Abstract
Aneurysmal subarachnoid hemorrhage (aSAH) is a serious condition with high mortality and a high permanent disability rate. In this study, we examined the association of clinical outcome with the Controlling Nutritional Status (CONUT) score during hospitalization in aSAH patients. A single-center, retrospective observational study was conducted at Gifu University Hospital. Patients transported to the emergency room for aSAH and diagnosed with World Federation of Neurosurgical Societies (WFNS) grade III and IV aSAH between April 2004 and March 2021 were enrolled. A logistic regression model was constructed to evaluate the association of the CONUT score with a modified Rankin scale (mRS) ≥ 3 and delayed cerebral ischemia (DCI). 127 patients diagnosed with WFNS grade III and IV aSAH were analyzed. CONUT score was significantly associated with mRS ≥ 3 during hospitalization. The score obtained by subtracting the CONUT score at admission from the maximum CONUT score was significantly associated with mRS ≥ 3 at discharge. Moreover, the score obtained by subtracting the CONUT score at admission from the maximum CONUT score during the first 14 days was significantly associated with DCI within 14 days from admission. These findings indicate that CONUT score during hospitalization may be a useful daily marker for predicting poor outcomes in aSAH patients.
Similar content being viewed by others
Introduction
Spontaneous subarachnoid hemorrhage (SAH) affects individuals with a mean age of 55 years and accounts for 5% of stroke cases. About 85% of SAHs are aneurysmal and 10% are non-aneurysmal perimesencephalic. Incidence of SAH is particularly high in East Asian countries, including Japan1. Aneurysmal subarachnoid hemorrhage (aSAH) is a serious condition with high mortality and a high permanent disability rate. The 30-day and 6-month mortality rate is 39% and 49%, respectively, and only 23% of patients have a favorable outcome, as evaluated using a modified Rankin scale (mRS) score of 0–22. The social and economic losses of patients with aSAH in the event of death or inability to return to society are therefore substantial3.
It well known that energy expenditure in patients with SAH dramatically increases after disease onset4,5,6. Several studies have reported that SAH patients’ nutritional status on admission7,8,9 and during hospitalization10,11 influences their clinical outcomes. Badjatia et al. reported in a prospective study of 58 SAH patients that a negative energy balance during the first 7 days after SAH correlated with the number of infectious complications10. These researchers also showed in a prospective observational study of 229 SAH patients that a negative nitrogen balance during the first 14 post-bleed days is a risk factor for infectious complications and is associated with poor outcomes (mRS ≥ 4) at 3 months11. Thus, it is important to identify a useful marker of the nutritional status of SAH patients during hospitalization.
The controlling nutritional status (CONUT) score, which is calculated based on patients’ serum albumin, total lymphocytes and total cholesterol, is associated with Full Nutritional Assessment (FNA) and was recently introduced as a nutritional screening tool12. Qi et al. demonstrated in a retrospectively analysis of 252 aSAH patients that a CONUT score < 4 at admission independently predicted patients’ functional outcome status at 3 months after aSAH7. However, it is unclear whether the CONUT score during hospitalization influences the functional outcomes of aSAH patients.
Here, we retrospectively analyzed the relationship between clinical outcomes and the CONUT score in aSAH patients during hospitalization.
Results
Patient demographics
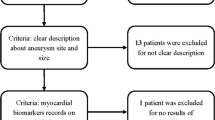
A flow diagram of patient recruitment is shown in Fig. 1. A total of 710 patients were admitted to our hospital with SAH during the study period. Of these, the number diagnosed with WFNS grade I, grade II, grade III, grade IV and grade V SAH were 252 (35.5%), 135 (19.0%), 17 (2.4%), 123 (17.3%) and 183 (25.8%), respectively. A total of 140 patients diagnosed with WFNS grade III and IV aSAH were enrolled in this study. Among these, 13 patients were excluded because they were transported to hospital 72 h and more after onset (n = 6), died before surgery (n = 6) or had an mRS of 4 before SAH onset (n = 1). This analysis therefore included 127 patients, of whom 16 and 111 patients were diagnosed with WFNS grade III and IV SAH, respectively.
