Abstract
An 8-week trial to examine the impacts of Arthrospira platensis and Chlorella vulgaris on the growth, nutrient aspects, intestinal efficacy, and antioxidants of 75 New Zealand white male rabbits (initial body weight = 665.93 ± 15.18 g). Herein the study was designed in one-way ANOVA to compare the effects of the two algae species with two levels of supplementations in the feeds of New Zealand white rabbits. The rabbits were divided into five groups (n = 15/group), where the first group was allocated as the control group (Ctrl) while the second and third groups received A. platensis at 300 or 500 mg/kg diet (Ap300 or Ap500). The fourth and fifth groups fed C. vulgaris at 300 or 500 mg/kg diet (Ch300 or Ch500). The basal diet rabbits exhibited the lowest values of weight, lipase, protease, and the highest feed conversion ratio, which improved noticeably with algae addition, particularly with Ap500, Ch300, and Ch500. All tested groups showed normal intestinal structure. Amylase potency, hematological indicators, and serum biochemistry revealed non-significant variation except for a higher serum total protein and lower total cholesterol in algal groups. The best GPx existed in groups fed algal diets, while favorable SOD and CAT efficiency occurred at the higher level of Arthrospira and both levels of Chlorella. In conclusion, incorporating Arthrospira or Chlorella in the diet of New Zealand white rabbits improved performance, nutrient utilization, intestinal efficacy, and antioxidants. Arthrospira (Ap500) and Chlorella (Ch300 or Ch500) have almost the same beneficial effect on rabbit performance.
Similar content being viewed by others
Introduction
Rabbits are one of the most profitable agricultural sectors involved in offering high-quality animal products with distinct merits, remarkably rapid growth, and sexual maturity with high fertility as well as a high gain of meat in carcass1. Finding promising tactics to ameliorate organisms' wellbeing and performance is vital for animal production sectors, specifically under stressful circumstances, and the foundation of success is efficient management2,3. Antibiotics were broadly employed as growth promoters, stress relievers, and remedies4. The usage of antibiotics as growth stimulants in animal production has been banned since 2006 within the European Union5. Antibiotics and other synthetic substances have been phased out in favor of more ecologically friendly methods for enhancing animal health, performance, and, eventually, ensuring the safety and superior quality of animal products6.
The foundation for good growth is a well-balanced diet, and one of the most effective approaches to altering an animal's growth is to modify the diet7. Utilizing functional feed supplements has become a commonly recognized approach for boosting the performance of animals8. Algae has the leverage to be a sustainable fountain of food and energy in the future. The majority of microalgal constituents are carbohydrates, lipids, proteins, minerals, vitamins, and bioactive substances9. Algal products in the diet of animals have been demonstrated to boost performance and meat goodness in ruminants and nonruminants. These results are greatly reliant on the form of microalgae and their level in the diet9.
Arthrospira (formerly Spirulina) and Chlorella are the two genera of algae that warrant a more thorough examination for nutritional purposes. Most microalgae protein fractions are stated to have the same or even better quality than typical plant protein fractions10. Arthrospira is well-known as a high-protein basis (60–70% of dry weight) with a high digestibility coefficient, with all essential amino acids accounting for about half of total protein11, essential fatty acids12, phytopigments (carotene—phycocyanin—phycocyanobilin chlorophyll and xanthophyll)13,14, water and lipid-soluble vitamins (B group, ascorbic acid, A, D, E, K) as well as minerals (Ca, Cr, Cu, Fe, K, Na, P, Se, Zn)15. Dry Chlorella has a 50–60% protein content, making it comparable to other sources, e.g., yeast, soy flour, and milk16. Also, Chlorella biomass provides basic nutrient, pigments, minerals, vitamins, and provitamins17. Moreover, dry Arthrospira and Chlorella microalgae contain a significant portion of lipids (up to 80%) and carbohydrates (12–57%)16. Arthrospira and Chlorella have been proposed as primary ingredients or dietary supplements to enhance the performance and health of animals. In this sense, rabbits treated with Arthrospira exhibited higher growth18,19,20,21,22,23, meat quality18,24, reproductive performance25, immunity18,19,26,27, and antioxidants18,19,21,28,29. Likewise, rabbits treated with Chlorella showed better growth30,31,32, immunity30,33, and antioxidants30,32.
