Abstract
Deep ocean water (DOW) exerts positive effects on the growth of marine organisms, suggesting the presence of unknown component(s) that facilitate their aquaculture. We observed that DOW suppressed plasma cortisol (i.e., a stress marker) concentration in Japanese flounder (Paralichthys olivaceus) reared under high-density condition. RNA-sequencing analysis of flounder brains showed that when compared to surface seawater (SSW)-reared fish, DOW-reared fish had lower expression of hypothalamic (i.e., corticotropin-releasing hormone) and pituitary (i.e., proopiomelanocortin, including adrenocorticotropic hormone) hormone-encoding genes. Moreover, DOW-mediated regulation of gene expression was linked to decreased blood cortisol concentration in DOW-reared fish. Our results indicate that DOW activated osteoblasts in fish scales and facilitated the production of Calcitonin, a hypocalcemic hormone that acts as an analgesic. We then provide evidence that the Calcitonin produced is involved in the regulatory network of genes controlling cortisol secretion. In addition, the indole component kynurenine was identified as the component responsible for osteoblast activation in DOW. Furthermore, kynurenine increased plasma Calcitonin concentrations in flounders reared under high-density condition, while it decreased plasma cortisol concentration. Taken together, we propose that kynurenine in DOW exerts a cortisol-reducing effect in flounders by facilitating Calcitonin production by osteoblasts in the scales.
Similar content being viewed by others
Introduction
Farmed fish are often reared at high densities, which stresses the fish and compromises their welfare1,2. Rearing density influences animal physiology, and increasing density elevates cortisol concentrations in the organisms1,3. Cortisol is a stress hormone released into the blood from inter-renal cells of the head kidney3. Cortisol synthesis and secretion are regulated by the hypothalamus–pituitary–inter-renal axis, which is involved in the stress response, making cortisol a suitable stress marker2,3. In fish, cortisol is an essential hormone that regulates several metabolic activities in the liver and muscles4,5,6. In marine fish, cortisol is also involved in mineral metabolism and osmoregulation7,8,9. However, excess cortisol can induce skeletal muscle atrophy, compromise immune response, and increase energy loss in fish3,6,10. Therefore, healthy and stress-free rearing of fish is necessary in fish farming.
Deep ocean water (DOW) is the water found 200 m below the Earth's ocean surface that has three major characteristics, low temperature (approximately 5–9 °C), rich inorganic nutrients (nitrogen, phosphorus, and silicate), and clean water (minimal to no bacterial activity and less phytoplankton photosynthesis)11,12. The growth of seaweeds13,14 and shrimp15 is reported to be improved by rearing them in DOW compared with the growth of rearing them in surface seawater (SSW). For example, the weight gain rate of the brown alga Sargassum fusiforme in DOW is higher than that in SSW14. Similar to S. fusiforme, juvenile sporophytes of Eisenia arborea and Eisenia cava grow faster in DOW than in SSW13. The deep-sea pelagic shrimp Sergia lucens grow for 185 and 17 days in DOW and SSW, respectively15. Therefore, DOW has some physiologically significant effects on aquatic organisms.
The health effects of mineral ions found in DOW on humans have been studied11,16,17. In addition to minerals, the DOW has been reported to contain organic compounds, which have gained attention. For example, a diterpene, sandaracopimarinol, produced by the Japanese cedar (Cryptomeria japonica) has been detected in the DOW (687 m) of Suruga Bay, Japan18. This finding suggests that terrestrial organic compounds are carried through rivers and deposited in DOW. These compounds may be present in DOW, either from terrestrial sources or from discharges of fish and other aquatic organisms. This study focused on indole compounds, which are ubiquitous in various animals and plants and have a bioactivity of stress-reducing effect19,20,21,22,23,24,25. For example, tryptophan supplementation in rainbow trout (Oncorhynchus mykiss), a fish that exhibits dominant behavior, suppresses aggressive behavior and reduces plasma cortisol concentration23. In addition, the indole compounds melatonin and serotonin, which are metabolites of tryptophan, are involved in the stress response21,22.
Kynurenine, a metabolite of tryptophan, has immune and inflammatory responses and is involved in stress responses in the brain24,25. Kynurenine is generally produced in response to stress and inflammation. The conversion of tryptophan to kynurenine requires an enzyme known as indoleamine 2,3-dioxygenase (IDO)26. IDO is expressed mainly in immune and neuronal cells and is induced by cortisol27, thereby linking the cortisol and kynurenine pathways. However, whether kynurenine is involved in the regulation of cortisol secretion remains unknown.
We compared the plasma cortisol, mineral, and component concentrations as well as gene expression profiles of brains between the Japanese flounders Paralichthys olivaceus reared under high-density condition in either SSW or DOW. The comparison indicated that DOW suppresses plasma cortisol concentration in the flounder reared under high-density condition and further that kynurenine, an indole compound presented in DOW but not in SSW, is involved in the cortisol-reducing effects of DOW. In addition, we provided evidence that kynurenine activates osteoblasts in the scales to produce Calcitonin, which is then transported to the brain and suppresses the cortisol secretion.
Results
Cortisol concentrations in flounders reared under density stress in SSW or DOW for 5 and 10 days
Plasma cortisol concentration was measured in flounders that had been transferred from a large to a small aquarium and reared under 6.25 times density stress in either SSW or DOW (Fig. 1A). Plasma cortisol concentrations in flounders reared in SSW increased significantly (P < 0.01; Fig. 1B), while that of flounders reared in DOW did not (P = 0.88; Fig. 1C).
Changes in the plasma cortisol concentrations in flounder at 5 and 10 days after rearing with surface seawater (SSW) or deep ocean water (DOW). (A) Schematic of experimental setup for Fig. 1B and C. Fish were kept under low density condition with SSW, then transferred to the high density condition with SSW (B) or DOW (C). Blood analytical samples were prepared from fish at the indicated points after the transfer to the high density condition. Black and white bars represent low density and high density conditions, respectively. Flounder in SSW and DOW, n = 7.