Table 1 shows the patient’s demographics. Among the 127 patients, 41 (32.3%) were male and 86 (67.7%) were female, median age was 67 years (range: 37–89), and median body mass index was 22.0 kg/m2 (IQR: 19.3–24.1). The median CONUT score, ALBI score, C-reactive protein/albumin ratio and mRS were 2 (IQR: 1–3), − 2.77 (IQR: − 3.08 to − 2.49), 0.043 (IQR: 0.015–0.161) and 5 (IQR: 5–5), respectively.
SAH treatment status
Treatment comprised coiling and clipping in 72 (56.7%) and 55 (46.3%) patients, respectively. The median length of hospital stay was 39 days (IQR: 27–57). The proportion of patients with an mRS of 0, 1, 2, 3, 4, 5 and 6 at discharge was 1 (0.8%), 25 (19.7%), 28 (22.0%), 15 (11.8%), 9 (7.1%), 42 (33.1%) and 7 (5.5%), respectively.
Relationship between mRS ≥ 3 and CONUT score during hospitalization
A total of 5834 data points obtained from the 127 patients were analyzed in this study. Figure 2A depicts the association of the proportion of patients with mRS ≥ 3 with CONUT score (0, 2, 4, 6, 8, 10 and 12) from the day of admission to discharge. The proportion of patients with mRS ≥ 3 was low among those with lower CONUT scores. According to multivariable logistic regression analysis, there was a significant association of mRS ≥ 3 with CONUT score after adjustment for period of hospital stay, age and sex [odd ratio (OR) = 1.49, 95% confidence interval (CI) = 1.27‒1.75, P < 0.001].
Figures 2B‒D show the association of the proportion of patients with mRS ≥ 3 with the three components of the CONUT score from the day of admission to discharge. Of the three components, serum albumin and total lymphocytes, but not total cholesterol, were significantly associated with mRS ≥ 3 (serum albumin: OR = 0.16, 95% CI = 0.07‒0.34, P < 0.001; total lymphocytes: OR = 0.93, 95% CI = 0.87‒0.98, P = 0.005; total cholesterol: OR = 0.99, 95% CI = 0.99‒1.00, P = 0.433; Fig. 2B–D).
Moreover, the score obtained by subtracting the CONUT score at admission from the maximum CONUT score was significantly association with mRS ≥ 3 at discharge in the multivariable logistic regression analysis after adjusting for age and sex (OR = 1.65, 95%CI = 1.04‒2.62, P = 0.006, Fig. 3A). In contrast, the score obtained by subtracting the CONUT score at discharge from the maximum CONUT score was not significantly associated with mRS ≥ 3 at discharge (OR = 1.01, 95% CI = 0.67‒1.53, P = 0.577; Fig. 3B).
Relationship between DCI and CONUT score during hospitalization
To assess the association of DCI within 14 days from admission with the CONUT score at admission, multivariable logistic regression analysis was performed with adjustment for age and sex. DCI within 14 days from admission was significantly associated with the score obtained by subtracting the CONUT score at admission from the maximum CONUT score during the first 14 days (OR = 1.27, 95% CI = 0.85‒1.90, P = 0.003, Fig. 4A) and the score obtained by subtracting the CONUT score on Day 14 from the maximum CONUT score during the first 14 days (OR = 1.18, 95% CI = 0.83‒1.70, P = 0.015; Fig. 4B).
Association between the predicted probability of DCI within 14 days from admission and the score obtained by subtracting CONUT score at admission from maximum CONUT score during the 14 days from admission (A) and the score obtained by subtracting CONUT score at Day 14 from maximum CONUT score during the 14 days from hospitalization.
Relationship between mRS ≥ 3 and DCI and the CONUT score at admission
According to multivariable logistic regression analysis adjusted for age and sex, mRS ≥ 3 at discharge and DCI within 14 days from admission were not significantly associated with CONUT score at admission (mRS: OR = 1.31, 95% CI = 0.78‒2.18, P = 0.114; Fig. 5A; DCI: OR = 1.19, 95% CI = 0.74‒1.92, P = 0.762; Fig. 5B).
Discussion
In this retrospective study, we identified a significant association between mRS ≥ 3 and CONUT score during hospitalization in aSAH patients. The score obtained by subtracting the CONUT score on hospitalization from the maximum CONUT score during hospitalization was also significantly associated with the proportion of patients with mRS ≥ 3 at discharge. Additionally, the incidence of DCI during the first 14 days was significantly associated with the score obtained by subtracting the CONUT score during hospitalization from maximum CONUT score during the first 14 days. To our knowledge, this is first report to show an association between a change in CONUT score during hospitalization and clinical outcome in aSAH patients.
