Abstract
Denitrification, as the main nitrogen (N) removal process in farmland drainage ditches in coastal areas, is significantly affected by saline-alkali conditions. To elucidate the effects of saline-alkali conditions on denitrification, incubation experiments with five salt and salt-alkali gradients and three nitrogen addition levels were conducted in a saline-alkali soil followed by determination of denitrification rates and the associated functional genes (i.e., nirK/nirS and nosZ Clade I) via N2/Ar technique in combination with qPCR. The results showed that denitrification rates were significantly decreased by 23.83–50.08%, 20.64–57.31% and 6.12–54.61% with salt gradient increasing from 1 to 3‰, 8‰, and 15‰ under 0.05‰, 0.10‰ and 0.15‰ urea addition conditions, respectively. Similarly, denitrification rates were significantly decreased by 44.57–63.24% with an increase of the salt-alkali gradient from 0.5 to 8‰. The abundance of nosZ decreased sharply in the saline condition, while a high salt level significantly decreased the abundance of nirK and nirS. In addition, the increase of nitrogen concentration attenuated the reduction of nirK, nirS and nosZ gene abundance. Partial least squares regression (PLSR) models demonstrated that salinity, dissolved oxygen (DO) in the overlying water, N concentration, and denitrifying gene abundance were key determinants of the denitrification rate in the saline environment, while pH was an additional determinant in the saline-alkali environment. Taken together, our results suggest that salinity and high pH levels decreased the denitrification rates by significantly inhibiting the abundance of the denitrifying genes nirK, nirS, and nosZ, whereas increasing nitrogen concentration could alleviate this effect. Our study provides helpful information on better understanding of reactive N removal and fertilizer application in the coastal areas.
Similar content being viewed by others
Introduction
Nitrogen (N) is an essential nutrient for crop growth, thus synthetic N fertilizers are commonly applied in the farmland to achieve high yield. However, yield increases that rely on increased N inputs likely result in a high risk of reactive N (Nr) losses1. Excess N fertilizer application in farmlands leads to substantial Nr releasing into rivers and lakes through runoff and drainage, which results in a series of environmental problems including agricultural non-point source pollution2,3,4. The drainage ditch system is a type of wetland in the agricultural ecosystem between the output of farmland field and the receiving water bodies (i.e., lakes and rivers). The migration and transformation of pollutants in these drainage ditches plays a vital role in controlling their final output load5. The urgency of eliminating Nr from farmland drainage ditches and reducing the environmental pollution from exogenous N in rivers and lakes has drawn substantial scientific and public attention. Denitrification which is the microbial reduction of nitrate/nitrite (NO3−/NO2−) to the gaseous products nitric oxide (NO), nitrous oxide (N2O), and dinitrogen (N2) under hypoxic conditions6, occurs essentially in all water-flooded systems and is considered the major N removal process in ditch systems7,8,9,10.
Mitigating agricultural non-point source N pollution is quite important in agricultural production areas since N fertilizer is easily lost therein. Saline-alkali soil in the coastal region of eastern China has become an important land resource for agriculture with increasing population growth and socioeconomic development11,12,13,14. However, due to its special physicochemical properties, the N fertilizer use efficiency of crops grown therein is generally very low, even to 5.63%15 and below the national average of 34.3%16 increasing the risk of Nr loss. Meanwhile, the salt and alkali in farmland soils can flow into the farmland drainage ditches with runoff and drainage, resulting in an increase of salt and alkali contents in these ditches. Salinization and alkalization can alter the fundamental physicochemical nature of the soil–water environment, not only in ionic concentrations but also in chemical equilibria and microbiological composition17,18. The increased ionic concentrations change physicochemical processes and chemical equilibria, facilitate aggregation and sedimentation of suspended solids, and induce the fast displacement of cations [e.g., ammonium (NH4+)] that are bound to the cation adsorption complex in the sediment18. Moreover, the microbial community structure and diversity of denitrifying bacteria in the soils are greatly affected by salinity19. Soil salinity adversely affects the activity of soil enzymes14 and the abundances of N cycling associated genes20,21. The altered physicochemical and biological properties induced by salinization and alkalization will inevitably influence N transformation processes therein. For instance, salinization can directly affect denitrification rate by increasing or decreasing N availability in the soil22,23. Some studies have also shown that the denitrifying community structure changes greatly under high salinity conditions and sometimes even loses its function24,25. Moreover, the high pH value of saline-alkali soil is another crucial factor affecting the composition of the denitrifying bacterial community26, enzyme activity27, and the abundance of associated functional genes6.
