clinical setting, at the patients’ homes, for example, through telemedicine. Enabling hearing aid access may also increase the patients’ acceptance and use of the devices. The proposed changes for over-the-counter hearing aids also require changing the standard processes for self-fitting16, 18. This clinical study is an effort to provide a method for patients to adjust their hearing aids outside of a clinical setting using their own hearing aids. Virtually all modern hearing aids are fitted using the patient’s audiogram, determined by pure tone audiometry in a controlled clinical setting, by trained personnel, using instruments specifically designed for this purpose19,20,21,22. The audiologist determines the patient’s hearing by incrementally approximating the lowest intensity for a pure tone that can still be perceived at a set of standard frequencies ranging from 125 Hz to 8 kHz23. At each frequency, a staircase “up 5 dB—down 10 dB” approach is used24. It takes an audiologist 10–15 min to complete an audiogram. Hearing aid amplification settings can be assigned using well-known prescriptive standards such as National Acoustic Laboratories (NAL), Desired Sensation Level (DSL), or others using the patient's audiogram25, 26. Despite pure-tone audiometry is an indispensable component of the hearing aid fitting process, eminent limitations of standard audiometry exist:
-
1)
A trained professional must administer a hearing test with specialized equipment, including a sound booth.
-
2)
A statistical estimate of confidence in the result is generally not available with standard audiometry.
-
3)
Standard test procedures do not use all information available in the data by assuming that the hearing thresholds at different frequencies are uncorrelated, leading to redundant measurements to achieve the desired accuracy and longer testing times.
-
4)
Restricting the stimuli to a fixed set of standard frequencies requires additional assumptions to estimate the threshold at other frequencies, particularly when fitting hearing aids with a large number of channels.
We test a novel approach with our clinical study, addressing the above limitations. The new procedure combines in-situ pure-tone audiometry with Bayesian statistical inference. With this, we name “In-situ hearing testing” as the use of hearing aids to administer a hearing test rather than specialized audiometry equipment. This approach uses a Gaussian Process Classification27, 28 to obtain continuous pure-tone threshold curves in less time than a traditional audiogram. The Gaussian Process Classification also provides a measure of confidence in its threshold estimates and requires only the hearing aids and a connected smartphone to conduct. In recent years, in-situ audiometry and Bayesian hearing tests have been separately compared to conventional pure-tone threshold testing, producing results comparable to a standard audiogram28,29,30,31,32,33. However, the threshold was tested using the same standard equipment in the clinic to assess hearing. A combined approach that uses the person’s hearing aids and probabilistic models to optimize in-situ hearing tests has not yet been explored.
Note that the audiogram is the starting point for the fitting process, and the need for alignment between the audiogram obtained by the audiologist or obtained by the self-fitting procedure is primarily to satisfy the need for documentation. Following this logic, no audiogram would be required for the fitting process. The fitting could start with an arbitrary audiogram, aligning somewhat with the patient's hearing ability. Using such an approach, the fitting will likely take more iterations. We suggest that a good alignment of the results from the self-fit will optimize the fitting procedure.
Results
Test subjects
Patients who had an appointment with the hearing clinic for a hearing assessment and had no severe-to-profound hearing loss were invited to participate in the study. Driven by patient availability, we did not control gender, ethnicity, and race. Among the participants, 37 (70%) were females, and 16 (30%) were males (Table 1). The age of the participants was similar for both genders. For females and males combined, the study participants’ age ranged from 24 to 81 years, with a median of 54 years, an average age of 51.3 years, a standard deviation of 16.6 years, and a 95% confidence interval between 46.9 and 55.8 years (Table 1). The female age ranged from 24 to 81 years, with a median of 54 years, an average age of 53.3 years with a standard deviation of 17.9 years, and a 95% confidence interval between 47.5 and 59.1 years (Table 1). The age of the males ranged from 25 to 81 years, with a median of 55 years, an average age of 53.9 years with a standard deviation of 17.9 years, and a 95% confidence interval between 45.1 and 62.7 years (Table 1). Using the t-test provided with the IGOR Pro8 statistical package, age differences between females and males, mean-agefemales ! = mean-agemales, were shown not to be significantly different (alpha = 0.05, the critical value t_stat = 1.5, nfemales = 37, nmales = 16, DFfemales = 36, DFmales = 15, P = 0.132).
Audiograms
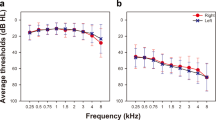
Figure 1 shows the standard audiogram (method M1), plotted along with the behavioral hearing threshold obtained “in-situ” with a hearing aid and the Gaussian Process classifier (method M2). While the standard audiogram collected values at 11 distinct frequencies: 125, 250, 500, 750, 1000, 1500, 2000, 3000, 4000, 6000, and 8000 Hz (Fig. 1a and b), hearing assessment with the Gaussian Process classifier provided data at many more frequencies. To compare the audiograms captured with the two methods, corresponding points to the standard audiogram are selected from the audiogram obtained with M2 (Fig. 1). The audiograms are shown in Fig. 1a to j for the left and right sides, respectively. The differences between the two audiograms obtained with M1 and M2 are shown in Figs. 1e,f,k and l. Data is shown for two subjects, one with normal hearing and the other with apparent high-frequency hearing loss.