Changes in plasma components of flounders reared under density stress with SSW or DOW
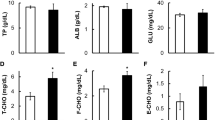
The total protein (TP), albumin (ALB), and urea nitrogen (UN) concentrations in the plasma of flounders at 10 days after rearing in SSW or DOW showed no significant differences (Fig. 2A). Similarly, the concentrations of plasma Na+, K+, and Cl− ions in flounders reared in SSW and DOW did not significantly differ from one another (Fig. 2B). In contrast, the plasma Ca2+ concentrations in flounders reared in DOW was significantly lower than that of flounders reared in SSW (Fig. 3A), although Ca2+ concentrations in SSW and DOW did not differ (Table S1). Furthermore, plasma Calcitonin concentrations of flounders reared in DOW were significantly higher than those of flounders reared in SSW (Fig. 3B).
RNA-sequencing analysis of the brain and skin of flounders reared under density stress in SSW or DOW
RNA-sequencing analysis showed lower expression levels of corticotropin-releasing hormone (crh)28 and pro-opiomelanocortin (pomc)29 in the brains of flounders reared in DOW compared with those in the brains of flounders reared in SSW (Table S2). Genes related to the analgesic action (Table S3) were extracted together with crh, pomc, and calcitonin receptor in the brain of flounders and further analyzed using Ingenuity Pathway Analysis tools. The results of pathway analysis are shown in Fig. 4, and the data are shown in Table S3. The genes involved in analgesia formed a network with pomc, crh, and the calcitonin receptor (calcr in Fig. 4).
Gene network analysis of flounder brain: Analysis of Calcitonin's analgesic action and stress response. Differentially expressed genes in the brain of flounder reared with DOW were compared to those in the brain of flounder reared with SSW using the Ingenuity Pathway Analysis tools. The network is represented graphically with nodes (genes) and edges (the biological associations between the nodes). bambi: bmp and activin membrane bound inhibitor, calcr: calcitonin receptor, cartpt: cart prepropeptide, chrm2: cholinergic receptor muscarinic 2, crh: corticotropin releasing hormone, Erk1/2: Extracellular signal-regulated kinase, ghr: growth hormone receptor, gper1: G protein-coupled estrogen receptor 1, grk3: G protein-coupled receptor kinase 3, grk5: G protein-coupled receptor kinase 5, grm1: glutamate metabotropic receptor 1, grm5: glutamate metabotropic receptor 5, nos1: nitric oxide synthase 1, oprd1: opioid receptor delta 1, pomc: proopiomelanocortin, trpv1: transient receptor potential cation channel subfamily V member 1.
Direct effects of SSW and DOW on osteoblasts of goldfish scales
RNA-sequencing analysis of genes expressed in the epidermis, including the scales of flounder, revealed elevated expression levels of calcitonin mRNA (Table S4). Therefore, we investigated the effects of DOW and SSW on the osteoblasts of flounder scales by adding DOW or SSW to the culture medium at a rate of 20% followed by incubation for 24 h. We observed higher osteoblast activity in goldfish (Carassius auratus) scales cultured with DOW compared with those cultured with SSW in the medium (Fig. 5A). mRNA expression levels of the osteoblastic marker (dlx5, Fig. 5B; col1a1, Fig. 5C) were also higher in fish scales cultured with DOW. For example, the expression level of dlx5 was significantly (P < 0.05) higher in fish scales cultured with DOW compared with that in fish scales cultured with SSW (Fig. 5B). Furthermore, calcitonin mRNA expression levels in fish scales treated with DOW were significantly higher than those in fish scales treated with SSW in the medium (Fig. 5D). The data of the increased expression of calcitonin, dlx5, and col1a1 mRNA of flounders reared in DOW compared with those of flounders in SSW (Table S4) were consistent with the data obtained from goldfish scales.
Indole compounds in SSW and DOW
We reported that an indole compound melatonin promoted Calcitonin secretion by scale osteoblasts30. Since there is no difference in trace minerals between DOW and SSW (Table S5), we speculated that an indole compound facilitating Calcitonin secretion exists in DOW but not in SSW. Accordingly, we analyzed for their indole compound concentrations of DOW and SSW (Table 1). The compounds N-acetyl-N-formyl-5-methoxykynuramine, kynuramine, and indole-3-acetic acid were detected in SSW, whereas kynurenine and indole-3-acetic acid were detected in DOW. Among these compounds, kynurenine was found only in DOW.
Stress-reducing effect of kynurenine in flounders
The effect of kynurenine on plasma cortisol concentrations of flounders reared in artificial seawater under density stress was investigated. After 5 days, plasma cortisol concentrations in kynurenine-treated flounders were significantly lower than those in control (Fig. 6A). Furthermore, the plasma Ca2+ concentrations in the kynurenine-treated flounders were significantly lower than that in the control flounders (Fig. 6B), whereas the plasma Calcitonin concentrations in the kynurenine-treated flounders were significantly higher than those in control (Fig. 6C).
Effect of kynurenine on calcitonin mRNA expression in osteoblasts of fish scales
Osteoblastic activity and calcitonin mRNA expression in osteoblasts were studied using the scales by in vitro assay to examine calcitonin mRNA expression in osteoblasts by kynurenine treatments. Figure 7A shows that the activity of osteoblasts increased significantly after incubating fish scales in a medium supplemented with kynurenine (10−10, 10−8, and 10−6 M). Similarly, calcitonin mRNA expression levels increased significantly with the addition of kynurenine (10−8 and 10−6 M) (Fig. 7B).
