Abstract
Neuromyelitis optica spectrum disorders (NMOSD) are severe inflammatory disorders of the central nervous system targeting aquaporin‐4 (AQP4). The risk factors for NMOSD remain to be determined, though they may be related to diet and nutrition. This study aimed to explore the possibility of a causal relationship between specific food intake and AQP4-positive NMOSD risk. The study followed a two-sample Mendelian randomization (MR) design. Genetic instruments and self-reported information on the intake of 29 types of food were obtained from a genome-wide association study (GWAS) on 445,779 UK Biobank participants. A total of 132 individuals with AQP4-positive NMOSD and 784 controls from this GWAS were included in our study. The associations were evaluated using inverse-variance-weighted meta-analysis, weighted-median analysis, and MR-Egger regression. A high consumption of oily fish and raw vegetables was associated with a decreased risk of AQP4-positive NMOSD (odds ratio [OR] = 1.78 × 10−16, 95% confidence interval [CI] = 2.60 × 10−25–1.22 × 10−7, p = 0.001; OR = 5.28 × 10−6, 95% CI = 4.67 × 10−11–0.598, p = 0.041, respectively). The results were consistent in the sensitivity analyses, and no evidence of directional pleiotropy was observed. Our study provides useful implications for the development of AQP4-positive NMOSD prevention strategies. Further research is needed to determine the exact causal relationship and mechanisms underlying the association between specific food intake and AQP4-positive NMOSD.
Similar content being viewed by others
Introduction
Neuromyelitis optica spectrum disorders (NMOSD) are a group of severe autoimmune demyelinating diseases of the central nervous system (CNS), characterized by optic neuritis and longitudinally extensive myelitis (LETM)1. The presence of autoantibodies against aquaporin 4 (AQP4) is a hallmark of NMOSD2, occurring in 80% of patients with this disease, which is also considered an autoimmune astrocytomosis3. NMOSD mostly affect young adults, particularly women4. NMOSD are caused by inflammation, and patients with these disorders are prone to peripheral and CNS inflammation caused by cytokines, particularly those produced by T helper (Th)2 and Th17 lymphocytes5.
Several risk factors have been associated with NMOSD, including environmental and genetic factors4,6. A thorough investigation on these associations has yet to be conducted; however, several environmental risk factors are known, such as specific dietary patterns in both sexes, and in women, history of abortion or trauma, low body mass index (BMI), and low physical activity levels7,8.
Different dietary patterns determine the variance in the gastrointestinal microbiome6. According to previous studies, a high sugar intake results in dysregulation and decrease in microbiota diversity9,10. A dysbiosis of gut microbiota can lead to systemic and neuro-inflammation by increasing the levels of interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-16,11,12. Additionally, a retrospective study showed that diets with high inflammatory potential are associated with an increased risk of NMOSD13.
Dietary risk factors associated with NMOSD progression have been identified in previous studies6,14, though the evidence was insufficient to establish causal roles. A cross-sectional study was conducted to determine the type of diet mostly associated with the incidence of NMOSD. However, these observations may have been confounded by unidentified factors, and the causality of the associations was not supported15. Randomized controlled trials (RCTs) are the gold standard to determine a causal relationship16,17, though they are challenging to implement due to ethical constraints. It is often impossible to link specific nutritional interventions to disease outcomes in long-term RCTs because of the difficulty in selecting appropriate control groups and blinding participants and researchers18. These limitations can be overcome using Mendelian randomization (MR).
A growing number of studies have used MR to examine the possible causal role of modifiable exposures on the incidence of several diseases. MR is a statistical framework used to estimate the effects of exposure using genetic variants19,20. In the MR model, different allelic compositions result in different exposures throughout life, as genetic variants alter or mimic nutritional exposures (such as circulating micronutrients, macronutrient intake, and dietary patterns). Therefore, these variants may also contribute to disease risk21. MR can help overcome the limitations typical of observational studies, such as residual confounding, reverse causation, and recall bias22.