The figure shows audiograms obtained from two study participants, one with normal hearing (panel (a) through (f)) and one with hearing loss (panel (h) through (l)). The traces in the top row with the “x” markers show the behavioral hearing thresholds at discrete frequencies, at which hearing was assessed during standard audiometry by a certified audiologist in a sound-reduced chamber with standard equipment. The green traces in the top row with the “o” markers show pure tone thresholds of the same patient obtained with a self-guided in-situ testing procedure using hearing aids in a sound-reduced environment. The second row shows the thresholds for the different methods at the same frequencies. The third row shows the difference between the methods. Both methods provided similar results for the hearing thresholds of the patients. The broken red line indicates the zero line or normal hearing.
The cumulative results for all study participants are shown in Table 2 and Fig. 2.
The panels show plots of all audiograms captured with M1 and M2 in a study participant's left and right ear. In panels (a–c), the results for M1 at the standard frequencies are shown. Panels (d–f) show the corresponding results obtained with M2. Panels (g–i) provide the difference between the results obtained with M1 and M2. The green lines are the individual difference; the red circles show the average ± one standard deviation. The broken red line indicates the zero line.
In Fig. 2, the left column shows the results from the left ears, the middle columns from the right ears, and the right column is the cumulative data from the left and right ears. The top row shows the standard audiograms from the study’s participants, with normal hearing to mild hearing loss. The second row shows the corresponding audiograms obtained with M2. The third row shows the differences for each audiogram determined by the two methods in green. The circles show the averages, and error bars indicate the standard deviation.
The average difference ± one standard deviation between the M1 and M2 threshold estimate across all subjects and frequencies was 3.8 ± 7.2 (N = 35) on the right and 2.8 ± 7.9 dB (N = 35) on the left side. They are close to zero at many frequencies, but lower frequencies tend to deviate more. It should be noted that while audiogram values collected from M1 could take on values below 10 dB HL, for statistical analysis, M1 values were limited to 10 dB HL to match the dynamic range of M2.
The distribution of the differences in the pure tone averages is shown in Fig. 3. The plot is a cumulative plot showing that 80% of the data obtained are within a range of ± 10 dB.
Statistical evaluation
Testing the pure tone threshold average differences for a mean equal to zero
The difference between the pure tone threshold averages (PTAs), obtained with M1 and M2, are normally distributed with an unknown mean and variance. The sample data was tested for matching a normal distribution using the Lilliefors test. The test is available in MATLAB, h = lillietest(x), where ‘h’ is the test decision for the hypothesis that the data ‘x’ come from a normal distribution with an unknown mean and variance (0 = true; 1 = false), at the 5% significance level. For our data, the test was h = 0 for the data from the right or left side. The t-test for a mean equal to zero and alpha = 0.05 in MATLAB, [h,p,ci,stats] = ttest(x), has the following results on the right side: h = 0, the probability p = 0.41; the confidence interval ci = (− 0.82, 6.5), and the test statistics, ‘stats’, produced a value for the statistics tstat = 2.12, with the degree of freedom df = 34, and an estimated population standard deviation sd = 7.9. The returned value h = 0 indicates that the t-test accepts the null hypothesis that the sample data comes from a population with a mean equal to zero at the 1% significance level.
For the left side, the values are: h = 1, the probability p = 0.004; the confidence interval ci = (0.49, 7.1), and the test statistics, ‘stats’, produced a value for the statistics tstat = 3.13, with the degree of freedom df = 34, and an estimated population standard deviation sd = 7.17. The returned value h = 1 indicates that the t-test rejects the null hypothesis that the sample data comes from a population with a mean equal to zero at the 1% significance level.
Comparing the audiograms with a multivariate analysis of variance (MANOVA)
Pure tone thresholds at ten different frequencies define the audiogram. Changes in the audiograms then show deviations from normal hearing. A multivariate version of the analysis of variance is therefore essential to determine whether the entire set of means of thresholds is different from one group to the next. Dependencies can be seen in the grouped plot matrix (Supplemental Figs. 1 and 2) generated with the MATLAB command gplotmatrix(x,[],group); ‘x’ denotes the groups, and ‘group’ the grouping variable. The MANOVA was performed with the MATLAB command [d,p,stats] = manova1(x,group); ‘x’ denotes the groups, ‘group’ the grouping variable, ‘d’ is an estimate of the dimension of the space containing the group means, ‘p’ is a vector of p-values testing whether the means lie in a space of dimension 0, 1, and so on. The command also returns ‘stats’, a structure with additional MANOVA results. The largest possible dimension is either the dimension of the space or one less than the number of groups. If the i-th p-value is near zero, it indicates that the group means do not lie on a space of i-1 dimensions. The choice of a critical p-value is specified by the value of the input argument alpha (0.05 or 0.01). For our data, p = 2.26*10–6 for the data from the left and p = 3.0*10–12 for the right side; the estimated dimension is 1 for each side, indicating that the data align along a line and are statistically different.
Credible interval analysis
A credible interval analysis was performed where the interval of interest was defined as the average difference at each frequency + /- 10 dB. The proportion of the probability distribution within the interval, assuming normality, was computed by integrating the posterior probability estimate between the interval values. This provided an estimate for the probability of future samples expected to be within 10 dB HL of the audiogram frequency.
No assumptions were made about the distribution over different values, so an uninformative prior distribution was used. Supplemental Fig. 3 shows an example of a credible interval being calculated. Supplemental Table 1 outlines the credible intervals for 10 different frequency values 250, 500, 750, 1000, 1500, 2000, 3000, 4000, 6000, and 8000 Hz.