Discussion
DOW has been known to positively influence the growth of seaweed13,14 and shrimp15. We have identified another positive influence of DOW, namely its cortisol-reducing effect on flounders. Our results provide several lines of evidence that DOW activates osteoblasts in the scales and promotes the production of Calcitonin, which has an important cortisol-reducing effect (Fig. 8). It is known that fish scales possess vascular tissue, including blood vessels31,32. In addition, Calcitonin has been shown to cross the blood–brain barrier in rats33. Given these findings, we propose that the Calcitonin produced by osteoblasts in the scales is transported to the brain via the bloodstream (Fig. 8). We further speculate that Calcitonin regulates the expression of genes involved in the regulation of cortisol secretion in the brain—such as crh and pomc —thereby resulting in lower blood cortisol concentration (Fig. 8).
Our analysis of gene expression profiles in the brains of flounders identified a candidate gene network involved in the calcitonin-dependent suppression of cortisol secretion. In this network, Calcitonin was thought to bind to the Calcitonin receptor and to thereby transduce a signal inhibiting the expression of the crh and pomc genes (Fig. 4). This signal transduction was likely mediated by several molecules, including opioid receptor delta 1 (oprd1), which directly associates with pomc. Opioid receptors are usually G-protein coupled receptors that bind to neurotransmitters and opioids outside the cell and trigger a response via G proteins inside the cell34. Moreover, opioids bound to opioid receptor delta molecules, thereby suppressing the pain response, exerting an analgesic effect35. Thus, the involvement of Oprd1 with the Calcitonin-mediated suppression of cortisol in the brain is consistent with the fact that Calcitonin also exerts an analgesic function in the central modulation of pain perception36,37.
In the current study, kynurenine activated osteoblasts and promoted their production of calcitonin in cultured fish scales (Fig. 7). In addition, it increased the blood Calcitonin concentrations of flounders in vivo in a manner similar to the phenomenon induced by DOW (Fig. 6). Based on these findings, we propose that kynurenine in DOW is a factor responsible for the DOW-induced activation of osteoblasts in the scales and the resulting Calcitonin production (Fig. 8); this therefore explains the cortisol-reducing effect of DOW on flounders. Kynurenine is metabolized to quinolinic acid (QA) via the other metabolites26,38. QA induces the release and inhibits the reuptake of glutamate causing excitotoxicity39, and this is hypothesized to act as a link between chronic stress, depression, and inflammation40. On the other hand, kynurenine is also metabolized to kynurenic acid, which is considered to be neuroprotective41. The current study revealed a novel mechanism in which kynurenine induces Calcitonin production in osteoblasts and increases the expression of analgesia genes in the brain to reduce blood cortisol levels (Fig. 8).
Previous study evaluated effect of stocking density on expression level of a stress marker gene Hsp70 in muscle of rainbow trout (Oncorhynchus mykiss)42 and sea bass (Dicentrarchus labrax, L.)43. In rainbow trout, the Hsp70 expression was found to increase under the high density conditions (24 and 44 kg/m3) compared to that under the low density condition (12 kg/m3). In addition, in sea bass, the Hsp70 expression was found to significantly over-express under the biomass of 100 kg/m3. Myosin is a ubiquitous motor protein and an ideal indicator for growth studies and myosin heavy chain (mhc) mRNA levels are known as a possible marker of trout muscle growth44. On other hand, myostatin (Mstn), is considered as a negative regulator of fish muscle growth45,46. In rainbow trout, expression of mhc decreased significantly under the high density condition (44 kg/m3) compared to that under the low density condition (12 kg/m3), while mstn-1a increased. Therefore, the density (24 kg/m3) we used is reasonable. In addition, in rainbow trout, a species with typically dominant behavior, tryptophan supplementation suppresses aggressive behavior and induces lower plasma cortisol concentration compared to unsupplemented rainbow trout23. This report is consistent with our findings, and can be attributed to the metabolism of tryptophan to kynurenine24,25, which thereafter exerts a stress-reducing effect.
Next, we also found that the plasma Ca2+ concentration was significantly lower in fish reared in DOW than in the fish reared in surface seawater (SSW), although these groups did not differ in their concentrations of plasma Na+, K+, or Cl− after ten days (Figs. 2B and 3A). Similarly, we also did not observe significant differences in plasma TP, ALB, and UN concentration between groups (Fig. 2A). The lower plasma Ca2+ levels in fish reared in DOW might be due to Calcitonin eliciting hypocalcemic activity37. In addition, flounders reared in DOW had significantly higher plasma Calcitonin concentration compared to flounders reared in SSW (Fig. 3B). Calcitonin is a hypocalcemic hormone that regulates plasma Ca2+ concentration to a constant level of approximately 10 mg/dL in teleosts and mammals37. Calcitonin is known to suppress osteoclastic activity in fish47 and mammals37 to reduce plasma Ca2+ concentration.
Aquaculture stresses fish because they are typically reared at high densities. Under these conditions, measures to reduce stress should be considered to improve fish welfare1,2. Furthermore, because excess cortisol induces skeletal muscle atrophy, compromises immune response, and increases energy waste in fish3,6,10, methods without stressing them are necessary. Finally, based on our results, the use of kynurenine or DOW for rearing fish may solve problems related to stress, and thereby promote fish welfare.
Conclusion
In this study we demonstrated that DOW reduces plasma cortisol (i.e., a stress marker) concentration in flounders reared under high-density condition. The cortisol-reducing action of DOW is attributed to Calcitonin produced by osteoblasts in the scales. In particular, we propose that Calcitonin produced in the scales is transported to the brain, where it contributes to the expression of genes involved in cortisol secretion. In addition, the indole component kynurenine in DOW was found to be responsible for osteoblast activation and the resulting production of Calcitonin, contributing to suppression of cortisol secretion. Overall, our results suggest a novel biological function of kynurenine, which can be used to reduce stress in fish reared in aquaculture environments.