The CONUT score was developed to screen for undernutrition in hospital populations12. Numerous studies have reported an association between short- and/or long-term clinical outcomes and CONUT score in various acute diseases, including heart failure13,14, coronary syndrome15, myocardial infarction16, pulmonary embolism17 and ischemic and hemorrhagic stroke7,18,19. In contrast, few reports have examined the association of changes in CONUT score during hospitalization with clinical outcomes. An observational study by Takada et al. that included 1705 hospitalized patients with heart failure showed that changes in CONUT score during hospitalization were associated with cardiovascular death and readmission for heart failure20. In the present study, multivariable logistic regression analysis adjusted for period of hospital stay, age and sex revealed a significant association between CONUT score and mRS ≥ 3 during hospitalization, and the proportion of patients with mRS ≥ 3 was low among those with lower CONUT scores during hospitalization. Additionally, the scores obtained by subtracting the CONUT score at admission from the maximum CONUT score and maximum CONUT score during the first 14 days were significantly associated with mRS ≥ 3 and DCI during the first 14 days. These findings indicate that CONUT score during hospitalization might be a useful predictor of clinical outcome in aSAH patients, and that an increase in CONUT score during hospitalization may be a risk factor for worse outcomes in these patients. Thus, early appropriate nutritional management, which improves CONUT score, during hospitalization might lead to improved clinical outcomes in aSAH patients.
The CONUT score reflects the body’s immune-nutritional status, and serum albumin levels, total lymphocyte counts, and total cholesterol content are markers of the body's nutritional status, immune function, and lipid metabolism parameters, respectively12. Of the components of CONUT, serum albumin and total lymphocytes were significantly associated with mRS ≥ 3 in this study. Consistent with our results, previous studies also reported an association of clinical outcomes with serum albumin21,22,23 and lymphocytes24,25. Poor nutritional status during hospitalization is associated with poor outcomes in SAH patients4,5,6. Additionally, it is well understood that immune inflammation in the central nervous system is an important mechanism of early brain injury after aSAH and plays a critical role in the development of cerebral vasospasm and DCI26. Moreover, several studies have reported the involvement of serum free fatty acid levels and the effectiveness of supplementation with omega-3 fatty acids for preventing complications such as DCI after SAH4,27,28. Omega-3 fatty acids have numerous pharmacologic actions, including anti-inflammatory activity, serum lipid-lowering effects and suppression of platelet aggregation. These previous findings indicate that, in addition to nutritional status, monitoring of immune status may also be important to preventing complications such as DCI after aSAH. Accordingly, CONUT score may a better marker of aSAH outcome than serum albumin and lymphocytes alone.
In the present study, mRS ≥ 3 at discharge and DCI within 14 days from admission were not significantly associated with CONUT score at admission, which is inconsistent with a previous report by Qi et al7. This discrepancy may be related to the difference in study populations: the present study enrolled patients with more severe SAH (WFNS grade III and IV) than that by Qi et al. Kasuya et al. also reported that the elevation of resting energy expenditure and the nitrogen deficits in patients with grade III, IV or V were significantly higher than in patients with grade I or II4. In critically ill patients, nutritional status during hospitalization may play a greater role in clinical outcome than nutritional status at onset. It is well known that, as with traumatic brain injury, patients with SAH display hypermetabolism, increased catabolism, nitrogen loss, insulin resistance in brain and elevation of resting energy expenditure29,30. Surgical clipping after SAH induces a catabolic state with significantly elevated resting energy expenditure and nitrogen deficit. Similarly, SAH patients treated with endovascular coiling were found to have significantly elevated resting energy expenditure4,31. Although low caloric intake and negative nitrogen balance during hospitalization are associated with a worse clinical outcome of SAH10,11, no optimal nutritional management for patients with SAH has yet been established. The present results may show that the implementation of nutritional management with monitoring of CONUT score during hospitalization is useful, and may lead to the establishment of optimal nutritional management in patients with SAH.