Single-nucleotide polymorphisms (SNPs) associated with dietary patterns and macronutrient intake can be used in MR analyses. Since SNPs cannot be modified, they are less susceptible to reverse causality due to Mendel’s second law, and thus MR using them is less likely to be affected by confounding factors and less prone to random or systematic measurement errors than other types of analysis. A key benefit of MR is that it allows to estimate the causal effect of an exposure on the occurrence of a disease using statistical analyses to mitigate the biases encountered in observational nutritional epidemiology.
A better understanding of how different types of foods affect the risk of NMOSD may help develop more effective prediction, treatment, and prevention strategies. The purpose of this study was to assess the causal relationship between dietary patterns and NMOSD using the MR method16.
Materials and methods
Genetic association between food intake and AQP4-positive NMOSD
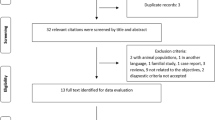
An overview of the study design is shown in a flowchart (Fig. 1). A large recent genome-wide association study (GWAS) has identified several SNPs associated with food intake and patterns. Full GWAS summary statistics are available at https://www.ebi.ac.uk/gwas/home. We examined 29 lifestyle and dietary factors, including consumption of alcohol, coffee, fish, fruit, vegetables, and beef. A summary of each type of exposure is provided in Table 1. Genetic association data for NMOSD were drawn from a GWAS meta-analysis of this disease (GCST006937); our study included 132 AQP4-positive patients and 784 controls. The presence of NMOSD was determined using the 2006 diagnostic criteria, which include optic neuritis, transverse myelitis, and two of the following three supportive elements: (1) longitudinally extensive lesions (≥ 3 vertebral segments in length); (2) magnetic resonance imaging of the brain with findings not consistent with multiple sclerosis; and (3) AQP4-IgG antibody seropositivity1.
Generic diet questionnaire
The genome associations of 29 food intake patterns were derived from a study by Pirastu et al.23. Analyses were conducted using data collected for the UK Biobank project (project no. 19655)24. A touchscreen dietary frequency questionnaire was used in the UK Biobank to assess dietary intakes and patterns23. The survey included questions regarding the frequency of consumption of specific foods and beverages.
All quantitative food and drink intake phenotypes were converted to weekly consumption; for example, drinking three cups of tea per day was converted to 21 cups per week. A semi-quantitative description25, such as never, two–four times per week, five–six times per week, and once or more per day, was converted to 0, 3, 5.5, and 7, respectively. Participants who chose not to answer or were not sure were excluded from the analysis.
All coffee traits were stratified by type (instant, ground, and decaffeinated) to account for differences in consumption patterns, such as cup size and caffeine concentration. We excluded participants who did not specify the type of coffee usually consumed.
Coffee consumption (any type of coffee, including unspecified) has a strong negative phenotypic correlation with water consumption; therefore, coffee consumption was treated as a covariate for water consumption. On the other hand, some semi-quantitative traits are not directly related to the amount or type of food or drink consumed. Non-dairy milk types (e.g., soy) were excluded from the calculation of milk fat content. The drink temperatures (very hot, hot, and warm) were converted to an arbitrary three-unit scale (3, 2, and 1, respectively). Individuals who did not consume hot drinks were excluded from the analysis. Supplementary Table S1 lists the number of samples used for each trait. Supplementary Table S2 provides a detailed description of the phenotypes.
Genetic instrumental variable selection
MR analyses use SNPs as instrumental variables (IVs) to estimate the causal associations between exposures and outcomes19. MR analysis is based on three critical assumptions: (i) the exposure is strongly associated with the IVs, (ii) confounders for exposures and outcomes should not affect the IVs, and (iii) exposure is the only factor that mediates the IV-outcome associations19.