Saline-alkali soil has a high salinity and pH28; however, previous studies on denitrification and denitrifying bacteria in saline-alkali soil were mostly restricted to low salinity soils19,20 and rarely involved alkaline environments27. It has been reported that high saline-alkali environments may induce physiological stress in denitrifying bacteria and can eventually result in large shifts in the composition of denitrifying bacteria and their associated ecosystem functions17. Furthermore, saline-alkali soil with a high salinity and pH often results in large losses of farmland fertilizer into the ditches, increasing the input of exogenous N into the ditches. A comprehensive understanding of how salt and alkali contents regulate denitrification by affecting the composition of denitrifying bacteria is critical to improve the removal of Nr in farmland drainage ditches and to reduce non-point source pollution in coastal areas. However, until recently, relatively little was known about the interaction effects of high saline-alkali conditions and exogenous N on the denitrification rate and the composition of denitrifying bacteria in farmland drainage ditches of coastal areas. To analyze the effect of saline-alkali condition on denitrifying bacteria, we focused on process-specific functional genes, mainly nirK, nirS, and nosZ, which encode pivotal reductases associated with each nitrate reduction pathway21 and are frequently used as functional markers for analyzing denitrifying microbe communities29. In this study, we examined the variation in denitrification rates of three urea levels under five salt and salt-alkali gradients (from low to high) through a flooded incubation experiment and investigated how the abundance of three denitrifying functional genes changed in saline and saline-alkali environments. Concurrently, we determined the controlling factors of the denitrification process using a partial least squares regression (PLSR) model and explored the key determinants of Nr removal in the drainage ditches of farmlands with saline-alkali soils. Our study addressed the question: what are the key determinants of denitrification in ditch systems under saline-alkali conditions and the underlying microbial mechanisms?
Materials and methods
Soils
The experimental soils were collected from a ditch in Liuzong Village (32°12′N, 120°42′E) in the town of Juegang, Rudong Country, Jiangsu Province. This area is a typical coastal reclamation area and is mainly used for agriculture. The sampled soils were transported to the laboratory, salt-leached, air-dried, ground and sieved to 5 mm. The soil had a silt loam texture with a pH of 8.0, a soil electrical conductivity (EC1:5) of 2.24 mS cm−1, a cation exchange capacity (CEC) of 4.99 cmol·kg-1, and an organic carbon content (OC) of 3.1 g kg−1. Additional details on the general chemistry of this soil can be found in Pan et al.30.
Experimental design
The incubation experiment was conducted in the Water-Saving Park of Hohai University (Nanjing, 31°57′N, 118°50′E, 144 m above sea level) from October 2020 to November 2020 to investigate the effects of different salt and salt-alkali gradients on denitrification rates under three N addition levels. Different salt (sodium chloride, NaCl)30 and salt-alkali (sodium bicarbonate, NaHCO3)31 gradients were set, including a control treatment (CK, without salt and alkali addition), four salt addition treatments (S1, S2, S3 and S4: 1‰, 3‰, 8‰ and 15‰ of soil mass, respectively) and four salt-alkali addition treatments (A1, A2, A3 and A4: 0.5‰, 1‰, 3‰ and 8‰ of soil mass, respectively). The exogenous N treatments included three analytically pure urea addition levels (N1, N2 and N3: 0.05, 0.10 and 0.15 g kg−1 soil, respectively). Each treatment was replicated three times.