This study has several limitations. First, we were unable to evaluate the daily amount of calories or contents consumed by patient. The adequacy of the daily amounts of calories or nutritional content balances required to decrease CONUT score therefore remain unknown. Revealing these will in turn lead to revealing the underlying mechanism of improving patient outcomes after SAH. Second, the sample size was small and data were obtained from a single institution. Several factors associated with clinical outcome in patients with SAH have been reported32,33. For example, endovascular coiling for aSAH is associated with higher 14-day case fatality and shorter length of hospital stay than surgical clipping for aSAH33,34. Additionally, surgical clipping is associated with a higher rate of discharge to rehabilitation in elderly patients with SAH35. Despite these several factors, confounding factors in all logistic regression models of this study examined to date have been limited to two factors—age and gender—to avoid overfitting for the small sample size. Additionally, it is important to note the generalizability of the present results, give that it is impossible to avoid the selection bias occurring in a single-institution studies. Finally, due to the retrospective nature of this study, potentially relevant confounders other than age and sex may have been missed. Thus, further large-scale prospective multicenter research is needed to confirm these findings and explore the underlying mechanisms.
These limitations notwithstanding, this study showed that the CONUT score during hospitalization may be a useful daily marker for predicting poor outcomes in aSAH patients. Additionally, an increase in CONUT score after the onset of SAH may be an important risk factor for poor outcomes in aSAH patients. Further large-scale studies are warranted to verify the usefulness of the CONUT score for predicting clinical outcomes.
Methods
Study design and patients
We conducted a single-center, retrospective study at Gifu University Hospital. Study participants included aSAH patients who were diagnosed with World Federation of Neurosurgical Societies (WFNS) grade III and IV aSAH between April 2004 and March 2021. Patients aged ≤ 16 y, patients who were transported ≥ 72 h from aneurysm rupture, patients whose aneurysm rupture date was unknown, patients whose SAH was induced by a cause other than aneurysm rupture, patients with mRS ≥ 4 from before aneurysm rupture, and patients who died before surgery for aSAH were excluded.
Treatment for aSAH
In this study, all subjects were treated based on the clinical practice guidelines for aSAH36. All patients with WFNS grade III and IV aSAH received 30 mg fasudil three times a day in the 14 days after surgery for aSAH, with dual antiplatelet therapy to prevent arterial thromboembolism implemented for one month, as appropriate. Intrathecal infusion of 60,000 units of urokinase for 3 days after coiling or clipping was usually performed to wash out the SAH. A ventriculoperitoneal shunt was placed for hydrocephalus.
Evaluation of clinical outcomes
All patients who suffered from aSAH were graded according to the WFNS37 by physicians (a neurologist or neurosurgeon) at admission. The mRS38 was used to assess neurological function, and was evaluated by physicians every day from admission to discharge. An mRS score ≥ 3 was used to indicate poor clinical outcomes in this study. The outcome in this study was mRS, and the primary outcome was a binary variable defined as poor prognosis when mRS ≥ 3. To determine serum albumin, total lymphocytes and total cholesterol12, blood sampling was performed at admission, every day while the patient was in the intensive care unit and every four days while the patient was in the general ward. Delayed cerebral ischemia (DCI) was defined as a decrease in Glasgow coma scale (GCS) score of ≥ 2 points from the day to next day39. GCS was determined through daily monitoring by physicians.
Data collection
The following data obtained during hospitalization were extracted from patients’ electronic medical records at the central database of our hospital and retrospectively analyzed: age, sex, height, weight, body mass index, serum albumin, alanine aminotransferase, aspartate aminotransferase, serum creatinine, C-reactive protein, total bilirubin, total cholesterol, total protein, white blood cell count, hemoglobin, platelet count, lymphocyte count, WFNS, mRS, GCS, time of aneurysm rupture, patient outcome (death), and cause of aneurysm rupture. Age, sex, height, weight, body mass index, and lymphocyte count in each patient were outputted from electronic medical records to the analysis sheet. The value of WFNS, mRS and GCS and the time of aneurysm rupture, patient outcome (death), and cause of aneurysm rupture were confirmed from the physician records in electronic medical records and posted to the analysis sheet by the researchers.
Creatinine clearance was calculated using the Cockcroft-Gault equation40, and the albumin-bilirubin (ALBI) score41 was calculated using the following equation: ALBI-score = log10Bilirubin (mmol/L) × 0.66 + Albumin (g/L) × (− 0.085).