A linkage disequilibrium (LD) occurs when an allele in one locus is disproportionately coinherited with an allele in a different locus. Due to Mendel's second law of random assortment, using several genetic variants in LD between them may introduce biases in MR studies. As a first step, we determined whether the chosen independent genetic variants were significantly associated with each instrument for each exposure (p < 5 × 10−8). We applied clumping with R2 < 0.001 and a window size > 5,000 kb to avoid LD26. Averaging SNP-specific F-statistics was used to avoid weak IVs, and IVs with F values > 10 were considered strong27,28. A list of the selected SNPs is provided in Supplementary Table S3.
Pleiotropy, heterogeneity, and sensitivity analysis
Genetic variants or genetic risk scores may be associated with other potential exposures or confounders; this phenomenon is known as pleiotropy. An estimate from an MR study could be unreliable if the genetic variants chosen are used in these circumstances29. We assessed the horizontal pleiotropy using MR-Egger regression, as indicated by the intercept19. A P-value < 0.05 indicates that the inverse variance-weighted (IVW) results might be invalid due to horizontal pleiotropy, and an MR Pleiotropy REsidual Sum and Outlier (MR-PRESSO) test should be conducted30. The degree of heterogeneity across all SNPs was evaluated using Cochran's Q statistic and leave-one-out analysis31. The results of the pleiotropy, heterogeneity, and sensitivity analyses are presented in Supplementary Tables S4, S5, and S6, respectively.
MR analysis
Our primary analysis used the IVW method32; thus, all the variants were assumed to be valid IVs, providing the most precise results. The weighted median and MR-Egger regression methods were used in complementary analyses33. If the result obtained using the IVW method is significant (P < 0.05), it can be regarded as a positive result, even when the results from other methods are not significant, if the beta values of the other methods are in the same direction34. If horizontal pleiotropy was identified without heterogeneity, the MR-Egger method was selected; if heterogeneity was identified without pleiotropy, the weighted median method or the multiplicative random-effects inverse variance weighting (mre-IVW) method was used for the analysis. All the MR analyses were performed in R 4.2.1 (R Core Team [2022]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/), using the TwoSampleMR and Mendelian Randomization packages35. Supplementary Table S7 presents the MR estimates obtained using the different methods.
Results
Our study included 26 types of food exposures, after excluding those without effective IVs (fortified wine consumption, spirit consumption, and vegetarianism). After a series of quality control steps, the SNPs chosen for each type of food ranged between 3 and 73. Among patients meeting the NMOSD diagnostic criteria, the ones with positive AQP4 antibodies were selected for the analysis. The F-statistic values were greater than the empirical threshold of 10, indicating that all SNPs were valid.
Higher intakes of oily fish and raw vegetables were associated with a lower risk of AQP4-positive NMOSD. For oily fish consumption, the results of the IVW and MR-Egger analyses were statistically significant (odds ratio [OR] = 0.006, 95% confidence interval [CI] = 4.18 × 10−5–0.885, p = 0.045; OR = 1.78 × 10−16; 95% CI = 2.60 × 10−25–1.22 × 10−7; p = 0.001, respectively). The Cochran’s Q test showed no heterogeneity. However, pleiotropy was identified through the MR-Egger intercept analysis (p = 0.004), whereas the MR-PRESSO test did not show significant pleiotropy (p = 0.081). The leave-one-out studies were used for the sensitivity analysis and demonstrated no significant influence from individual studies. Considering the above findings, we selected the result from the MR-Egger method as the main one, as it was statistically significant. For raw vegetable consumption, the result from the IVW analysis was statistically significant (OR = 5.28 × 10−6; 95% CI = 4.67 × 10−11–0.598; p = 0.041). Heterogeneity and pleiotropy were not observed in this analysis. Leave-one-out studies were used for the sensitivity analysis and demonstrated no influence from individual studies. Overall, the consumption of oily fish and raw vegetables was associated with a lower risk of AQP4-positive NMOSD. Figure 2 shows the forest plots for exposure to oily fish and raw vegetables. The scatter plots, leave-one-out plots, and funnel plots are summarized in Supplementary Figs. S1 and S2. The results are summarized in Table 2.