Considering the high nutritional value of algae, the purpose of the current trial was to contrast the impacts of dry Chlorella vulgaris and Arthrospira platensis as dietary supplements on the growth, nutrient efficiency, intestinal health, blood indices, and antioxidant capacity in New Zealand white rabbits.
Materials and methods
Isolation of algal species
Two algal species were used in this study, namely, Chlorella vulgaris and Arthrospira platensis. The green alga Chlorella vulgaris was isolated from a site in the Damietta branch (Drainage of sewage Omar Buck for 10 km in the city of Mansoura), while the cyanobacterial Arthrospira platensis species was isolated from wadi-elnatrun brackish ponds. The isolated algae were developed primarily in a 250-ml Erlenmeyer conical container comprising 100 ml of growth media. For the growth of Chlorella vulgaris, Bold`s Basal Medium (BBM) with a final pH of 6.3 was used, while A. platensis was enriched in spirulina medium. Unialgal strains were acquired by picking up the clonal population from an algal medium agar plate which was obtained by serial dilution of the primary inoculum.
Morphological Identification of algal species
The isolated algal species were identified morphologically according to features described by Deyab et al.34 using a Zeiss (Axiolab 5) light microscope. For more accurate morphological characterization, the isolated species were examined using a JEOL JSM 6510 scanning electron microscope (Figs. 1 and 2).
Cultivation of algal species
All culture media were incubated on an orbital shaker (130 rpm) at 25 ± 2 °C, with a light intensity of 1.2 Klux, and an illumination of 16:8 h for a week with continuous aeration. The separated algal species were grown in a 2L flask, each comprising 1000 ml of medium, and developed under similar circumstances for 21 days to achieve biomass. To obtain dried biomass, thin layers of wet biomass of both algal species were dehydrated using a Binder Hot oven at 60 °C for 12 h. The chemical content of the tested algae supplements was evaluated following standard analysis techniques35.
Chemical profiling of the algal extract using GC–MS
For the extraction of algae, 1 g of freeze-dried biomass for each alga was extracted twice using 10 ml methanol according to Deyab et al.34. To obtain cell-free supernatant, the extracts were centrifuged at 6000 rpm for 20 min, then concentrated using a rotary evaporator at 40 °C. The dried residues were redissolved using 3 ml of methylene chloride and kept at 4 °C until GC–MS analysis. The crude extracts were analyzed using Varian GC–MS (Varian Chrompack CP-3800 GC/MS/MS-2000, Germany). The GC–MS was equipped with a split-splitless injector in addition to a DB-5.625 GC column (30 m × 0.25 mm i.d., 0.25 µm film thickness). The active chemical compounds were identified by matching their recorded spectra with the data bank mass spectra (Saturn and NIST library databases) provided by the instrument software. The concentration (% content) of the components of the extract was computed by integrating their peak areas in the total ion current (TIC) chromatograms, assuming a unity response by all components.
Animals and management
The experiment was conducted for 8 weeks from December 2020 to January 2021 on a private farm under the supervision of the Animal Production Department, Faculty of Agriculture at Tanta University in Egypt. The ethical committee of the Faculty of Agriculture at Tanta University approved the experimental protocol and all methods in the present study for treating animals for scientific purposes (Approval No. AY2019-2020/Session 6/2020.01.13). All experiments were performed in accordance with relevant guidelines and regulations. Our reporting of research involving animals follows the recommendations of the ARRIVE guidelines. Seventy-five five weeks old New Zealand white male rabbits were chosen for litter weight at weaning (665.93 ± 15.18 g) and placed into five experimental groups (n = 15/group). All rabbits were maintained separately in galvanized wire pens (35 × 35 × 60 cm) with freely accessible feeders (ad libitum) and a freshwater outlet under the same management and hygienic conditions, namely a regimen of 12 h light and 12 h dark, natural ventilation, an average temperature of 17.29 ± 0.27 °C and a relative humidity of 59.96 ± 0.42 (Table 1).
The rabbits in the reference group (Ctrl) were fed a basal diet with no additions (Table 2), whereas the remainder of the groups were provided a basal diet with 300 or 500 mg of Arthrospira platensis (Ap300 or Ap500) or Chlorella vulgaris (Ch300 or Ch500).
Performance variables
The weight of the rabbits at the start and conclusion of the trial and the amount of feed consumed were recorded as follows:
where WT = Final weight; W0 = Initial weight; FI = Feed intake.