Methods
Statements on the ethical treatment of animals
This study was carried out in strict accordance with the recommendations in the ethical guidelines of Kanazawa University. All experimental protocols in this study were approved by the Animal Welfare Committee of Kanazawa University. In addition, all experimental protocols in this study were in strict accordance with the ARRIVE guidelines 2.048. We made maximum efforts to avoid causing pain and distress to experimental animals. When biological samples were collected from the animals, pain was minimized by anesthesia preoperatively. Particular attention was paid to the use of anesthesia to alleviate discomfort.
Animals
Flounders (P. olivaceus) were purchased from Marinetech Co. Ltd. (Aichi, Japan) and they were cultivated. Goldfish (C. auratus) purchased from Higashikawa Fish Farm (Yamatokoriyama, Japan) were used to examine the effects of the bioactive substance in DOW on fish scales. The DOW was pumped from 320 m at a facility (Aquas Noto, Ishikawa prefecture, Japan). After the aeration of DOW in an aquarium, the dissolved oxygen was maintained at approximately 7 mg/L. SSW was pumped from Tsukumo Bay (Noto Peninsula, Ishikawa Prefecture), where our marine laboratory is located, and was stored in an aquarium with bubbling air. The dissolved oxygen of SSW was also maintained at approximately 7 mg/L. A system of DOW and SSW rearing was used in which wastes were filtered out in a filtration tank. Each of fish was reared at a density of 24 kg/m3 in a single aquarium (60 cm × 25 cm × 30 cm). Fish were collected sequentially from the same tank.
Flounders acclimated in SSW at 20 °C for 1 week (mean density: 241 g/62.5 L; 200 cm × 100 cm × 65 cm) were used. Meanwhile, goldfish acclimated in freshwater at 25 °C for approximately 1 week were used for in vitro experiments. To prepare the biological samples for analyses, the fish were anesthetized with a 0.04% 2-phenoxyethanol (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) or MS-222 (Sigma-Aldrich, Inc. St. Louis, MO, USA) and then were decapitated. MS-222 was neutralized with sodium bicarbonate.
Rearing of marine teleost in DOW or SSW
The density during acclimation was 3.85 kg/m3. After acclimation, flounders were reared in aquaria (mean density: 241 g/62.5 L; 200 cm × 100 cm × 65 cm) for 1 week. The flounders were grouped in separate aquaria containing either SSW or DOW (n = 7) at mean densities of 24 g/L, which was 6.25 times the stress density. The value for density stress threshold was defined based on the previous study42. Fish were reared with SSW or DOW for 10 days at 20 ± 1 °C under a 12 h:12 h light–dark cycle. Each day, 1/10th of the volume of water in each aquarium was replaced. The fish were fed with artificial feed (4% body weight; Otohime, Marubeni Nisshin Feed Co., Ltd., Tokyo, Japan) once every morning. Prior to rearing in the small aquaria, the fish were anesthetized with a 0.04% 2-phenoxyethanol (FUJIFILM Wako Pure Chemical Corporation) to avoid causing pain, and their blood was sampled from caudal vessels using a heparinized syringe. The same blood sampling procedure was followed at 5 and 10 days after rearing. The collected blood was transferred into a 1.5-mL tube and then centrifuged at 5200 × g for 5 min. The separated plasma was immediately frozen and kept at − 80 °C until use.
Measurement of plasma cortisol and Calcitonin
A five-fold volume of diethyl ether was added to the plasma sample, and the ether layer was evaporated with nitrogen gas. The dried sample was dissolved in assay buffer (50 mM H3BO3, 0.2% bovine serum albumin, 0.01% thimerosal, pH 7.8), and the plasma cortisol concentration was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Cosmo Bio Co. Ltd., Tokyo, Japan).
The competitive ELISA procedure for Calcitonin was performed as described by Suzuki49. In separate glass tubes, 250 μL of diluted antibody (400,000 times) with a diluting solution (10 mM phosphate-buffered solution containing 0.1% bovine serum albumin and 0.1% NaN3, pH 7.4) was pre-incubated with the same volume of serially diluted synthetic salmon Calcitonin (6.25, 12.5, 25, 50, 100, 200, 400, and 800 pg/mL) or plasma samples diluted three times with a diluting solution for 3 days at 4 °C. Thereafter, salmon Calcitonin-coated plates were washed four times with a washing buffer solution (10 mM phosphate-buffered solution containing 0.05% Tween 20). Aliquots (100 μL) of the pre-incubated synthetic salmon Calcitonin or plasma samples were transferred to the coated plates and incubated at 4 °C for 24 h. After washing four times with the washing buffer solution, color development was performed. Eight milligrams of o-phenylenediamine dihydrochloride was added to 12 mL 0.1 M citric acid and 0.2 M Na2HPO4 solution (pH 4.5) containing 2.4 μL of hydrogen peroxide (30% solution, FUJIFILM Wako Pure Chemical Corporation). Aliquots of 100 μL of the solution were added and incubated in wells with constant agitation at room temperature for 5 min. After incubation, the reaction was stopped, followed by the addition of 50 μL 3 N sulfuric acid. The optical density of the reacted triplicate samples in plates was measured at 492 nm using a microplate reader (MTP-500, Corona Electric Co. Ltd., Tokyo, Japan). After color development, a regression curve for synthetic salmon Calcitonin was drawn using Excel (Microsoft Office, Microsoft Corporation, San Francisco, CA, USA) to determine the concentration of the sample.
The specificity of the antibody was checked using peptide hormones 1–34 bovine PARATHYROID HORMONE and human CALCITONIN GENE-RELATED PEPTIDE49.
Measurement of plasma components and seawater (DOW and SSW)
Ten days after rearing flounders in SSW or DOW, their plasma samples were analyzed by Oriental Yeast Co., Ltd. (Tokyo, Japan), where plasma TP, ALB and UN were measured using L-type Wako TP, L-type Wako ALB-BCP, and L-type Wako UN-V kits (FUJIFILM Wako Pure Chemical Corporation), respectively. In addition, plasma levels of Na+, Cl−, and K+ were measured by an ion electrode method with a Hitachi 7180 automatic analyzer (Hitachi High Technologies Corporation, Tokyo, Japan). The plasma Ca2+ concentration was determined using the Ca II assay kit (Shino-Test Corporation, Tokyo, Japan). Mineral components, such as Na+, Cl−, K+, and Ca2+, in DOW and SSW were measured as described above.