Power calculations
Sample size and data sets not included in the analysis
The target enrollment was 55 participants. Between 2018 and 06-26 and 2018-10-25, 55 test participants/patients were enrolled. The standard audiograms for three subjects obtained with M1 were not available at the analysis time. Fourteen results using M2 were not available and could not be analyzed at the analysis time. A total of 17 data sets were excluded from the data analysis for these reasons. Three data sets acquired with M2 could not be used because the results suggested a significant error in the test procedure. For data analysis, we considered 35, with the power of the study being approximately 90%.
Discussion
With the advent of over the counter hearing aids, and the aim to better serve patients needing amplification devices, it becomes necessary to fit those devices to their needs18, 34,35,36,37,38,39,40. This study validates the Gaussian Process classification as a robust procedure for assessing hearing with a hearing aid for in-situ fitting. The hearing threshold curves collected from M1 and M2 for all analyzed subjects divided between left and right ears showed a conventional pattern. Most subjects with hearing loss displayed sloping high-frequency hearing loss. It is visible in these curves that M2 may provide higher threshold estimates in the lower frequencies, which could be related to the fact that M2 was conducted outside of a sound booth. Note that no correction has been implemented to compensate for potential reductions in sound pressure levels originating from the transducer in the hearing aid at frequencies below 500 Hz. The calibration was accepted as provided by the hearing manufacturer, Soundworld.
When computing the difference between M1 and M2 curves across all subjects, the results suggest that the experimental in-situ procedure is similar to the standard audiometric method, where the average difference was 3.8 ± 7.2 and 2.8 ± 7.9 dB on the right and left side, respectively. These numbers represent an average across the ten frequencies where a difference value was obtained for each subject. When looking at the difference values separated across frequency, the average differences were minor at most frequencies, although deviations occurred more frequently at frequencies below 500 Hz and above 6 kHz. It is suggested that the discrepancy in the lower frequencies may be due to the difference in the testing environment between M1 and M2. It can be argued that 3.8 ± 7.2 on the right side and 2.8 ± 7.9 dB on the left side are small amounts considering test–retest variability for a traditional audiogram can be higher than this value41. Our data are not as good as published results on the difference between automated and manual audiometry. Swanepoel et al.42 showed that hearing thresholds obtained with automated and manual audiometry were within 5 dB or less in 87% of the normal hearing and 97% in the hearing-impaired group. Their largest overall average absolute difference across frequencies was 3.6–3.9 dB for the normal-hearing group and 3.3–2.4 for the hearing-impaired group. We must emphasize that the differences in outcomes likely originate from the methods; while our study is an in-situ study and compares audiograms obtained in the booth with standard equipment and the subjects hearing aids outside a sound-reduced hearing enclosure, Swanepoel compared the audiologist driven and the self-fitting with the same equipment bot acquired in a sound reduced chamber.
With the assumption that the audiogram is the starting point for the fitting process, any starting point could be selected, and the alignment of the audiogram, which was obtained by a certified audiologist or a self-fitting procedure, is of secondary nature. The answer we will not be able to provide from our results is how well and how fast the fitting procedure will converge and whether differences in the quality of the fits will exist. Further tests are required to address the question. The audiogram is necessary for the documentation of hearing loss for insurance purposes. It is not clear whether an audiogram obtained through a self-fitting procedure that differs by more than 10 dB from the audiogram of the same subject obtained by an audiologist must be considered as not aligned.
The returned value h = 1 from the results indicates that the t-test rejects the null hypothesis that the sample data comes from a population with a mean equal to zero at the 5% significance level. Using the average difference (3.8 ± 7.2 dB on the right side and 2.8 ± 7.9 dB on the left side) as calibration, the results of M2 can be shifted by this amount as a calibration factor. The t-test of the adjusted data shows that h = 0, indicating that the data comes from a population with a mean equal to zero at the 5% significance level. In other words, with a modest scalar calibration, the sample data representing the difference between M1 and M2 can be said to come from a population with a mean equal to zero, indicating that the M1 and M2 are similar.
The output d estimates the dimension of the group means. d = 1 indicates that the means differ but fall along a line. The two most-left columns and the top two rows of SFigures 1 and 2, corresponding to 250 and 500 Hz, show differences. These data indicate that the lower frequency differences, compared to the control responses in the standard audiometric method, appear more pronounced, confirming what the difference curves in Fig. 2g–i suggest.
The credible interval analysis assumed an interval between + /− 10 dB when estimating the probability distribution of the difference values between M1 and M2 using an uninformative prior. This analysis suggests that most subjects’ M2 measurements are within 10 dB of the M1 measurement.
Previous studies suggested that hearing thresholds obtained in-situ with hearing aids are equivalent to currently accepted audiometric procedures43,44,45. Our results agree with those studies. DiGiovanni and Pratt reported significant differences between hearing thresholds obtained with standard and in-situ procedures38. It is not clear whether the differences of 6–9 dB at 500, 1000, and 2000 Hz are caused by differences in sound level produced by the transducers.
This study is not the first report on combining probabilistic modeling and information gain. Özdamar et al.46 published their results on classifying audiograms by sequential testing using a dynamic Bayesian approach. The procedure included the clinician’s a priori knowledge to efficiently select the next intensity and frequency. Selections were based on a probability table and the results from previous trials. More recent work has shown that a probabilistic model of the response-generating process can provide this information. Hearing threshold estimation becomes a Bayesian posterior inference using the probabilistic modeling approach. Intensity and frequency are sequentially selected by maximizing the information gained during each iteration46, in particular using Gaussian Process models28, 33, 47,48,49,50,51,52,53.