The other 24 food intake exposures considered were not associated with the risk of AQP4-positive NMOSD (consumption of beef, beer or cider, spread on bread, bread, champagne or white wine, cheese, cooked vegetables, decaffeinated coffee, dried fruit, fresh fruit, ground coffee, instant coffee, lamb, non-oily fish, pork, poultry, processed meat, red wine, added salt, tea, poultry, and water, as well as water consumption corrected for coffee, drink temperature, and milk fat percentage). The intake differences between patients and controls were not significant using all methods.
Discussion
Environmental factors are believed to influence significantly the risk and progression of NMOSD, particularly in women who are predominantly affected, with a female-to-male ratio of up to 9:1. Several putative risk factors have been suggested in previous studies, including ethnic or racial background, other autoimmune conditions, smoking, and infections36.
In susceptible individuals, certain foods may increase or decrease the risk of NMOSD13,37. We found that a higher consumption of oily fish was associated with a lower risk of AQP4-positive NMOSD. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are omega-3 polyunsaturated fatty acids (PUFAs) found in oily fish38. According to animal experiments and clinical intervention studies, omega-3 fatty acids exert anti-inflammatory effects via intracellular signaling pathways, transcription factor activity, and gene expression39. Several studies have examined the benefits of dietary supplements containing fish oils on inflammatory and autoimmune conditions, including ulcerative colitis, Crohn's disease, and rheumatoid arthritis40. In several placebo-controlled trials, fish oil has been shown to reduce the activity of chronic inflammatory diseases and the need for anti-inflammatory medications. According to experimental studies, the gut microbiota, omega-3 PUFAs, and the immune system play key roles in maintaining intestinal wall integrity40. Consequently, high oily fish consumption can improve the microflora profile and suppress the inflammatory process, and thus modification of the dietary patterns may reduce the susceptibility for NMOSD, according to the "gut-brain axis" hypothesis12.
On the other hand, raw vegetables have low energy density in addition to vitamins, fiber, folate, potassium, lignans, flavonoids, minerals, and other bioactive phytochemicals41. In a recent study, green salads were found to reduce the risk of all-cause mortality42. According to another study, raw vegetable consumption is inversely related to the level of DNA damage caused by oxidative stress43. Our statistical analyses revealed an association between high raw vegetable intake and low risk of AQP4-positive NMOSD. This finding can be explained by the abundant antioxidants found in raw vegetables. A high total antioxidant capacity (TAC) reduces the incidence of NMOSD and the risk of seropositivity44. As these disorders are astrocytopathy-mediated, patients with NMOSD are protected from free-radical damage by the antioxidants produced by astrocytes45. Astrocytes exert their effects in several ways, such as regulating the glutamate levels; nerve cells can be damaged by high levels of glutamate, a stimulatory neurotransmitter44. Astrocytes also produce glutathione (GSH), which protects neurons against oxidative damage. Finally, astrocytes activate the Nrf2–KEP1–ARE pathway in response to oxidative stress to protect neurons46. Thus, astrocytes may have a stronger activity against disturbances with the support of more antioxidants from the diet. We speculate that raw vegetables may reduce the risk of NMOSD through their anti-inflammatory properties, though few studies were conducted on this type of food.
Conclusion and limitation
In conclusion, oily fish and raw vegetable consumption can decrease the risk of AQP4-positive NMOSD. The main strength of our study was the MR design, suitable for causal inference. RCTs on NMOSD are difficult to design and perform; therefore, an MR study may provide valuable insights into the risk of developing NMOSD associated with specific dietary components. Our study included 26 food types; some of the factors included, such as the consumption of processed psychoactive drinks, were not previously examined using MR. Therefore, future research on the relationship between food intake and disease risk may benefit from the results of this study.