Sampling procedure
After 8 weeks of feeding, 5 rabbits/group were allocated for blood collection and slaughter. Blood samples were drawn without anesthesia from the lateral saphenous superficial vein of the back leg after wetting the fur with alcohol using a 1 ml syringe with heparin for hematological measurements or without anticoagulants to separate the serum. Heparin-treated blood was employed for hematocrit (Ht) quantification using microhematocrit tubes and rotary centrifugation (13,000 rpm for 5 min)36. Non-heparinized blood was centrifuged [3000 rpm undercooling (4 °C) for 10 min] to harvest serum. Hematological and biochemical blood indices were measured using CBC Micros ABX, France automatic analyzer with P500 kinetic & Quality control Diatron Q.C kits according to package guidelines. The liver and small intestine were separated on an ice layer, cleaned with regular saline solution (0.90%; pH 7.5), and subjected directly to the determination of hepatic antioxidants, intestinal structure, and digestive enzyme activities.
Intestinal enzymes and histology assessment
Parts of the collected intestine (duodenum) were finely homogenized in freezing iced NaCl (0.86%) using VEVOR, FSH-2A device, and centrifuged at 8000 rpm for 5 min, 4 °C. The filtrate was employed for the colorimetric detection of amylase and lipase at A714 and A5407. Protease potency was measured using a non-specific protease vigor methodology utilizing casein37. For histological evaluation, samples (duodenum, jejunum, ileum) were fixed in a neutral buffered (10% formalin solution) for 72 h, dehydrated in rising grades of ethanol (60–100%), cleared in xylene, embedded in paraffin wax (24 h), and then sectioned with Rotary Microtome 2145, Leica Microsystems at a 3–5 μm in thickness.
Hepatic antioxidants
Liver samples (5 rabbits/treatment) were finely homogenized in cold iced potassium phosphate buffer (pH 7.4, 10% w/v) using VEVOR, FSH-2A device, and centrifuged at 4 °C, 12,000 rpm for 10 min. The filtrate was employed for the colorimetric detection (Jenway UV–Vis spectrophotometer 7415, Staffordshire, UK) of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) at 550, 280, 412 nm using Biodiagnostic and research reagents, Dokki, Giza, Egypt.
Statistical analysis
The study was designed in one-way ANOVA to compare the effects of the two algae species with two levels of supplementations in the feeds of New Zealand white rabbits. The rabbits were divided into five groups. The first group received a diet without either A. platensis or C. vulgaris (Control group, Ctrl). Conversely, the second group was given a diet with 300 mg/kg of A. platensis (Ap300), the third group a diet with 500 mg/kg of A. platensis (Ap500), the fourth group a diet with 300 mg/kg of C. vulgaris (Ch300), and the fifth group a diet with 500 mg/kg of C. vulgaris (Ch500). The data was examined using the IBM® SPSS® Inc., IL, USA program (IBM SPSS Statistics Ver. 26.0). The Shapiro–Wilk and Levene tests were employed to verify variance normality and homogeneity. The outcomes of the one-way ANOVA and Duncan's post hoc test were presented as a mean of three replicates with standard errors.
Approval for animal experiments
The ethical committee of the Faculty of agriculture at Tanta University approved the experimental protocol and all methods in the present study for treating animals for scientific purposes (Approval No. AY2019-2020/Session 6/2020.01.13). All experiments were performed in accordance with relevant guidelines and regulations. Our reporting of research involving animals follows the recommendations of the ARRIVE guidelines.
Results
Chemical composition of the Algal supplements
Arthrospira platensis dry biomass comprises 56.4 ± 3.3, 6.6 ± 0.6, and 26.2 ± 0.98% of protein, lipids, and carbohydrates, compared to Chlorella vulgaris's proportions of 43.6 ± 2.4, 20.19 ± 1.2, and 23.8 ± 0.94%. A total of 25 active chemical compounds were characterized in the extracts of both algae. The identified chemical products with their retention time and % peak area of both extracts were shown in Table 3. The chromatograms of both extracts were shown in Fig. 3.