In his 1998 paper, Neal strongly advocated using the Gaussian Process for regression analysis and classification54. He argued that the Gaussian Process is the most straightforward method of defining Bayesian regression and classification models. The strengths of the models include the variety of possible covariance functions to select from. For audiometry, the most favorable kernel is the squared-exponential covariance kernel over linear or periodic kernels because it best represents the data sets. Other strengths of the Gaussian Process relate to the efficiency in data sampling55, the fact that the Gaussian Process provides a posterior distribution of the model’s belief of the underlying function value for a given test point. The model is a two-dimensional binary classifier in the frequency and by a psychometric function in the intensity dimension, which provides uncertainty bands on the resulting threshold estimate. The model’s output provides objective stopping criteria and allows for optimizing the next intensity and frequency selection, which is based on the previous data collected.
Conclusions
The present study measured the similarity between the hearing threshold estimates provided by a standard audiogram procedure versus an experimental in-situ hearing threshold assessment. The average difference between the threshold estimates provided by these two methods was 3.8 ± 7.2 on the right side and 2.8 ± 7.9 dB on the left side. For the fitting procedure, the method provides a reasonable starting point for the actual gain finally preferred and used by the patient. The in-situ method has advantages because the systems used for testing are the same as those for delivery.
Summary of Experimental In-Situ Procedure:
-
1)
It is designed to be self-administered only using wireless-enabled hearing aids and a smartphone.
-
2)
It uses a probabilistic model to estimate the uncertainty in the estimate, which may be used to determine if a test is valid or should be retaken.
-
3)
It uses probability theory to optimize which tones are presented to the subject using prior response information.
-
4)
Output is a smooth, continuous curve representing the threshold estimate for each as a function of frequency.
Materials and methods
Ethics declaration
All experimental procedures with human subjects followed the institutional research committee's ethical standards and the 1964 Helsinki Declaration and its later amendments. The Institutional Review Boards at Northwestern University Feinberg School of Medicine reviewed and approved all procedures (STU00205797). The subjects gave informed consent before participating in this study.
Summary description
This study collected hearing threshold curves from the left and right ears in volunteers using two methods for each subject. Method 1 (M1) was a standard pure tone audiogram, which measured the subject’s hearing sensitivity to acoustic tones at different frequencies. The assessment focused on pure-tone air conduction thresholds inside a sound booth with a certified audiologist of the hearing clinic at Northwestern Memorial Hospital (NMH). The audiologist conducted the test in a sound booth and is part of the clinical assessment for which the patient came to the clinic. Each ear was tested separately using various transducers such as headphones or inserted earphones. As it is a behavioral test, its outcome depends on the individual's response. To estimate a person's pure tone hearing threshold, the sound level of the pure tone with a given frequency was changed incrementally until the lowest level was determined that could still be perceived. A set of standard frequencies ranging from 0.125 to 8 kHz was used, and the sound level was modified before each presentation of a short tone stimulus using a staircase “up 5 dB—down 10 dB” approach24, 56. Sound levels start at soft, which is barely or non-audible. After assessing their hearing with a standard audiogram, patients who fulfilled the inclusion criteria were offered to participate in the study. The audiologist immediately connected interested patients with this study's designated investigator, as described in the IRB (STU00205797).
Method 2 (M2) estimated pure tone thresholds with a self-guided in-situ testing procedure, where wireless-enabled hearing aids (HD100, Soundworld, Oakbrook, IL, USA) connected to a mobile app on a handheld device (Apple iPhone 8 Plus smartphone) played pure tones. The mobile app contains software that can run a Gaussian Process classification algorithm to determine the pure tones and their corresponding intensities to play throughout the test. The algorithm then returns and stores a probabilistic estimate of the hearing threshold for the left and right ears. Sound levels were between 10 and 80 dB HL. After each presentation of the pure tone, the subject was asked to press a button on the screen of an iPhone, indicating on which side the tone was heard. The software then determined the subsequent frequency and sound level based on the previously recorded responses. The following tones and corresponding sound levels were sent to the hearing aid via Bluetooth, playing the pure tone at the given sound level. The results were stored locally on the iPhone. The data were erased from the iPhone after transferring the de-identified data to a secured server at Northwestern University. The hearing aid limited the maximum possible sound levels of the hearing aid. The software used here could not override the hearing aid’s safety limits.
Hearing aids
The hearing aid was the HD100 from Soundworld. Our custom-written software sets the pure tone frequencies and their corresponding sound levels via a Bluetooth connection between an Apple iPhone 8 Plus smartphone and the hearing aid during the hearing tests. The software could not override the internal safety measures of the hearing aid. The maximum level of the pure tones delivered was 80 dB hearing level (HL), where HL was referenced to sound levels in dB re 20 µPa obtained from Ref.57.