However, the study also has some limitations. First, findings on several common types of NMOSD tend to have low statistical power, reducing their reliability31. The second limitation is that three of the food intake types considered had insufficiently effective IVs. Additionally, we examined single food items, whereas these elements may act synergistically or antagonistically in complex diets47. Furthermore, the existing literature on oily fish and vegetables and the associated risk of NMOSD is scarce, and this association should be explored with randomized controlled trials in the future. Researchers should examine various dietary patterns with MR studies to determine whether they affect the risk of developing NMOSD. We believe that several of the potential causal relationships described here have potential for further investigation22.
Data availability
The human data used in this study are publicly available. All databases were obtained from the following website: GWAS Catalog (https://www.ebi.ac.uk/gwas/). The GWAS ID in Table 1 can be entered in the website to query and download the GWAS dataset used in this article.
References
Wingerchuk, D. M., Lennon, V. A., Pittock, S. J., Lucchinetti, C. F. & Weinshenker, B. G. Revised diagnostic criteria for neuromyelitis optica. Neurology 66(10), 1485–1489. https://doi.org/10.1212/01.wnl.0000216139.44259.74 (2006).
Fujihara, K. Neuromyelitis optica spectrum disorders: Still evolving and broadening. Curr. Opin. Neurol. 32(3), 385–394. https://doi.org/10.1097/wco.0000000000000694 (2019).
Yick, L. W., Tang, C. H., Ma, O. K., Kwan, J. S. & Chan, K. H. Memantine ameliorates motor impairments and pathologies in a mouse model of neuromyelitis optica spectrum disorders. J. Neuroinflammation 17(1), 236. https://doi.org/10.1186/s12974-020-01913-2 (2020).
Eskandarieh, S. et al. Comparing epidemiology and baseline characteristic of multiple sclerosis and neuromyelitis optica: A case-control study. Mult. Scler. Relat. Disord. 12, 39–43. https://doi.org/10.1016/j.msard.2017.01.004 (2017).
Wang, X. et al. Resolution of inflammation in neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 27, 34–41. https://doi.org/10.1016/j.msard.2018.09.040 (2019).
Rezaeimanesh, N. et al. The association between dietary sugar intake and neuromyelitis optica spectrum disorder: A case-control study. Mult. Scler. Relat. Disord. 31, 112–117. https://doi.org/10.1016/j.msard.2019.03.028 (2019).
Baek, S. H. et al. Low body mass index can be associated with the risk and poor outcomes of neuromyelitis optica with aquaporin-4 immunoglobulin G in women. J. Neurol. Neurosurg. Psychiatry 89(11), 1228–1230 (2018).
Eskandarieh, S. et al. Environmental risk factors in neuromyelitis optica spectrum disorder: A case-control study. Acta Neurol. Belg. 118(2), 277–287. https://doi.org/10.1007/s13760-018-0900-5 (2018).
Beilharz, J. E., Kaakoush, N. O., Maniam, J. & Morris, M. J. The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain Behav. Immun. 57, 304–313. https://doi.org/10.1016/j.bbi.2016.07.151 (2016).
Wang, X. et al. Role of gut microbiota in multiple sclerosis and potential therapeutic implications. Curr. Neuropharmacol. 20(7), 1413–1426. https://doi.org/10.2174/1570159x19666210629145351 (2022).
Esposito, S., Bonavita, S., Sparaco, M., Gallo, A. & Tedeschi, G. The role of diet in multiple sclerosis: A review. Nutr. Neurosci. 21(6), 377–390. https://doi.org/10.1080/1028415x.2017.1303016 (2018).
Lombardi, V. C. et al. Nutritional modulation of the intestinal microbiota; future opportunities for the prevention and treatment of neuroimmune and neuroinflammatory disease. J. Nutr. Biochem. 61, 1–16. https://doi.org/10.1016/j.jnutbio.2018.04.004 (2018).
Paz, É. S. et al. Excess weight, central adiposity and pro-inflammatory diet consumption in patients with neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 54, 103110. https://doi.org/10.1016/j.msard.2021.103110 (2021).