In general, the identified chemical compounds belong to seven major chemical groups, including esters, fatty acids, fatty alcohol, hydrocarbons, ketone, steroids, and terpenes. Chlorella vulgaris extract contains more esters (53.35%), fatty compounds (21.82), and hydrocarbons than Arthrospira platensis extract (31.00, 1.72, and 20.25, respectively). Meanwhile, Arthrospira platensis extract contains more ketone (19.96%), cholesterol (4.64%), and terpenes (20.24%) than Chlorella vulgaris extract (5.94, 0.00, and 5.92%, respectively).
Performance variables
Table 4 shows the growth and nutrient efficiency of New Zealand white rabbits fed experimental diets for 8 weeks. Rabbits fed the basal diet exhibited the lowest final weights and weight gains and the highest feed conversion ratio, which improved noticeably with algae addition, particularly with Ap500, Ch300, and Ch500. Feed intake did not change with treatments except for the high level of Chlorella vulgaris (Ch500), which showed the lowest FI value.
Intestinal efficiency
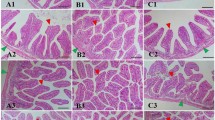
Figure 4 shows the intestinal structure of New Zealand white rabbits fed experimental diets for 8 weeks. All rabbit groups showed intact and normal intestinal structures with no pathological alterations such as degeneration, necrosis, hemolysis, edema, congestion, hemorrhages, and hypertrophy. The efficiency of intestinal enzymes is shown in Table 4. A remarkable enhancement in the efficiency of lipase and protease occurred in algal groups compared to the control, while the efficiency of amylase did not change between the experimental groups.
Intestinal structure (duodenum, jejunum, ileum, H&E = 40 X) of New Zealand white rabbits fed experimental diets for 8 weeks. Ctrl = the control group; Ap300 and Ap500 = Arthrospira platensis inclusion levels at 300 and 500 mg/kg; Ch300 and Ch500 = Chlorella vulgaris inclusion levels at 300 and 500 mg/kg.
Blood health
Table 5 exhibits the blood profile of New Zealand white rabbits after 8 weeks of feeding trial. Hematological indicators comprising hematocrit (Ht), hemoglobin (Hb), red blood cells (RBCs), and white blood cells (WBCs) showed non-significant variation with dietary treatments. Similarly, serum biochemistry displayed no alteration in glucose, triglyceride, alanine transaminase (ALT), and aspartate transaminase, while a significant alteration occurred in total protein and total cholesterol. Rabbits treated with Arthrospira (Ap300 and Ap500) and Chlorella (Ch300 and Ch500) exhibited higher total protein and lower total cholesterol compared with the reference group. The lowest level (P < 0.05) of cholesterol was found in the blood of rabbits given a high level of Arthrospira (Ap500) and both levels of Chlorella (Ch300 and Ch500).
Hepatic antioxidants
Figure 5 displays the hepatic superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities in New Zealand white rabbits after 8 weeks of feeding trial. Rabbits fed the basal diet exhibited the poorest antioxidant potency (SOD, CAT, and GPx). The best GPx existed in all groups fed algal diets, while the favored SOD and CAT efficacy appeared at higher Arthrospira (Ap500) and both levels of Chlorella (Ch300 and Ch500).
Hepatic superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities in New Zealand white rabbits fed experimental diets for 8 weeks. Ctrl = the control group; Ap300 and Ap500 = Arthrospira platensis inclusion levels at 300 and 500 mg/kg; Ch300 and Ch500 = Chlorella vulgaris inclusion levels at 300 and 500 mg/kg.
Discussion
Stimulating the maximum production of animals necessitates specific procedures to ensure quantity, quality, and animal health7. Nutraceuticals feed additives in the animal production business as natural substitutes for antibiotics have risen in importance6. Rabbit production is an appropriate agricultural investment because of its low production costs, superior fertility, short generation intervals, and ability to utilize a range of forages1.