The Soundworld HD100 Hearing Aid is a printed circuit assembly powered by a rechargeable lithium-ion battery. The main processing unit of HD100 is an open − programmable DSP − based hybrid specifically designed for wireless, high − performance hearing aids. The PCB incorporates a low-power Bluetooth 4.0 radio and all necessary passive components for interfacing with transducers. The Digital Signal Processor (DSP) core processor supports wireless protocols and combines an open − programmable controller with hardware accelerators for audio coding and error correction support. A Filter Engine enables time-domain filtering and supports an ultra − low − delay audio path. The design allows for 16-channel compression, feedback cancellation, 16-channel noise reduction, and several environmental modes. Only proprietary software written and distributed by Soundworld and its affiliates can be installed on the HD100. “Source code” (both firmware and software) is solely controlled by Soundworld and is not distributed to end-users. The HD100 employs two MEMS microphones, allowing for directional detection of sound. Three pushbuttons allow to power the device on and off, modify the volume, and change the program options. The output consists of a Receiver-in-the-Canal (RIC) speaker employed in the user’s ear canal. The HD100 is housed in a weatherized, plastic enclosure and houses the tuned antenna inside.
The otoTune software platform
The otoTune app provides a user interface to connect the phone's left and right HD100 hearing aids. Once the devices are connected, another app mode can be accessed, displaying a left and right button on the smartphone’s screen to collect feedback from the subject. Mathematical code was implemented to perform the large computations necessary to compute GP classification on the mobile device’s hardware. Once the evaluation was completed, the results were saved to the smartphone's memory with any personal identifying information removed. The software also provided a mode to view a list of the collected data files, which could then be securely and wirelessly exported to a personal computer to conduct data analysis. All data were transferred to an encrypted hard drive and removed from the smartphone at the study's end.
Hearing assessment
The process to obtain a threshold estimate using GP classification can be summarized by the following steps:
-
1.
A pure tone was played through the left or right hearing aid. It was labeled “audible” or “non-audible”, depending on the subject pressing a button on the user interface, indicating that a tone was heard on the corresponding side. The 3-tuple represented each trial (frequency, sound level (dB HL), and response label (1 = heard, 0 = not heard).
-
2.
A list of pre-specified tones was played to generate an initial probabilistic estimate of the subject’s hearing threshold curve.
-
3.
The probabilistic estimate was used to select the next tone determined by which area in the space of tones and frequency the algorithm was most uncertain about.
-
4.
A tone at this most uncertain point was played, and the subject provided feedback on whether this tone was heard or not.
-
5.
The probabilistic estimate was computed again to generate a new point that the algorithm was most uncertain about, and that tone was played in the subsequent presentation.
-
6.
The process continued iteratively until additional tones only provided marginal new information based on the change in the uncertainty estimate. The model could be viewed as a binary classifier for any pair of possible tones, with a decision boundary in the frequency-intensity space interpreted as the hearing threshold curve.
The Gaussian process classification
The testing procedure used a Gaussian process classification model for each ear to estimate the probability that a tone can be heard over the space of dB HL intensity and frequency values. The Gaussian Process is outlined in Eq. (1).
where f is a function, X is the design matrix, \(\mathcal{N}\) p(f|μ,K) is (the function f has a) Gaussian distribution with mean vector μ and covariance matrix K, μ is the mean function, and K is the covariance matrix.
The GP requires selecting a covariance function or covariance kernel; a squared-exponential covariance kernel was used in this application, shown in Eq. (2).
where κ (xi, xj) is the squared-exponential covariance function (kernel) evaluated at xi, xj, σ2f is the variance of the (noise free) signal (σ determines the average distance of the function away from the mean), and l is the characteristic length scale, which determines the ripples in the function.
The standard GP model must be modified using a logistic function to perform classification to obtain a function whose output is between 0 and 1, which can be seen in Eq. (3)54.
where L is the log-likelihood, \(x\) is input, and k is a constant.
Pressing the corresponding left or right button during the tone delivery provided the user’s input. Each response was recorded and was 1 for pressing the correct button and 0 otherwise. With the patient’s prior responses, a continuous two-dimensional curve over the space of tones (dB HL x frequency) can be computed at any time during the test. The function range is a value between 0 and 1, interpreted as a probability estimate that the user can hear a tone at that intensity and frequency.
The GP also provides an uncertainty estimate for every possible probability estimate, where the uncertainty estimate curve has an identical domain as the probability estimate curve. This uncertainty estimate serves to determine the next tone and intensity. The selection is accomplished by choosing the point on the uncertainty estimate curve over the tones' space with the highest value. It is the tone that the probability model is most uncertain about and will be the tone that is played next, therefore maximizing the amount of information obtained. Once this next tone is played, its response is recorded as heard or unheard and added to the test dataset.
Another Gaussian process is then computed to obtain a new probability and uncertainty estimate curve for which a new tone will be selected. The process is repeated iteratively until approximately 35 responses per ear are collected. A final output curve for the left and right ear is obtained from the GP uncertainty curve given the complete dataset by selecting the maximum value from the uncertainty estimate curve across the entire range of frequencies. The algorithm is based on a probabilistic model, viewed as a two-dimensional binary classifier specified by a GP. The GP outputs a continuous estimate; therefore, a smooth function of frequency is obtained.
Study participants and study design
The study was a single-center study. The criteria to include the patient in the study were: (1) the study participant is 21 years or older, (2) the study participant has English language proficiency, (3) the study participant can follow written instructions in English, (4) the study participant had an audiogram in the past six months, including the day of the patient’s visit to the clinic. All study participants with or without hearing loss were eligible if they did not have a severe-to-profound hearing impairment and had none of the exclusion criteria outlined below.