Trace minerals in flounder plasma (pooled sample for two individuals) were also analyzed. After the removal of protein by 10% trifluoroacetic acid and centrifugation, the supernatant was passed through a 0.22-μm filter. The filtrate was used to measure the trace minerals in their plasma by inductively coupled plasma mass spectrometry (X7, Thermo Inc., Waltham, MA, USA)50.
Analysis of mRNA expression in the brain and skin after rearing flounders in SSW or DOW
Flounders anesthetized with 0.04% 2-phenoxyethanol and reared in either SSW (n = 10) or DOW (n = 10) were dissected after decapitation, and the brain and skin samples were collected. The separated samples were placed in RNAlater (Sigma-Aldrich Inc., St. Louis, MO, USA) and frozen at − 80 °C for mRNA analysis.
Total RNA was extracted using a NucleoSpin RNA II kit (Takara Bio Inc., Otsu, Japan) following the manufacturer’s instructions. Genomic DNA was removed from the extract using an RNase-Free DNase Set (Takara Bio Inc.). Total RNA extracted from 10 individuals was diluted to 2 nM, mixed in equal amounts (10 µL each), and used as one sample for RNA-sequencing. RNA-seq libraries for directional paired-end reads (100-bp paired-end) were constructed from flounder mRNAs using TruSeq RNA Sample Prep Kit v2 and sequenced on a HiSeq 2500 platform (Illumina, San Diego, CA, USA) using cluster generation. Quality control on raw reads was performed using FastQC ( http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ )51, and high-quality reads (> Q20 and > 36 bp) without adaptors were extracted using Trimmomatic52. Further, a transcriptome was assembled de novo with the filtered reads using Trinity with default parameters to establish the reference sequence of flounder transcripts53. Sequence reads were pseudomapped to the reference sequence using kallisto with default parameters; the pseudoalignment rates were approximately 92%54. The assembled sequences were annotated using Blastx and Blastn against the NCBI database. The mapped and annotated read counts were normalized using the Transcripts Per Million reads method by kallisto. Gene ontology, including biological processes, molecular functions, and gene networks of sequence data (accession no. DRA015483) were analyzed using Ingenuity Pathway Analysis tools (Qiagen, Venlo, Netherlands)55.
Direct effects of SSW and DOW on the osteoblasts of fish scales
The direct effects of SSW and DOW on fish scales were analyzed using an in vitro assay system consisting of goldfish scales47,56. Teleost scales regenerate after being removed. The osteoblastic activity in regenerating scales is considerably higher than that in normal scales57,58,59. Therefore, we used regenerating scales to examine the influence of SSW and DOW on osteoblasts.
Goldfish (n = 24) were anesthetized with MS-222 (Sigma-Aldrich, Inc.) solution neutralized with sodium bicarbonate, and a total of 32 scales were collected from both sides of each goldfish. The scales comprised eight from each side of one row of scales above and below the lateral line. Ten days after the removal of the scales, the goldfish were anesthetized again, and the regenerating scales were removed and used to investigate the influence of SSW and DOW on osteoblastic enzymatic activity and mRNA expression. These regenerating scales were incubated at 15 °C for 24 h in Leibovitz’s L-15 Medium (Thermo Fisher Scientific Inc., Grand Island, NY, USA) containing 20% of either SSW or DOW and 1% antibiotic mixture (10,000 units/mL of penicillin and 10,000 µg/mL of streptomycin; Thermo Fisher Scientific, Inc.). After incubation, some scales were analyzed for alkaline phosphatase, which is an osteoblastic marker47,56,57, while the other scales were placed in RNAlater (Sigma-Aldrich Inc.) and frozen at − 80 °C for mRNA analysis.
Total RNAs were extracted from goldfish scales using NucleoSpin RNA II kit (Takara Bio Inc.). Genomic DNA was removed from the extract using RNase-Free DNase Set (Takara Bio Inc.). Complementary DNA was synthesized using a PrimeScript II 1st strand cDNA Synthesis Kit (Takara Bio Inc.). The gene-specific primers for amplifying dlx5, col1a1, and calcitonin are listed in Table S6. The elongation factor1α (ef1α) cDNA was amplified as a housekeeping gene using the primer set listed in Table S6. PCR amplifications were performed using a real-time PCR apparatus (Mx3000p; Agilent Technologies, Santa Clara, CA, USA). The mRNA levels of dlx5, col1a1, and calcitonin were normalized to the ef1α mRNA level60.
Analysis of indole compounds in SSW and DOW
DOW and SSW (each 5 L) were adsorbed on an ultrafiltration system (3 M 2215 C18 Empore Extraction Disks, GL Sciences Inc., Tokyo, Japan) with a quantity of ultrafiltrated water and eluted with 10 mL of ethanol and 30 mL of benzene. The eluted solution was dried up by nitrogen gas and analyzed by liquid chromatography–tandem mass spectrometry (LC–MS/MS, LCMS-8050; Shimadzu Co., Kyoto, Japan). Each of the dried samples was resuspended in 100 μL of Milli-Q water and centrifuged. The supernatant was passed through a 0.22-µm filter, and the filtrate was analyzed by LC–MS/MS. The indole compounds in SSW or DOW were simultaneously analyzed as described 61.
Stress-reducing effect of kynurenine in flounder
Flounders were reared in artificial sea water (SEALIFE, Marinetech Co. Ltd.) with or without kynurenine (10−6 M; FUJIFILM Wako Pure Chemical Corporation) at a stress density described above. Flounders were kept directly in the aquarium without a filtration tank. The water was changed daily. Five days after rearing, blood samples were collected from the caudal vessels of anesthetized (0.04% 2-phenoxyethanol) fish using a heparinized syringe. The collected blood was transferred into a 1.5 mL tube, and the tube was centrifuged at 5200 × g for 5 min. The separated plasma was immediately frozen and stored at − 80 °C until use. The plasma Cortisol, Ca2+, and Calcitonin concentrations were measured as described above.