Results of growth variables showed that rabbits fed the basal diet exhibited the poorest performance, which improved noticeably with algae addition, particularly with Ap500, Ch300, and Ch500 (Table 4). The high nutritional value of algae may be one of the reasons for the enhanced performance in animals fed with algae supplements. In this sense, Mahmoud et al.18 found that soybean substitution by A. platensis at levels of 20, 40, and 60% in rabbit feed did not show any negative outcomes and maintained indicators of growth, health, and meat quality. Furthermore, Seyidoglu et al.26 found an enhancement in the immune system of growing rabbits with A. platensis-diets. In contrast, Gerencsér et al.38 assumed that Arthrospira (5%) and thyme (3%), either alone or in combination, did not substantially alter the growth or health of growing rabbits. Chlorella has been suggested to boost the performance and health of animals17,30,39. In a previous study, Hassanein et al.40 compared the influence of Arthrospira (Spirulina) platensis and Chlorella vulgaris at levels of 0.75 and 1.5 g/kg diet on growing New Zealand white rabbits and concluded that both levels of A. platensis improved the growth and reduced liver enzyme, cholesterol and total lipids contents in serum in comparison with Chlorella vulgaris supplements. Moreover, An et al.39 demonstrated that adding 0.15% dried Chlorella vulgaris powder to Ross broiler chicks' feed considerably improved growth, blood cell counts, and declined total lipids in serum. According to Abdelnour et al.30, adding 1.0 g of Chlorella vulgaris to the diet of growing New Zealand white rabbits could boost their immunological and antioxidant health, as well as reduce blood lipid accumulation. Despite these valuable results and to the authors' best knowledge, so far there are no planned studies that have compared the potentials of Arthrospira and Chlorella on intestinal histology, digestive enzyme potency, and hepatic antioxidants of New Zealand white rabbits. Consequently, the current trial was designed to cover these parameters.
Likewise, the improvement in weight with algae supplements may be linked to a change in the feed conversion ratio (↓ FCR). The detected reduction in FCR may be linked with the amended intestinal efficiency (Table 4), particularly digestive enzymes (lipase and protease). Several studies have demonstrated that adding algal biomass or extracts improves growth and nutrient use. In Arthrospira impacts regard, Alazab et al.20 found that adding Spirulina platensis (SP) to the diet of growing rabbits at a level of 0.6 g/kg diet resulted in considerably better growth performance parameters and enhanced feed conversion ratio in comparison to those provided the low level (0.3 g/kg diet) or those fed a basal diet. Moreover, Aladaileh et al.21 highlighted that exogenous supplementation of SP enhanced the growth traits of rabbits subjected to Pb. In addition, Peiretti and Meineri22,23 demonstrated that rabbits receiving Arthrospira at a level of 10% exhibited higher feed consumption. Regarding the effect of Chlorella, Sikiru et al.31 noted that dietary implementation of the Chlorella vulgaris amidst 200 and 500 mg/kg diet considerably raised the rabbits’ weights without substantial alteration in feed intakes, but substantially enhanced feed to gain ratio. In another study by Sikiru et al.32 on New Zealand white rabbits, a significant positive boost in the final body weight and feed intake with the addition of Chlorella vulgaris. In contrast to the findings of the current study, no alteration was observed in the growth aspects with the dietary incorporation of Arthrospira (Spirulina)22,23,24,38 or Chlorella30 and this may be due to the different conditions of the experiment.
Blood status is a precise sign of the welfare and health status of animals, hence are direct reflectors of stressors and external stimuli41. Hematological indicators and serum biochemistry showed non-significant variation except for serum total protein and total cholesterol (Table 5). Rabbits treated with Arthrospira and Chlorella had higher total protein (TP) and lower total cholesterol than the reference group. The higher levels of TP in rabbits fed algae may suggest an improvement in rabbit health. In this context, Hassan et al.19 reported an enrichment in plasma total protein in rabbits provided a diet enhanced with Zn-Se- rich Spirulina compared to the reference group. A similar improvement in glycoprotein appeared with Chlorella treatment33. The hypocholesterolemic effect of algae could explain the lower cholesterol levels associated with supplementation. In line with the present results, Cheong et al.27 suggested that spirulina consumption can reduce hypercholesterolemic atherosclerosis by lowering total serum cholesterol in New Zealand White rabbits. Also, Hassan et al.19 found low levels of total cholesterol, LDL- and VLDL-cholesterol in Se-rich Spirulina and Zn-Se- rich Spirulina groups of New Zealand White male rabbits. Similar impacts on cholesterol were reported with Chlorella incorporation. In this regard, Abdelnour et al.30 found a reduction in serum VLDL in the Chlorella-treated groups relative to those in the control group.