The criteria to exclude the patient in the study were (1) the study participant was not able to consent, (2) for the study participant, English is a second language, (3) the study participant was legally blind (central visual acuity of 20/200 or less in the better eye with best possible correction and a visual field of 20 degrees or less, (3) the study participant has a severe-to-profound hearing loss (> 70 dB HL), (4) the study participant cannot understand the written and verbal instructions given by the study software or by the study coordinator.
The study investigator brought the patient into a quiet examination room. Informed consent documentation was provided to the participant. The study responsible investigator explained the study participant answered questions from the patient regarding the study. Written consent to participate in the research projects was sought and obtained directly from the participants. Documentation consisted of a signed statement of informed consent approved by the Institutional Review Board (IRB). Participants were informed of the tasks and time involved in the study. They were also told that their participation is strictly voluntary and that choosing not to participate would not harm their relationship with their clinical care providers. They were also informed that if they decide to participate, they can withdraw from the research project at any time, for any reason, and without penalty. Participants were given a signed copy of their consent form. A four-digit random number was assigned to each patient.
All written and recorded data were de-identified. Upon entry into the study, participants received a randomized code number, used on all material generated by the research protocols and for data presentation in scientific and professional meetings. Computers used on the project were encrypted, password-protected, and access to files required two-factor authentication.
Data analysis
The primary goal of this study was to determine if the audiogram values obtained from the standard clinical procedure were similar to those hearing thresholds collected using the self-guided in-situ test method.
The study has a cross-over design where each subject was used as their own control. M1 was used as described above and represented the control group. The experimental condition used M2. Statistical analysis included graphing the threshold curves obtained from M1 and M2 along with the difference curves, computing t-tests, running a MANOVA, and an analysis of credible intervals based on pure tone threshold differences at 250, 500, 750 1000, 1500, 2000, 3000, 4000, 6000, and 8000 Hz.
The outcome measure was the hearing sensitivity of each patient for a frequency range between 250 and 8000 Hz. The M1 procedure produced up to 11 discrete audiogram frequencies, whereas M2 produced a continuous function of frequency. Therefore, the difference between M1 and M2 were evaluated at the frequencies from M1 subtracted from M2 at those same frequencies to obtain differences.
The analysis included the following steps: (1) Graph the M1 standard audiometric data for all subjects, (2) plot the M2 experimental in-situ data for all subjects, (3) compare difference values at 250, 500, 750, 1000, 1500, 2000, 3000, 4000, 6000, and 8000 Hz for all subjects, (4) calculate the average threshold difference and corresponding standard deviations.
Since the study approach includes repeated measures (multiple frequencies for each evaluation method), a MANOVA was used. The Lilliefors test was applied to determine whether the sample data derived from the differences measured between M1 and M2 come from a distribution in the normal family. A credible interval analysis of the difference values across frequency. The power of the study was calculated.
The sample size for the study was determined before the start of the study using MATLAB-2018b, MATLAB-2019a (Mathworks, Natick, MA, USA), G*Power (RRID:SCR_013726), and IgorPro 8 (WaveMetrics Inc, Lake Oswego). After plotting and visually inspecting the plots, the actual power was calculated after the completion of the study with the same software. The a priori calculations for the sample size and power assuming a medium effect size (0.47) and an alpha of 0.05 suggested a target enrollment of 55 participants.
Data availability
All data analyzed have been shown in the paper. The raw data are available upon request from the corresponding author.
References
Goman, A. M. & Lin, F. R. Prevalence of hearing loss by severity in the United States. Am. J. Public Health 106, 1820–1822. https://doi.org/10.2105/AJPH.2016.303299 (2016).
Yamasoba, T. et al. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hear Res. 303, 30–38. https://doi.org/10.1016/j.heares.2013.01.021 (2013).
McNeal, M. Say what? The affordable care act, medicare, and hearing aids. Harvard J. Legisl. 53, 621–670 (2016).
Chien, W. & Lin, F. R. Prevalence of hearing aid use among older adults in the United States. Arch. Intern. Med. 172, 292–293. https://doi.org/10.1001/archinternmed.2011.1408 (2012).
Reed, N. S., Garcia-Morales, E. & Willink, A. Trends in hearing aid ownership among older adults in the United States From 2011 to 2018. JAMA Intern. Med. 181, 383–385. https://doi.org/10.1001/jamainternmed.2020.5682 (2021).
Madans, J. H., Weeks, J. D. & Elgaddal, N. Hearing difficulties among adults: United States, 2019. NCHS Data Brief, 1–8 (2021).
McCormack, A. Why do people fitted with hearing aids not wear them?. Int. J. Audiol. 52, 360–368 (2013).
Assi, L., Reed, N. S., Nieman, C. L. & Willink, A. Factors associated with hearing aid use among medicare beneficiaries. Innov. Aging 5, igab021. https://doi.org/10.1093/geroni/igab021 (2021).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6 (2020).
Livingston, G. et al. Dementia prevention, intervention, and care. Lancet 390, 2673–2734. https://doi.org/10.1016/S0140-6736(17)31363-6 (2017).
Lin, F. R. et al. Hearing loss and incident dementia. Arch. Neurol. 68, 214–220. https://doi.org/10.1001/archneurol.2010.362 (2011).
Gallacher, J. et al. Auditory threshold, phonologic demand, and incident dementia. Neurology 79, 1583–1590. https://doi.org/10.1212/WNL.0b013e31826e263d (2012).