Effect of kynurenine on the osteoblasts of fish scales
The effects of kynurenine on the osteoblasts of fish scales were investigated using an in vitro assay system with regenerating goldfish scales. The regenerating scales were prepared as described above, and placed in Leibovitz’s L-15 Medium (Thermo Fisher Scientific Inc.) containing 10−12, 10−10, 10−8, and 10−6 M kynurenine. The osteoblasts’ activities of the scales in the same individual were similar in goldfish58. Scales for the experiment with each dose were obtained from the same fish. The half of collected scales were then used for the control experiment and the other half for the kynurenine-treated experiment. The scales were incubated at 15 °C for 24 h, and the osteoblastic activity was measured as described above.
The calcitonin mRNA expression levels of the control and kynurenine-treated groups were compared within one individual goldfish and investigated by incubating regenerating scales in a medium supplemented with 10−10, 10−8, and 10−6 M kynurenine at 15 °C for 24 h. After incubation, the scales were placed in RNAlater (Sigma-Aldrich Inc.) and frozen at − 80 °C for mRNA analysis.
Total RNA was extracted using NucleoSpin RNA II kit (Takara Bio Inc.) following the manufacturer’s instructions. Genomic DNA was removed from the extract using an RNase-Free DNase Set (Takara Bio Inc.). Complementary DNA was synthesized using a PrimeScript II 1st strand cDNA Synthesis Kit (Takara Bio Inc.). The expression of calcitonin mRNA was examined using real-time PCR methods as described above.
Statistical analysis
All results were expressed as the mean ± standard error. Based on the Shapiro–Wilk test, cortisol levels at each time point did not follow a normal distribution. Therefore, cortisol concentration was compared by a nonparametric Friedman test in R62. The statistical significance between the control and experimental groups was assessed using an independent sample t-test or paired t-test. In all cases, the selected significance level was P < 0.05.
Data availability
The raw sequence reads were deposited at the DNA Data Bank of Japan (DDBJ) under the DDBJ Sequence Read Archive (DRA) accession no. DRA015483 (https://ddbj.nig.ac.jp/resource/sra-submission/DRA015483).
References
Conte, F. S. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 86, 205–223. https://doi.org/10.1016/j.applanim.2004.02.003 (2004).
Raposo de Magalhães, C. et al. Protein changes as robust signatures of fish chronic stress: A proteomics approach to fish welfare research. BMC Genomics 21, 309. https://doi.org/10.1186/s12864-020-6728-4 (2020).
Ellis, T. et al. Cortisol and finfish welfare. Fish Physiol. Biochem. 38, 163–188. https://doi.org/10.1007/s10695-011-9568-y (2012).
Vijayan, M. M., Pereira, C. & Moon, T. W. Hormonal stimulation of hepatocyte metabolism in rainbow trout following an acute handling stress. Comp. Biochem. Physiol. C. 108, 321–329. https://doi.org/10.1016/0742-8413(94)00024-5 (1994).
Tripathi, G. & Verma, P. Pathway-specific response to cortisol in the metabolism of catfish. Comp. Biochem. Physiol. B. 136, 463–471. https://doi.org/10.1016/S1096-4959(03)00249-5 (2003).
Lawrence, M. J. et al. Cortisol modulates metabolism and energy mobilization in wild-caught pumpkinseed (Lepomis gibbosus). Fish Physiol. Biochem. 45, 1813–1828. https://doi.org/10.1007/s10695-019-00680-z (2019).
McLeese, J. M. et al. Seasonal changes in osmoregulation, cortisol, and cortisol receptor activity in the gills of parr/smolt of steelhead trout and steelhead-rainbow trout hybrids Oncorhynchus mykiss. Gen. Comp. Endocrinol. 93, 103–113. https://doi.org/10.1006/gcen.1994.1012 (1994).
Tsui, W. C., Chen, J. C. & Cheng, S. Y. The effects of a sudden salinity change on cortisol, glucose, lactate, and osmolality levels in grouper Epinephelus malabaricus. Fish Physiol. Biochem. 38, 1323–1329. https://doi.org/10.1007/s10695-012-9620-6 (2012).
Hu, Y. C., Chu, K. F., Hwang, L. Y. & Lee, T. H. Cortisol regulation of Na+, K+-ATPase β1 subunit transcription via the pre-receptor 11β-hydroxysteroid dehydrogenase 1-like (11β-Hsd1L) in gills of hypothermal freshwater milkfish, Chanos chanos. J. Steroid Biochem. Mol. Biol. 192, 105381. https://doi.org/10.1016/j.jsbmb.2019.105381 (2019).
Aedo, J. E. et al. mRNA-seq reveals skeletal muscle atrophy in response to handling stress in a marine teleost, the red cusk-eel (Genypterus chilensis). BMC Genomics 16, 1024. https://doi.org/10.1186/s12864-015-2232-7 (2015).
Mohd Nani, S. Z. et al. Potential health benefits of deep sea water: A review. Evid. Complement. Alternat. Med. 2016, 6520475. https://doi.org/10.1155/2016/6520475 (2016).
Hunt, J. D. et al. Deep seawater cooling and desalination: Combining seawater air conditioning and desalination. Sustain. Cities Soc. 74, 103257. https://doi.org/10.1016/j.scs.2021.103257 (2021).
Nimura, K., Omamoto, K. & Takase, S. Effect of flow rate on the growth of juvenile Eisenia arborea and Ecklonia cava (Laminariales, Phaeophyceae) cultured in Suruga Bay deep and surface seawaters. Deep Ocean Water Res. 7, 7–11. https://doi.org/10.11174/dowas2000.7.2_7 (2006).