The oxidative state of the animal is positively related to its immunity and wellbeing42. Oxidative stress is caused by an imbalance in the generation and clearance of reactive oxygen species (ROS)43. Several enzymes in the oxidative system, such as SOD, CAT, and GPx aid in the elimination of ROS and the maintenance of cell homeostasis44. In the present trial, algae dietary application mediates a substantial rise in SOD, CAT, and GPx activities. This may be due to the unique compositions of Arthrospira and Chlorella that are rich in effective compounds with an antioxidant impact, e.g., minerals, vitamins, β carotene, β-glucan, linolenic acid, tocopherols, phycocyanin, flavonoids, and phenols. Similar interpretations were reported for New Zealand White rabbits fed Arthrospira by Hassan et al.19 or Chlorella by Abdelnour et al.30. Several studies have found enhanced antioxidant enzymes in rabbits fed Arthrospira21,28,29 and Chlorella30,32.
The content of active chemicals in feed additives is mostly responsible for their beneficial effects. The overall results indicated that including Chlorella surpassed Arthrospira additives in New Zealand white rabbit feeds. These results indicated superior improvements in the growth performance, feed efficiency, and intestinal and blood health of New Zealand white rabbits fed Chlorella. These observations could be associated with the content of Chlorella and its effect on intestinal health and body immunity in rabbits. The GC–MS analysis of the crude extracts of the two algae showed the presence of 25 chemical substances with known favorable bioactivity on rabbits19,20,21,31 and humans45. It is challenging to explain the effects of algae dietary supplements at the level of a single ingredient because algal extracts contain a significant number of active compounds and the best strategy is to classify them into major categories. Arthrospira exceeded Chlorella in its content of ketone, cholesterol, and terpenes, but Chlorella surpassed Arthrospira in its content of esters, fatty compounds, and hydrocarbon. Both extracts contain the majority of the active chemicals, albeit in different amounts which amply explain the convergence of the impacts supporting the performance and wellbeing of New Zealand white rabbits. In this context, phytol is a diterpene compound found in almost all crude extracts of the used algae and known for its anticancer and antioxidative properties46. The hydrocarbon pentadecane and the fatty acid Pentanoic acid, 4- methyl- are known for their antimicrobial activity47 and antitumor activity48, as well as a growth promoter49.
Conclusions
The present study sheds light on the potential of algae feed additives (Arthrospira platensis VS Chlorella vulgaris) on the performance and wellbeing of New Zealand white rabbits. Incorporating Arthrospira platensis at 500 mg/kg diet or Chlorella vulgaris at levels of 300 and 500 mg/kg diet improved growth, nutrient aspects, intestinal enzyme efficiency, blood health, and antioxidants of New Zealand white rabbits. It will be vital for rabbit production in the future to monitor molecular responses to external feeds and/or supplements and to concentrate on obtaining a precise nutritional formula for algal biomass in rabbit feeding without compromising performance or health.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Gorlov, I. F., Semenova, I. A., Knyazhechenko, O. A., Mosolov, A. A. & Karpenko, E. V. Assessment of the impact of new complex feed additives in the production of rabbit meat. IOP Conf. Ser. Earth Environ. Sci. 548, 82073 (2020).
Oladimeji, A. M., Johnson, T. G., Metwally, K., Farghly, M. & Mahrose, K. M. Environmental heat stress in rabbits: implications and ameliorations. Int. J. Biometeorol. https://doi.org/10.1007/s00484-021-02191-0 (2021).
Yue, Z. et al. Vitamin A alleviates heat stress-induced damage to hair follicle development in Rex rabbits. J. Sci. Food Agric. 102, 2291 (2021).
Roth, N. et al. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 98, 1791–1804 (2019).
Millet, S. & Maertens, L. The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet. J. 187, 143–144 (2011).
Abd El-Hack, M. E. et al. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: A comprehensive review. Poult. Sci. 101, 101584 (2022).
Khattab, A. A. A., El Basuini, M. F. M., El-Ratel, I. T. & Fouda, S. F. Dietary probiotics as a strategy for improving growth performance, intestinal efficacy, immunity, and antioxidant capacity of white Pekin ducks fed with different levels of CP. Poultry Science vol. 100 (Poultry Science Association Inc., 2021).
Calik, A. et al. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms 7, 257 (2019).
Madeira, M. S. et al. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 205, 111–121 (2017).
Becker, E. W. Micro-algae as a source of protein. Biotechnol. Adv. 25, 207–210 (2007).
Gutiérrez-Salmeán, G., Fabila-Castillo, L. & Chamorro-Cevallos, G. Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. 32, 34–40 (2015).