Statistical analysis
Baseline characteristics are described as median with interquartile range (IQR) for continuous variables, and frequency and percentage for categorical variables. Logistic regression models were used to confirm the association of CONUT score, serum albumin, total lymphocytes, and cholesterol with mRS ≥ 3 over with the course of hospitalization. To address repeated data, Huber-White sandwich variance estimators were used to calculate covariance. Each model included one component of the CONUT score (serum albumin, total lymphocytes, or total cholesterol) and days after hospitalization and their interaction terms. Age and gender are common patient characteristics and are thought to be factors associated with mRS and/or CONUT score32,42. Multicollinearity was checked using the variance inflation factor (VIF) and the model was fixed based on the fact that all values were less than 0.2. Associations of DCI within 14 days from admission and mRS ≥ 3 at discharge with the maximum CONUT score at discharge were confirmed using logistic regression models adjusted for age and sex. The association of DCI within 14 days from admission and mRS ≥ 3 at discharge with CONUT score at admission was confirmed using the same logistic regression model.
In all logistic regression models, nonlinear relationships between outcomes and explanatory variables were assessed using restricted cubic spline curves. A two-sided p-value less than 0.05 was considered statistically significant. All analyses were performed using IBM SPSS version 22 (IBM Japan Ltd., Tokyo, Japan) and R software version 4.2.1 (www.r-project.org).
Ethics statement
The investigation conformed with the principles outlined in the Declaration of Helsinki43. The present study was performed in accordance with the guideline for human studies adopted by the Medical Review Board of Gifu University Graduate School of Medicine and notified by the Japanese government. The experimental protocol was approved by the Medical Review Board of Gifu University Graduate School of Medicine (institutional review board approval number: 2021-080). Due to the retrospective nature of the study, the Medical Review Board of Gifu University Graduate School of Medicine waived the need of obtaining informed consent.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ALB:
-
Albumin
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- CRE:
-
Creatinine
- CRP:
-
C-reactive protein
- T-Bil:
-
Total bilirubin
- T-CHO:
-
Total cholesterol
- TP:
-
Total protein
- WBC:
-
White blood cells
- HGB:
-
Hemoglobin
- PLT:
-
Platelets
- CONUT:
-
Controlling nutritional status
- ALBI:
-
Assessment of albumin–bilirubin
- mRS:
-
Modified Rankin scale
References
Macdonald, R. L. & Schweizer, T. A. Spontaneous subarachnoid haemorrhage. Lancet 389, 655–666. https://doi.org/10.1016/S0140-6736(16)30668-7 (2017).
Konczalla, J. et al. Outcome after Hunt and Hess Grade V subarachnoid hemorrhage: A comparison of pre-coiling era (1980–1995) versus post-ISAT era (2005–2014). J. Neurosurg. 128, 100–110. https://doi.org/10.3171/2016.8.JNS161075 (2018).
Ridwan, S. et al. Health care costs of spontaneous aneurysmal subarachnoid hemorrhage for rehabilitation, home care, and in-hospital treatment for the first year. World Neurosurg. 97, 495–500. https://doi.org/10.1016/j.wneu.2016.09.123 (2017).
Kasuya, H., Kawashima, A., Namiki, K., Shimizu, T. & Takakura, K. Metabolic profiles of patients with subarachnoid hemorrhage treated by early surgery. Neurosurgery 42, 1268–1274; discussion 1274–1265. https://doi.org/10.1097/00006123-199806000-00038 (1998).
Esper, D. H., Coplin, W. M. & Carhuapoma, J. R. Energy expenditure in patients with nontraumatic intracranial hemorrhage. JPEN J. Parenter. Enteral Nutr. 30, 71–75. https://doi.org/10.1177/014860710603000271 (2006).
Nyberg, C., Engstrom, E. R., Hillered, L. & Karlsson, T. Daily systemic energy expenditure in the acute phase of aneurysmal subarachnoid hemorrhage. Ups. J. Med. Sci. 124, 254–259. https://doi.org/10.1080/03009734.2019.1659888 (2019).
Qi, H. et al. Clinical value of controlling nutritional status score in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. 126, e1352–e1358. https://doi.org/10.1016/j.wneu.2019.03.100 (2019).
Kimura, Y. et al. Combination of low body mass index and low serum albumin level leads to poor functional recovery in stroke patients. J. Stroke Cerebrovasc. Dis. 26, 448–453. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.10.008 (2017).