Hara, Y. et al. Growth and arsenic content of “Hijiki” Sargassum fusiforme cultivated with deep seawater from Suruga Bay. Deep Ocean Water Res. 10, 19–26. https://doi.org/10.11174/dowas.10.19 (2009).
Okamoto, K. Comparison of survival and growth in adult pelagic shrimp Sergia lucens between deep and surface seawater cultures. Deep Ocean Water Res. 7, 1–7. https://doi.org/10.11174/dowas2000.7.1_1 (2006).
Keen, D. A., Constantopoulos, E. & Konhilas, J. P. The impact of post-exercise hydration with deep-ocean mineral water on rehydration and exercise performance. J. Int. Soc. Sports Nutr. 13, 17. https://doi.org/10.1186/s12970-016-0129-8 (2016).
Harris, P. R. et al. Fluid type influences acute hydration and muscle performance recovery in human subjects. J. Int. Soc. Sports Nutr. 16, 15. https://doi.org/10.1186/s12970-019-0282-y (2019).
Ishikawa, H. et al. A diterpene, sandaracopimarinol, produced by Japanese cedar and found from the deep seawater pumped up from the Suruga bay. Deep Ocean Water Res. 6, 47–50. https://doi.org/10.11174/DOWAS2000.6.1_47 (2005).
Hay-Schmidt, A. The evolution of the serotonergic nervous system. Proc. Biol. Sci. B. 267, 1071–1079. https://doi.org/10.1098/rspb.2000.1111 (2000).
Lee, J. H., Wood, T. K. & Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 23, 707–718. https://doi.org/10.1016/j.tim.2015.08.001 (2015).
Field, T. et al. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 115, 1397–1413. https://doi.org/10.1080/00207450590956459 (2005).
Chojnowska, S. et al. Salivary biomarkers of stress, anxiety and depression. J. Clin. Med. 10, 517. https://doi.org/10.3390/jcm10030517 (2021).
Lepage, O., Larson, E. T., Mayer, I. & Winberg, S. Serotonin, but not melatonin, plays a role in shaping dominant-subordinate relationships and aggression in rainbow trout. Horm. Behav. 48, 233–242. https://doi.org/10.1016/j.yhbeh.2005.02.012 (2005).
Kennedy, P. J., Cryan, J. F., Dinan, T. G. & Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112(Pt B), 399–412. https://doi.org/10.1016/j.neuropharm.2016.07.002 (2017).
Martin, K. S., Azzolini, M. & Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 318, 818–830. https://doi.org/10.1152/ajpcell.00580.2019 (2020).
Johnston, J. N. et al. Inflammation, stress and depression: An exploration of ketamine’s therapeutic profile. Drug Discov. Today 28, 103518. https://doi.org/10.1016/j.drudis.2023.103518 (2023).
O’Connor, J. C. et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiat. 14, 511–522. https://doi.org/10.1038/sj.mp.4002148 (2009).
Lee, J. H. & Torpy, D. J. Adrenal insufficiency in pregnancy: Physiology, diagnosis, management and areas for future research. Rev. Endocr. Metab. Disord. 24, 57–69. https://doi.org/10.1007/s11154-022-09745-6 (2023).
Tritos, N. A. & Miller, K. K. Diagnosis and management of pituitary adenomas: A review. JAMA 329, 1386–1398. https://doi.org/10.1001/jama.2023.5444 (2023).
Ikegame, M. et al. Melatonin is a potential drug for the prevention of bone loss during space flight. J. Pineal Res. 67, e12594. https://doi.org/10.1111/jpi.12594 (2019).
Suzuki, N., Kitamura, K. I. & Hattori, A. Fish scale is a suitable model for analyzing determinants of skeletal fragility in type 2 diabetes. Endocrine 54, 575–577. https://doi.org/10.1007/s12020-016-1153-9 (2016).
Hirayama, J. et al. Physiological consequences of space flight, including abnormal bone metabolism, space radiation injury, and circadian clock dysregulation: Implications of melatonin use and regulation as a countermeasure. J. Pineal Res. 74, e12834. https://doi.org/10.1111/jpi.12834 (2023).
Kalafateli, A. L. et al. An amylin and calcitonin receptor agonist modulates alcohol behaviors by acting on reward-related areas in the brain. Prog. Neurobiol. 200, 101969. https://doi.org/10.1016/j.pneurobio.2020.101969 (2021).
Lamberts, J. T. & Traynor, J. R. Opioid receptor interacting proteins and the control of opioid signaling. Curr. Pharm. Des. 19, 7333–7347. https://doi.org/10.2174/138161281942140105160625 (2013).
Machelska, H. & Celik, M. Ö. Advances in achieving opioid analgesia without side effects. Front. Pharmacol. 9, 1388. https://doi.org/10.3389/fphar.2018.01388 (2018).
Gennari, C. Analgesic effect of calcitonin in osteoporosis. Bone 30(Suppl 1), 67–70. https://doi.org/10.1016/S8756-3282(02)00713-5 (2002).
Suzuki, N. Calcitonin. In Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research 2nd edn (eds Ando, H. et al.) 405–408 (Academic Press, 2021).
Lugo-Huitrón, R. et al. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013, 104024. https://doi.org/10.1155/2013/104024 (2013).
Schwarcz, R. Kynurenines and glutamate: Multiple links and therapeutic implications. Adv. Pharmacol. 76, 13–37. https://doi.org/10.1016/bs.apha.2016.01.005 (2016).
Dantzer, R. & Walker, A. K. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression?. J. Neural. Transm. 121, 925–932. https://doi.org/10.1007/s00702-014-1187-1 (2014).
Foster, A. C., Vezzani, A., French, E. D. & Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 48, 273–278. https://doi.org/10.1016/0304-3940(84)90050-8 (1984).