Rezaeimanesh, N., Saeedi, R., Sahraian, M. A., Razeghi Jahromi, S. & Naser Moghadasi, A. The possible beneficial effects of higher vitamin B6 intake from diet on cognitive function of patients with neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 42, 102132. https://doi.org/10.1016/j.msard.2020.102132 (2020).
Steeger, C. M., Buckley, P. R., Pampel, F. C., Gust, C. J. & Hill, K. G. Common methodological problems in randomized controlled trials of preventive interventions. Prev. Sci. 22(8), 1159–1172. https://doi.org/10.1007/s11121-021-01263-2 (2021).
Chen, B., Han, Z. & Geng, L. Mendelian randomization analysis reveals causal effects of food intakes on inflammatory bowel disease risk. Front. Immunol. 13, 911631. https://doi.org/10.3389/fimmu.2022.911631 (2022).
West, S. G. & Thoemmes, F. Campbell’s and Rubin’s perspectives on causal inference. Psychol. Methods 15(1), 18–37. https://doi.org/10.1037/a0015917 (2010).
Mirmiran, P., Bahadoran, Z. & Gaeini, Z. Common limitations and challenges of dietary clinical trials for translation into clinical practices. Int. J. Endocrinol. Metab. 19(3), e108170. https://doi.org/10.5812/ijem.108170 (2021).
Smith, G. D. & Ebrahim, S. “Mendelian randomization”: Can genetic epidemiology contribute to understanding environmental determinants of disease?. Int. J. Epidemiol. 32(1), 1–22. https://doi.org/10.1093/ije/dyg070 (2003).
Bottigliengo, D. et al. A Mendelian randomization study investigating the causal role of inflammation on Parkinson’s disease. Brain 145(10), 3444–3453. https://doi.org/10.1093/brain/awac193 (2022).
Davey Smith, G. & Ebrahim, S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures?. BMJ 330(7499), 1076–1079. https://doi.org/10.1136/bmj.330.7499.1076 (2005).
Taba, N. et al. Mendelian randomization identifies the potential causal impact of dietary patterns on circulating blood metabolites. Front. Genet. 12, 738265. https://doi.org/10.3389/fgene.2021.738265 (2021).
Pirastu, N. et al. Using genetic variation to disentangle the complex relationship between food intake and health outcomes. PLoS Genet. 18(6), e1010162. https://doi.org/10.1371/journal.pgen.1010162 (2022).
Sudlow, C. et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12(3), 1001779. https://doi.org/10.1371/journal.pmed.1001779 (2015).
Bradbury, K. E., Young, H. J., Guo, W. & Key, T. J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 7, e6. https://doi.org/10.1017/jns.2017.66 (2018).
Park, S. et al. Atrial fibrillation and kidney function: A bidirectional Mendelian randomization study. Eur. Heart J. 42(29), 2816–2823. https://doi.org/10.1093/eurheartj/ehab291 (2021).
Bowden, J. et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 45(6), 1961–1974. https://doi.org/10.1093/ije/dyw220 (2016).
Burgess, S. & Thompson, S. G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40(3), 755–764. https://doi.org/10.1093/ije/dyr036 (2011).
Swerdlow, D. I. et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int. J. Epidemiol. 45(5), 1600–1616 (2016).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50(5), 693–698. https://doi.org/10.1038/s41588-018-0099-7 (2018).
Shi, X., Wei, T., Hu, Y., Wang, M. & Tang, Y. The associations between plasma soluble Trem1 and neurological diseases: a Mendelian randomization study. J. Neuroinflammation 19(1), 218. https://doi.org/10.1186/s12974-022-02582-z (2022).
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N. & Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27(8), 1133–1163. https://doi.org/10.1002/sim.3034 (2008).
Larsson, S. C. et al. Type 2 diabetes, glucose, insulin, BMI, and ischemic stroke subtypes: Mendelian randomization study. Neurology 89(5), 454–460 (2017).
Chen, X. et al. Depression and prostate cancer risk: A Mendelian randomization study. Cancer Med. 9(23), 9160–9167. https://doi.org/10.1002/cam4.3493 (2020).