Different concentrations of NaCl and NaHCO3 solutions were gently sprayed onto the sieved soil samples to prevent soil agglomeration, and sprayed and mixed several times to prevent an influence of uneven salt distribution on the results. Soil samples were placed into incubators (PVC, 5 mm thickness, 340 × 270 × 130 mm internal size) after being naturally dried, and each incubator was filled with 8.0 kg soil. Details on procedure of the incubation are provided in previous studies30. The incubation was maintained in a shallow water layer of approximately 5 cm for two weeks to stabilize the soil properties and restore microbial communities. Three urea concentration solutions were subsequently applied to the corresponding incubator. An undisturbed sediment sampler was used to collect the overlying water–sediment samples after 24 h of fertilization.
Denitrification rate measurements
The denitrification (DNF) rates were measured using membrane inlet mass spectrometry (MIMS, Bay Instruments, Easton, MD, USA) according to the protocol by Kana et al.32, which quantifies changes in dissolved N2:Ar ratios within the water overlying the sediments. Details on this procedure are provided in previous studies6,33 and Supplementary Fig. S1. Nitrogen removal efficiency (RE) is the ratio of the nitrogen removed by denitrification within 24 h to the nitrogen added in the incubator. More details on the calculation of Nitrogen removal efficiency are shown in Supplementary Text S1.
Environmental factor measurements
After 24 h of fertilization, before measuring the denitrification rates, overlying water samples (100 mL each) and sediment samples (0–5 cm layer, approximately 20 g for each) were collected to measure environmental parameters, including dissolved oxygen (DO) content, EC, pH, sodium ion (Na+) and inorganic nitrogen concentration of the overlying water–sediment system. The NH4+-N and NO3–N of the filtered water and soil extracts were analyzed using a flow injection analyzer (Skalar Analytical, Breda, The Netherlands). More details on these environmental factor measurements are provided in a previous study30 and the summary of the results showed in Supplementary Table S1.
DNA extraction and quantification of denitrifying gene abundance
The sediment samples were immediately collected from the surface layer of the overlying water–sediment interface at a depth of 5 mm for cryopreservation to quantitative analysis of denitrifying gene abundance after the denitrification rates measurement. Total genomic deoxyribonucleic acid (DNA) samples were extracted from frozen sediment subsamples using an Ultra Clean Soil DNA Isolation kit (MoBio Laboratory, Carlsbad, CA, USA) according to the manufacturer’s instructions. For PCR amplification of all functional genes, a microfluidics Fluidigm Gene Expression chip was used to quantify all genes simultaneously34. The gene-specific primers are listed in Table 1, and more details are shown in Supplementary Text S2.
Statistical analysis
One-way analysis of variance (ANOVA) was carried out and examined with Duncan’s test to investigate the statistical significance of the impact of different salt or salt-alkali gradients on denitrification rates and gene abundances under the same urea level. Variations in the data for denitrification rates and gene abundance affected by the interactions of salt or salt-alkali and N were evaluated by two-way ANOVA. The normality of all data was checked and met before the ANOVA was performed. Pearson’s correlation analysis was conducted to determine the relationship between the denitrification rate, environmental parameters and denitrifying gene abundance. Statistical significance was determined at a probability level of 0.05. Redundancy analysis (RDA) was performed with salinity, pH, DO and N concentration as variables to identify the primary environmental factors affecting the abundance of denitrifying genes. A classical regression method was not ideal for this dataset because some variables may be highly correlated, which could lead to reduced statistical power of the classical regression model and even cause a misinterpretation of regression coefficients. A partial least squares regression (PLSR) model which allows for a strong collinearity between the variables was used to examine the effect of the variables on the denitrification rate38. All the above statistical analyses were performed using SPSS 22.0 (SPSS IBM., Armonk, NY, USA). All figures were created using Origin 9.0 (OriginLab Corporation, USA).