Shen, H. C. et al. Impact of nutritional status on long-term functional outcomes of post-acute stroke patients in Taiwan. Arch. Gerontol. Geriatr. 53, e149-152. https://doi.org/10.1016/j.archger.2010.08.001 (2011).
Badjatia, N. et al. Relationship between energy balance and complications after subarachnoid hemorrhage. JPEN J. Parenter. Enteral Nutr. 34, 64–69. https://doi.org/10.1177/0148607109348797 (2010).
Badjatia, N. et al. Inflammation, negative nitrogen balance, and outcome after aneurysmal subarachnoid hemorrhage. Neurology 84, 680–687. https://doi.org/10.1212/WNL.0000000000001259 (2015).
Ignacio de Ulibarri, J. et al. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 20, 38–45 (2005).
Nishi, I. et al. Nutritional screening based on the controlling nutritional status (CONUT) score at the time of admission is useful for long-term prognostic prediction in patients with heart failure requiring hospitalization. Ibaraki Cardiovascular Assessment Study-Heart Failure Investigators. Heart Vessels 32, 1337–1349. https://doi.org/10.1007/s00380-017-1001-8 (2017).
Raposeiras Roubín, S. et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 76, 828–840. https://doi.org/10.1016/j.jacc.2020.06.058 (2020).
Takahashi, T. et al. Prognostic significance of the controlling nutritional (CONUT) score in patients with acute coronary syndrome. Heart Vessels. 36, 1109–1116. https://doi.org/10.1007/s00380-021-01792-4 (2021).
Chen, B. et al. Correlations and prognostic roles of the nutritional status and neutrophil-to-lymphocyte ratio in elderly patients with acute myocardial infarction undergoing primary coronary intervention. Int. Heart J. 61, 1114–1120. https://doi.org/10.1536/ihj.20-138 (2020).
Yıldırım, B. et al. Controlling Nutritional Status (CONUT) score predicts in-hospital mortality in acute pulmonary embolism. Med. Princ. Pract. 31, 439–444. https://doi.org/10.1159/000525240 (2022).
Kokura, Y., Kimoto, K., Okada, Y. & Kawakita, S. The Controlling Nutritional Status score as a functional prognostic marker in patients with acute stroke: A multicenter retrospective cohort study. Nutrition 79–80, 110889. https://doi.org/10.1016/j.nut.2020.110889 (2020).
Akimoto, T., Hara, M., Morita, A., Uehara, S. & Nakajima, H. Relationship between nutritional scales and prognosis in elderly patients after acute ischemic stroke: Comparison of controlling nutritional status score and geriatric nutritional risk index. Ann. Nutr. Metab. 77, 116–123. https://doi.org/10.1159/000515212 (2021).
Takada, T. et al. Nutritional status during hospitalization is associated with the long-term prognosis of patients with heart failure. ESC Heart Fail 8, 5372–5382. https://doi.org/10.1002/ehf2.13629 (2021).
Shang, F. et al. Predictive value of the serum albumin level on admission in patients with spontaneous subarachnoid hemorrhage. Front. Surg. 8, 719226. https://doi.org/10.3389/fsurg.2021.719226 (2021).
Wang, P. et al. Association between serum albumin and hospital-acquired infections after aneurysmal subarachnoid hemorrhage. Neurocrit. Care 37, 424–434. https://doi.org/10.1007/s12028-021-01421-y (2022).
Dicpinigaitis, A. J. et al. Low serum albumin as a risk factor for delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage: eICU collaborative research database analysis. J. Neurosurg. Sci. https://doi.org/10.23736/S0390-5616.22.05604-1 (2022).
Giede-Jeppe, A. et al. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 132, 400–407. https://doi.org/10.3171/2018.9.JNS181975 (2019).
Gusdon, A. M. et al. Time course of peripheral leukocytosis and clinical outcomes after aneurysmal subarachnoid hemorrhage. Front. Neurol. 12, 694996. https://doi.org/10.3389/fneur.2021.694996 (2021).
Zeyu, Z., Yuanjian, F., Cameron, L. & Sheng, C. The role of immune inflammation in aneurysmal subarachnoid hemorrhage. Exp. Neurol. 336, 113535. https://doi.org/10.1016/j.expneurol.2020.113535 (2021).
Shirao, S. et al. Inhibitory effects of eicosapentaenoic acid on chronic cerebral vasospasm after subarachnoid hemorrhage: Possible involvement of a sphingosylphosphorylcholine-rho-kinase pathway. Cerebrovasc. Dis. 26, 30–37. https://doi.org/10.1159/000135650 (2008).