Mener, D. J., Betz, J., Genther, D. J., Chen, D. & Lin, F. R. Hearing loss and depression in older adults. J. Am. Geriatr. Soc. 61, 1627–1629. https://doi.org/10.1111/jgs.12429 (2013).
Hopper, T., Slaughter, S. E., Hodgetts, B., Ostevik, A. & Ickert, C. Hearing loss and cognitive-communication test performance of long-term care residents with dementia: Effects of amplification. J. Speech Lang. Hear Res. 59, 1533–1542. https://doi.org/10.1044/2016_JSLHR-H-15-0135 (2016).
Chung, S. Association between sudden sensorineural hearing loss and anxiety disorder: A population-based study. Eur. Arch. Otorhinolaryngol. 272, 2673–2678 (2015).
Keidser, G. Self-Fitting Hearing Aids. Trends Hear. 20, 233121651664328 (2016).
FDA, D. o. H. a. H. S. in 21 CFR Parts 800, 801, 808, and 874 (2021).
Convery, E., Keidser, G., Seeto, M., Yeend, I. & Freeston, K. Factors affecting reliability and validity of self-directed automatic in situ audiometry: Implications for self-fitting hearing AIDS. J. Am. Acad. Audiol. 26, 5–18. https://doi.org/10.3766/jaaa.26.1.2 (2015).
Ravn, G. & Preves, D. Hearing aid-related standards and test systems. Semin. Hear. 36, 29–48. https://doi.org/10.1055/s-0034-1396925 (2015).
ANSI. Methods for manual pure-tone threshold audiometry. ANSI S3.21–1978 (American National Standards Institute, New York, 1978).
ASHA. Guidelines for Manual Pure-tone Audiometry. American Speech-Language-Hearing Asociation ASHA 20, 297–301 (1978).
Hughson, W. & Westlake, H. Manual for Program Outline for Rehabilitation of Aural Casualties Both Military and Civilian. Trans. Am. Acad. Opthal. Otolaryngol., 1–15 (1944).
Yost, W. A. Fundamentals of Hearing: An Introduction 3rd edn. (Academic Press, 1994).
Carhart, R. & Jerger, J. Preferred method for clinical determination of pure-tone thresholds. J. Speech Hear. Disorders 24, 330–345 (1959).
Byrne, D., Dillon, H., Ching, T., Katsch, R. & Keidser, G. NAL-NL1 procedure for fitting nonlinear hearing aids: Characteristics and comparisons with other procedures. J. Am. Acad. Audiol. 12, 37–51 (2001).
Keidser, G. & Convery, E. Outcomes with a self-fitting hearing aid. Trends Hear. 22, 2331216518768958. https://doi.org/10.1177/2331216518768958 (2018).
Yang, J., Zhu, H., Choi, T. & Cox, D. D. Smoothing and mean-covariance estimation of functional data with a Bayesian hierarchical model. Bayesian Anal. 11, 649–670. https://doi.org/10.1214/15-ba967 (2016).
Cox, M. & de Vries, B. Bayesian pure-tone audiometry through active learning under informed priors. Front. Digit Health 3, 723348. https://doi.org/10.3389/fdgth.2021.723348 (2021).
Durisala, N. In-situ audiometry: How close is it to conventional audiometry?. Hear. Balance Commun. 13, 8–14 (2015).
Kiessling, J. et al. A comparison of conventional and in-situ audiometry on participants with varying levels of sensorineural hearing loss. J. Am. Acad. Audiol. 26, 68–79. https://doi.org/10.3766/jaaa.26.1.8 (2015).
Kiessling, J., Leifholz, M., Pons-Kuhnemann, J., Jespersen, C. T. & Pedersen, J. N. A comparison of conventional and in-situ audiometry on participants with varying levels of sensorineural hearing loss. J. Am. Acad. Audiol. 26, 68–79 (2015).
Dox, M. & de Vries, B. A Bayesian binary classification approach to pure tone audiometry. . arXiv (2016).
Song, X. D. et al. Fast, continuous audiogram estimation using machine learning. Ear Hear. 36, e326-335. https://doi.org/10.1097/AUD.0000000000000186 (2015).
Boymans, M. & Dreschler, W. A. In situ hearing tests for the purpose of a self-fit hearing aid. Audiol. Neurootol. 22, 15–23. https://doi.org/10.1159/000457829 (2017).
Convery, E., Keidser, G., Dillon, H. & Hartley, L. A self-fitting hearing aid: Need and concept. Trends Amplif. 15, 157–166. https://doi.org/10.1177/1084713811427707 (2011).
Convery, E., Keidser, G., Hickson, L. & Meyer, C. Factors associated with successful setup of a self-fitting hearing aid and the need for personalized support. Ear Hear. 40, 794–804. https://doi.org/10.1097/AUD.0000000000000663 (2019).
Cox, R. M. & Alexander, G. C. Evaluation of an in-situ output probe-microphone method for hearing aid fitting verification. Ear Hear. 11, 31–39. https://doi.org/10.1097/00003446-199002000-00008 (1990).
Digiovanni, J. J. & Pratt, R. M. Verification of in situ thresholds and integrated real-ear measurements. J. Am. Acad. Audiol. 21, 663–670. https://doi.org/10.3766/jaaa.21.10.6 (2010).
Keidser, G., Dillon, H., Zhou, D. & Carter, L. Threshold measurements by self-fitting hearing aids: Feasibility and challenges. Trends Amplif. 15, 167–174. https://doi.org/10.1177/1084713812438700 (2011).