Mendes, R. L., Nobre, B. P., Cardoso, M. T., Pereira, A. P. & Palavra, A. F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorganica Chim. Acta 356, 328–334 (2003).
Gong, R., Ding, Y., Liu, H., Chen, Q. & Liu, Z. Lead biosorption and desorption by intact and pretreated spirulina maxima biomass. Chemosphere 58, 125–130 (2005).
Bermejo, P., Piñero, E. & Villar, Á. M. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulinaplatensis. Food Chem. 110, 436–445 (2008).
Farag, M. R., Alagawany, M., Abd El-Hac, M. E. & Dhama, K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int. J. Pharmacol. 12, 36–51 (2015).
Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306 (2007).
Kotrbáček, V., Doubek, J. & Doucha, J. The chlorococcalean alga Chlorella in animal nutrition: a review. J. Appl. Phycol. 27, 2173–2180 (2015).
Mahmoud, A. E., Naguib, M. M., Higazy, A. M., Sultan, Y. Y. & Marrez, D. A. Effect of Substitution Soybean by Blue Green Alga Spirulina platensis on Performance and Meat Quality of Growing Rabbits. Am. J. Food Technol. 12, 51–59 (2016).
Hassan, F. et al. Zinc and/or selenium enriched spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals 11, 756 (2021).
Alazab, A. M. et al. Effect of Spirulina platensis supplementation in growing Rabbit’s diet on productive performance and economic efficiency. J. Anim. Poult. Prod. 11, 325–330 (2020).
Aladaileh, S. H. et al. Spirulina platensis ameliorates the sub chronic toxicities of lead in rabbits via anti-oxidative, anti- inflammatory, and immune stimulatory properties. Sci. Total Environ. 701, 134879 (2020).
Peiretti, P. G. & Meineri, G. Effects of diets with increasing levels of Spirulina platensis on the performance and apparent digestibility in growing rabbits. Livest. Sci. 118, 173–177 (2008).
Peiretti, P. G. & Meineri, G. Effects of diets with increasing levels of Spirulina platensis on the carcass characteristics, meat quality and fatty acid composition of growing rabbits. Livest. Sci. 140, 218–224 (2011).
Dalle Zotte, A., Sartori, A., Bohatir, P., Rémignon, H. & Ricci, R. Effect of dietary supplementation of Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) on growth performance, apparent digestibility and health status of companion dwarf rabbits. Livest. Sci. 152, 182–191 (2013).
Abadjieva, D., Shimkus, A., Shimkiene, A., Rashev, P. & Kistanova, E. Transgenerational beneficial effect of Arthrospira (Spirulina) platensis on the rabbit ovaries. J. Appl. Phycol. 30, 1691–1700 (2018).
Seyidoglu, N., Galip, N., Budak, F. & Uzabaci, E. The effects of Spirulina platensis ( Arthrospira platensis ) and Saccharomyces cerevisiae on the distribution and cytokine production of CD4+ and CD8+ T-lymphocytes in rabbits. Austral J. Vet. Sci. 49, 185–190 (2017).
Cheong, S. H. et al. Spirulina prevents atherosclerosis by reducing hypercholesterolemia in rabbits fed a high-cholesterol diet. J. Nutr. Sci. Vitaminol. (Tokyo) 56, 34–40 (2010).
Abdelnour, S. A. et al. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 19, 1046–1056 (2020).
Sabry, M. M., Abdel-Rahman, R. F., El-Shenawy, S. M., Hassan, A. M. & El-Gayed, S. H. Estrogenic activity of Sage (Salvia officinalis L.) aerial parts and its isolated ferulic acid in immature ovariectomized female rats. J. Ethnopharmacol. 282, 114579 (2022).
Abdelnour, et al. Impacts of enriching growing rabbit diets with chlorella vulgaris microalgae on growth, blood variables, carcass traits, immunological and antioxidant indices. Animals 9, 788 (2019).
Sikiru, A. B., Arangasamy, A., Alemede, I. C., Egena, S. S. A. & Bhatta, R. Dietary supplementation effects of Chlorella vulgaris on performances, oxidative stress status and antioxidant enzymes activities of prepubertal New Zealand White rabbits. Bull. Natl. Res. Cent. 43, 162 (2019).
Sikiru, A. B. et al. Chlorella vulgaris supplementation effects on performances, oxidative stress and antioxidant genes expression in liver and ovaries of New Zealand White rabbits. Heliyon 5, e02470 (2019).