Yoneda, H. et al. Does eicosapentaenoic acid (EPA) inhibit cerebral vasospasm in patients after aneurysmal subarachnoid hemorrhage?. Acta Neurol. Scand. 118, 54–59. https://doi.org/10.1111/j.1600-0404.2007.00983.x (2008).
Abdelmalik, P. A., Dempsey, S. & Ziai, W. Nutritional and bioenergetic considerations in critically Ill patients with acute neurological injury. Neurocrit. Care. 27, 276–286. https://doi.org/10.1007/s12028-016-0336-9 (2017).
Schmidt, J. M. et al. Nutritional support and brain tissue glucose metabolism in poor-grade SAH: A retrospective observational study. Crit Care. 16, R15. https://doi.org/10.1186/cc11160 (2012).
Nagano, A., Yamada, Y., Miyake, H., Domen, K. & Koyama, T. Increased resting energy expenditure after endovascular coiling for subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 25, 813–818. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.12.008 (2016).
Lantigua, H. et al. Subarachnoid hemorrhage: Who dies, and why?. Crit. Care 19, 309. https://doi.org/10.1186/s13054-015-1036-0 (2015).
Lindgren, A. et al. Outcome after clipping and coiling for aneurysmal subarachnoid hemorrhage in clinical practice in Europe, USA, and Australia. Neurosurgery 84, 1019–1027. https://doi.org/10.1093/neuros/nyy223 (2019).
Zhang, X. et al. Total hospital costs and length of stay of endovascular coiling versus neurosurgical clipping for unruptured intracranial aneurysms: Systematic review and meta-analysis. World Neurosurg. 115, 393–399. https://doi.org/10.1016/j.wneu.2018.04.028 (2018).
Bekelis, K. et al. Surgical clipping versus endovascular coiling for elderly patients presenting with subarachnoid hemorrhage. J. Neurointerv. Surg. 8, 913–918. https://doi.org/10.1136/neurintsurg-2015-011890 (2016).
Connolly, E. S. Jr. et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43, 1711–1737. https://doi.org/10.1161/STR.0b013e3182587839 (2012).
Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J. Neurosurg. 68, 985–986. https://doi.org/10.3171/jns.1988.68.6.0985 (1988).
Newcommon, N. J., Green, T. L., Haley, E., Cooke, T. & Hill, M. D. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 34, 377–378; author reply 377–378 https://doi.org/10.1161/01.str.0000055766.99908.58 (2003).
Vergouwen, M. D. et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 41, 2391–2395. https://doi.org/10.1161/STROKEAHA.110.589275 (2010).
Cockcroft, D. W. & Gault, M. H. Prediction of creatinine clearance from serum creatinine. Nephron 16, 31–41. https://doi.org/10.1159/000180580 (1976).
Johnson, P. J. et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 33, 550–558. https://doi.org/10.1200/JCO.2014.57.9151 (2015).
Drolz, A. et al. Gender-specific differences in energy metabolism during the initial phase of critical illness. Eur. J. Clin. Nutr. 68, 707–711. https://doi.org/10.1038/ejcn.2013.287 (2014).
Rickham, P. P. Human experimentation. Code of ethics of the world medical association. Declaration of helsinki. Br. Med. J. 2, 177. https://doi.org/10.1136/bmj.2.5402.177 (1964).
Acknowledgements
We thank DMC Corp. (www.dmed.co.jp <http://www.dmed.co.jp/ >) for providing English language editing of this paper.
Funding
This study did not receive funding from any funding source.
Author information
Authors and Affiliations
Contributions
S.S., Y.S., K.S., M.I., A.R. and A.S. developed the study concept and design. S.S. and A.S. wrote the manuscript. S.S., S.N. and R.K. created the database. T.I. performed the statistical analysis. T.H. and Y.E. supervised the study. S.S., Y.S., K.S., M.I., T.I. A.R., R.K. and A.S. revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimizu, S., Hanai, T., Egashira, Y. et al. Controlling nutritional status score during hospitalization as a predictor of clinical outcome in patients with aneurysmal subarachnoid hemorrhage. Sci Rep 13, 12758 (2023). https://doi.org/10.1038/s41598-023-39938-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39938-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.