Mondelli, M. F., Garcia, T. M., Hashimoto, F. M. & Rocha, A. V. Open fitting: Performance verification of receiver in the ear and receiver in the aid. Braz. J. Otorhinolaryngol. 81, 270–275. https://doi.org/10.1016/j.bjorl.2014.08.013 (2015).
de Swanepoel, W., Mngemane, S., Molemong, S., Mkwanazi, H. & Tutshini, S. Hearing assessment-reliability, accuracy, and efficiency of automated audiometry. Telemed. J. e Health 16, 557–563 (2010).
de Swanepoel, W., Mngemane, S., Molemong, S., Mkwanazi, H. & Tutshini, S. Hearing assessment-reliability, accuracy, and efficiency of automated audiometry. Telemed. J. e Health 16, 557–563. https://doi.org/10.1089/tmj.2009.0143 (2010).
O’Brien, A., Keidser, G., Yeend, I., Hartley, L. & Dillon, H. Validity and reliability of in-situ air conduction thresholds measured through hearing aids coupled to closed and open instant-fit tips. Int. J. Audiol. 49, 868–876. https://doi.org/10.3109/14992027.2010.503664 (2010).
Smith-Olinde, L., Nicholson, N., Chivers, C., Highley, P. & Williams, D. K. Test-retest reliability of in situ unaided thresholds in adults. Am. J. Audiol. 15, 75–80. https://doi.org/10.1044/1059-0889(2006/009) (2006).
Winter, M. & Kuk, F. Using a DSP instrument fitting protocol for paediatric cases. Hear. Rev. 15, 71–73 (1998).
Özdamar, O., Eilers, R. E., Miskiel, E. & Widen, J. Classification of audiograms by sequential testing using a dynamic Bayesian procedure. J. Acoust. Soc. Am. 88, 2171–2179. https://doi.org/10.1121/1.400114 (1990).
Gardner, J. R., Malkomes, G., Garnett, R., Weinberger, K. Q. & Barbour, D. L. 2015 In: Advances in Neural Information Processing Systems. C. Cortes et al. (eds) (Curran Associates, Inc, UK). 2386–2394
Cox, M. & de Vries, B. A. Bayesian binary classification approach to pure tone audiometry. https://arXiv.org/quant-ph/151108670 (2015).
Gardner, J. R., Song, X. D., Weinberger, K. Q., Barbour, D. L. & Cunningham, J. P. Psychophysical detection testing with bayesian active learning., 286–295 (2015).
Barbour, D. L. et al. Online machine learning audiometry. Ear Hear. 40, 918–926. https://doi.org/10.1097/AUD.0000000000000669 (2019).
Barbour, D. L. et al. Conjoint psychometric field estimation for bilateral audiometry. Behav. Res. Methods 51, 1271–1285. https://doi.org/10.3758/s13428-018-1062-3 (2019).
Schlittenlacher, J., Turner, R. E. & Moore, B. C. J. Audiogram estimation using Bayesian active learning. J. Acoust. Soc. Am. 144, 421. https://doi.org/10.1121/1.5047436 (2018).
Schlittenlacher, J., Turner, R. E. & Moore, B. C. J. A hearing-model-based active-learning test for the determination of dead regions. Trends Hear. 22, 2331216518788215. https://doi.org/10.1177/2331216518788215 (2018).
Neal, R. M. Regression and Classification Using Gaussian Process Priors 475–501 (Oxford University Press, 1998).
Rasmussen, C. E. & Williams, C. K. I. Gaussian Processes for Machine Learning (The MIT Press, 2006).
Jerger, J. F., Carhart, R., Tillman, T. W. & Peterson, J. L. Some relations between normal hearing for pure tones and for speech. J. Speech Hear. Res. 2, 126–140. https://doi.org/10.1044/jshr.0202.126 (1959).
Lee, J. et al. Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear. 33, 315–329. https://doi.org/10.1097/AUD.0b013e31823d7917 (2012).
Funding
Resonance Medical, LLC and Soundwave Hearing, LLC.
Author information
Authors and Affiliations
Contributions
C.B.: Conceptualization; methodology; data acquisition; formal analysis; investigation; project administration; writing, review & editing. R.R.: Conceptualization; methodology; writing, review & editing. J.B.: Methodology; writing, review & editing. M.P.: Methodology; data acquisition; writing, review & editing. A.M.: Methodology; data acquisition; writing, review & editing. C.P.R.: Conceptualization; methodology; data acquisition; formal analysis; investigation; project administration; writing the original draft; writing, review & editing.
Corresponding author
Ethics declarations
Competing interests
Yes, the authors have competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper. These interests are listed in a paragraph at the end of the manuscript. "Christopher Boven, Reagan Roberts, Jeff Biggus, and Claus-Peter Richter are members of the Medical Resonance technical team. Christopher Boven, Reagan Roberts, and Claus-Peter Richter own shares from Resonance Medical. Claus-Peter Richter is a co-founder and CSO of Resonance Medical. Christopher Boven is a co-founder of Soundwave Hearing. Malini Patel, Akihiro J. Matsuoka, have no conflict of interest".
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boven, C., Roberts, R., Biggus, J. et al. In-situ hearing threshold estimation using Gaussian process classification. Sci Rep 13, 14667 (2023). https://doi.org/10.1038/s41598-023-40495-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40495-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.