Lesyk, I. V., Fedoruk, R. S. & Dolaĭchuk, O. P. Immunobiological blood parameters in rabbits after addition to the diet suspensions of chlorella, sodium sulfate, citrate and chromium chloride. Fiziol. Zh. 59, 78–84 (2013).
Deyab, M., Mofeed, J., El-Bilawy, E. & Ward, F. Antiviral activity of five filamentous cyanobacteria against coxsackievirus B3 and rotavirus. Arch. Microbiol. 202, 213–223 (2020).
AOAC. Official Methods of Analysis of AOAC International. (Associations of Analytical Chemists, International, 2007).
Goldenfarb, P. B., Bowyer, F. P., Hall, E. & Brosious, E. Reproducibility in the hematology laboratory: the microhematocrit determination. Am. J. Clin. Pathol. 56, 35–39 (1971).
Cupp-Enyard, C. Sigma’s non-specific protease activity assay—Casein as a substrate. J. Vis. Exp. 899, 1–3 (2008).
Gerencsér, Z. et al. Effect of dietary supplementation of spirulina (Arthrospira platensis) and thyme (Thymus vulgaris) on apparent digestibility and productive performance of growing rabbits. World Rabbit Sci. 22, 1 (2014).
An, B.-K., Kim, K.-E., Jeon, J.-Y. & Lee, K. W. Effect of dried Chlorella vulgaris and Chlorella growth factor on growth performance, meat qualities and humoral immune responses in broiler chickens. Springerplus 5, 718 (2016).
Hassanein, H., Arafa, M. M., Abo Warda, M. A. & Abd-Elall, A. Effect of Using Spirulina platensis And Chlorella vulgaris as Feed Additives on Growing Rabbit Performance. Egypt. J. Rabbit Sci. 24, 413–431 (2014).
El-Tarabany, M. S., Ahmed-Farid, O. A. & El-Tarabany, A. A. Impact of space allowance on performance traits, brain neurotransmitters and blood antioxidant activity of New Zealand White rabbits. Prev. Vet. Med. 163, 44–50 (2019).
El-Gindy, Y., Zeweil, H., Zahran, S., El-Rahman, M. A. & Eisa, F. Hematologic, lipid profile, immunity, and antioxidant status of growing rabbits fed black seed as natural antioxidants. Trop. Anim. Health Prod. 52, 999–1004 (2020).
Lee, M. T., Lin, W. C. & Lee, T. T. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals—A review. Asian-Australas. J. Anim. Sci. 32, 309–319 (2019).
Chauhan, S. S., Rashamol, V. P., Bagath, M., Sejian, V. & Dunshea, F. R. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 65, 1231–1244 (2021).
Wollina, U. et al. Arthrospira Platensis - Potential in Dermatology and Beyond. Open Access Maced. J. Med. Sci. 6, 176–180 (2018).
Gliszczyńska, A. et al. Synthesis of novel phytol-derived γ-butyrolactones and evaluation of their biological activity. Sci. Rep. 11, 4262 (2021).
Omage, K., Azeke, M. A. & Omage, S. O. Evaluation of the efficacy of Acalypha wilkesiana leaves in managing cardiovascular disease risk factors in rabbits exposed to salt-loaded diets. Clin. Phytoscience 4, 1 (2018).
Essien, E. E., Ogunwande, I. A., Setzer, W. N. & Ekundayo, O. Chemical composition, antimicrobial, and cytotoxicity studies on S. erianthum and S. macranthum essential oils. Pharm. Biol. 50, 474–480 (2012).
Martignon, M., Burel, C., Cauquil, L., Combes, S. & Gidenne, T. Impact of feed restriction and fragmented feed distribution on performance, intake behaviour and digestion of the growing rabbit. Animal 15, 100270 (2021).
Acknowledgements
The first author would like to thank all staff members of the Animal Production Department, Faculty of Agriculture, Tanta University, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding is provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Basuini, M.F., Khattab, A.A.A., Hafsa, S.H.A. et al. Impacts of algae supplements (Arthrospira & Chlorella) on growth, nutrient variables, intestinal efficacy, and antioxidants in New Zealand white rabbits. Sci Rep 13, 7891 (2023). https://doi.org/10.1038/s41598-023-34914-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34914-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.