Yavorska, O. O. & Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46(6), 1734–1739. https://doi.org/10.1093/ije/dyx034 (2017).
Rotstein, D. L. et al. A national case-control study investigating demographic and environmental factors associated with NMOSD. Mult. Scler. 29(4–5), 521–529. https://doi.org/10.1177/13524585231151953 (2023).
Hajianfar, H., Mirmossayeb, O., Mollaghasemi, N., Nejad, V. S. & Arab, A. Association between dietary inflammatory index and risk of demyelinating autoimmune diseases. Int. J. Vitam. Nutr. Res. https://doi.org/10.1024/0300-9831/a000754 (2022).
Calder, P. C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 45(5), 1105–1115. https://doi.org/10.1042/bst20160474 (2017).
Singh, J. E. Dietary sources of omega-3 fatty acids versus omega-3 fatty acid supplementation effects on cognition and inflammation. Curr. Nutr. Rep. 9(3), 264–277. https://doi.org/10.1007/s13668-020-00329-x (2020).
Parolini, C. Effects of fish n-3 PUFAs on intestinal microbiota and immune system. Mar. Drugs https://doi.org/10.3390/md17060374 (2019).
Eichholzer, M., Lüthy, J., Gutzwiller, F. & Stähelin, H. B. The role of folate, antioxidant vitamins and other constituents in fruit and vegetables in the prevention of cardiovascular disease: The epidemiological evidence. Int. J. Vitam. Nutr. Res. 71(1), 5–17. https://doi.org/10.1024/0300-9831.71.1.5 (2001).
Kwok, C. S. et al. Dietary components and risk of cardiovascular disease and all-cause mortality: A review of evidence from meta-analyses. Eur. J. Prev. Cardiol. 26(13), 1415–1429 (2019).
Møller, P. et al. Fish and salad consumption are inversely associated with levels of oxidatively damaged DNA in a Danish adult cohort. Mutat. Res. Genet. Toxicol. Environ. Mutagen 843, 66–72. https://doi.org/10.1016/j.mrgentox.2018.11.003 (2019).
Rezaeimanesh, N., Jahromi, S. R., Sahraian, M. A., Rafiee, P. & Moghadasi, A. N. The association between dietary total antioxidant capacity and NMO-IgG seropositivity in patients with neuromyelitis optica spectrum disorder. Clin. Neurol. Neurosurg. 209, 106903. https://doi.org/10.1016/j.clineuro.2021.106903 (2021).
Rezaeimanesh, N., Jahromi, S.R., Moghadasi, A.N., Rafiee, P., Sahraian, M.A.J.N. & Science F., Dietary total antioxidant capacity and neuromyelitis optica spectrum disorder susceptibility. 2019. ahead-of-print(ahead-of-print).
Chen, Y. et al. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 53, 12781 (2020).
Hu, F. B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 13(1), 3–9. https://doi.org/10.1097/00041433-200202000-00002 (2002).
Funding
The study was supported by the Science and Technology Planning Project of Jilin Province (No. 20180520110JH), Basic Research of Free Exploration—Natural Science Foundation of Jilin Province (Surface Project (Medical Science field)) (No. YDZJ202301ZYTS028) and Outstanding Young Teacher Training Program of Jilin University.
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to the study. S.W. drafted the manuscript and contributed to its editing and revision. L.P., W.M., and M.X. downloaded the datasets and conducted the bioinformatic analysis. X.Z. and H.Z. performed the analysis. R.W. and Y.S. contributed to the figures and tables. M.Z. edited the manuscript. All the authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Pan, L., Wu, R. et al. Oily fish and raw vegetable consumption can decrease the risk of AQP4-positive neuromyelitis optica spectrum disorders: a Mendelian-randomization study. Sci Rep 13, 9372 (2023). https://doi.org/10.1038/s41598-023-36372-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36372-1
This article is cited by
-
Sex hormones and neuromyelitis optica spectrum disorder: a bidirectional Mendelian randomization study
Neurological Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.