Zahedi, S. et al. Efect of stocking density on growth performance, plasma biochemistry and muscle gene expression in rainbow trout (Oncorhynchus mykiss). Aquaculture 498, 271–278. https://doi.org/10.1016/j.aquaculture.2018.07.044 (2019).
Gornati, R. et al. Rearing density influences the expression of stress-related genes in sea bass (Dicentrarchus labrax, L.). Gene 341, 111–118. https://doi.org/10.1016/j.gene.2004.06.020 (2004).
Overturf, K. & Hardy, R. W. Myosin expression levels in trout muscle: A new method for monitoring specific growth rates for rainbow trout Oncorhynchus mykiss (Walbaum) on varied planes of nutrition. Aquac. Res. 32, 315–322. https://doi.org/10.1046/j.1365-2109.2001.00582.x (2001).
Garikipati, D. K., Gahr, S. A. & Rodgers, B. D. Identification, characterization, and quantitative expression analysis of rainbow trout myostatin-1a and myostatin-1b genes. J. Endocrinol. 190, 879–888. https://doi.org/10.1677/joe.1.06866 (2006).
Gabillard, J. C., Biga, P. R., Rescan, P. Y. & Seiliez, I. Revisiting the paradigm of myostatin in vertebrates: Insights from fishes. Gen. Comp. Endocrinol. 194, 45–54. https://doi.org/10.1016/j.ygcen.2013.08.012 (2013).
Suzuki, N., Suzuki, T. & Kurokawa, T. Suppression of osteoclastic activities by calcitonin in the scales of goldfish (freshwater teleost) and nibbler fish (Seawater teleost). Peptides 21, 115–124. https://doi.org/10.1016/S0196-9781(99)00181-3 (2000).
Percie du Sert, N. et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PloS. Biol. 18, e3000411. https://doi.org/10.1371/journal.pbio.3000411 (2020).
Suzuki, N. Calcitonin-like substance in the plasma of Cyclostomata and its putative role. Comp. Biochem. Physiol. B. 129, 319–326. https://doi.org/10.1016/S1096-4959(01)00338-4 (2001).
Gankhurel, B. et al. Arsenic and uranium contamination of Orog Lake in the valley of Gobi lakes, Mongolia: Field evidence of conservative accumulation of U in an alkaline, closed-basin lake during evaporation. J. Hazard Mater. 436, 129017. https://doi.org/10.1016/j.jhazmat.2022.129017 (2022).
Andrews S. FastQC: A quality control tool for high throughput sequence data (2010). http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. https://doi.org/10.1093/bioinformatics/btu170 (2014).
Haas, B. J. et al. De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat. Protoc. 8, 1494–1512. https://doi.org/10.1038/nprot.2013.084 (2013).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527. https://doi.org/10.1038/nbt.3519 (2016).
Tabuchi, Y. et al. Genetic networks responsive to sodium butyrate in colonic epithelial cells. FEBS Lett. 580, 3035–3041. https://doi.org/10.1016/j.febslet.2006.04.048 (2006).
Suzuki, N. & Hattori, A. Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J. Pineal Res. 33, 253–258. https://doi.org/10.1034/j.1600-079X.2002.02953.x (2002).
Yoshikubo, H. et al. Osteoblastic activity and estrogenic response in the regenerating scale of goldfish, a good model of osteogenesis. Life Sci. 76, 2699–2709. https://doi.org/10.1016/j.lfs.2004.10.063 (2005).
Suzuki, N. et al. Response of osteoblasts and osteoclasts in regenerating scales to gravity loading. Biol. Sci. Space 23, 211–217. https://doi.org/10.2187/bss.23.211 (2009).
Thamamongood, T. A. et al. Expression of osteoblastic and osteoclastic genes during spontaneous regeneration and autotransplantation of goldfish scale: A new tool to study intramembranous bone regeneration. Bone 50, 1240–1249. https://doi.org/10.1016/j.bone.2012.03.021 (2012).
Sato, M. et al. Sodium fluoride influences calcium metabolism resulting from the suppression of osteoclasts in the scales of nibbler fish Girella punctata. Fish. Sci. 83, 543–550. https://doi.org/10.1007/s12562-017-1086-0 (2017).
Iwashita, H. et al. The melatonin metabolite N1-acetyl-5-methoxykynuramine facilitates long-term object memory in young and aging mice. J. Pineal Res. 70, e12703. https://doi.org/10.1111/jpi.12703 (2021).
R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria (2022). https://www.R-project.org/.
Acknowledgements
This study was supported in part by grants to Nobuo Suzuki (Grant-in-Aid for Scientific Research [C] No. 20K06718 by JSPS and Adaptable and Seamless Technology Transfer Program through Target-driven R&D No. JPMJTM19AP by JST), to Hajime Matsubara (Grant-in-Aid for Scientific Research [C] No. 21K05725 by JSPS), and to Kazuki Watanabe (Grant-in-Aid for JSPS Fellows No. 22J01508 by JSPS). This work was partly supported by the cooperative research program of the Institute of Nature and Environmental Technology, Kanazawa University, Accept. Nos. 22009, 22015, 22016, 22017, and 22044, by the Salt Science Research Foundation (No. 2209), and by National University Management Reform Promotion Project (MEXT, Japan).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation (T.I., M.A.R., S.O., K.K., K.H., T.S., R.K.), data collection (J.H., K.W., A.S.), analysis (K.F., Y.K., H.M., A.N., T.N., A.S.), and discussion (A.K.S, Y.F., Y.T., Y.M., A.H. M.A.Y., K.T., N.S.) were performed. The first draft of the manuscript was written by N.S., Y.F., T.I., and J.H. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ikari, T., Furusawa, Y., Tabuchi, Y. et al. Kynurenine promotes Calcitonin secretion and reduces cortisol in the Japanese flounder Paralichthys olivaceus. Sci Rep 13, 8700 (2023). https://doi.org/10.1038/s41598-023-35222-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35222-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.