Results
Variation in denitrification rates
The denitrification rates of the salt and salt-alkali treatments were 180.33–536.93 μmol N2 m−2 h−1 and 146.51–241.13 μmol N2 m−2 h−1, respectively (Fig. 1a). Both salt and salt-alkali treatments significantly affected denitrification rates under the same urea level, and the denitrification rates decreased with increasing salt and salt-alkali addition. Compared with the CK treatment, the denitrification rates decreased rapidly under the salt-alkali treatments and were much lower than those under the salt treatments. An increase in urea significantly increased the denitrification rates (Fig. 1a). Two-way ANOVA showed that the interactions between urea and salt/salt-alkali treatments significantly (p < 0.01) affected the denitrification rates (Table 2).
Denitrification rates (a) and nitrogen removal efficiency caused by denitrification (b) under various salt/salt-alkali gradients and urea levels within 24 h. The different letters above the bars indicate significant differences between the treatments within salt/salt-alkali gradients under the same urea level. Note: N1, N2, and N3 represent 0.05‰, 0.10‰ and 0.15‰ urea addition, respectively. Bars represent denitrification rates; error bars denote standard deviations of three independent repetitions (n = 3). Different letters indicate the significant differences among treatments under the same nitrogen condition (p < 0.05, one-way ANOVA followed by Duncan’s test).
With increased salt or salt-alkali addition, the N removal efficiency within 24 h of denitrification decreased gradually (Fig. 1b). For the salt treatments, the N removal efficiency of N1 was significantly higher than that of N2 and N3. Moreover, there was no significant difference in N removal efficiency between the N2 and N3 levels under the same salt conditions. However, under salt-alkali conditions, the N removal efficiency of the N2 level treatments was significantly higher than that of the N3 level (Fig. 1b).
Response of denitrifying gene abundance to salt and salt-alkali gradients
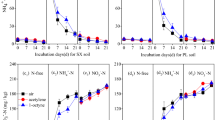
Two-way ANOVA showed that there was no significant interactive effect between urea and salt, nor between urea and salt-alkali content, on the denitrifying gene abundance (Table 2). Therefore, one-way ANOVA was used to test whether salt and salt-alkali treatments had significant impacts on denitrifying gene abundance under each urea level. For the salt treatments, the abundances of the nirK, nirS and nosZ genes were between 2.93 × 108–1.44 × 109 copies g−1, 5.60 × 107–2.62 × 108 copies g−1 and 8.28 × 108−2.75 × 109 copies g−1 of dry sediment, respectively (Fig. 2a–c). The abundance of all three denitrifying functional genes decreased with increasing salt addition. Salt and urea levels had no significant effect on the abundance of the nirK gene at salt additions of 1‰ and 3‰, while at 1‰ salt additions, salt and urea levels had no significant effect on nirS gene abundance. When the salt gradient was greater than 3‰, the abundance of the nirK and nirS genes decreased significantly, and the urea level had a significant and positive impact on the nirK and nirS gene abundance (Fig. 2a,b). Compared to nirK and nirS, nosZ gene abundance was more sensitive to salt and decreased significantly when salt increased slightly (Fig. 2c).
Abundance of the denitrifying functional genes nirK (a), nirS (b), and nosZ (c) under various salt/salt-alkali gradients and urea levels. The different letters above the bars indicate significant differences between the treatments within salt/salt-alkali gradients under the same urea level. Note: N1, N2, and N3 represent 0.05‰, 0.10‰ and 0.15‰ urea addition, respectively. Bars represent abundance of the denitrifying functional genes; error bars denote standard deviations of three independent repetitions (n = 3). Different letters indicate the significant differences among treatments under the same nitrogen condition (p < 0.05, one-way ANOVA followed by Duncan’s test).
For the salt-alkali treatments, the abundances of the nirK, nirS and nosZ genes were within the range of 2.62 × 108–1.36 × 109 copies g-1, 6.78 × 107–1.96 × 108 copies g−1 and 1.12 × 109–2.49 × 109 copies g−1 of dry sediment, respectively (Fig. 2a–c), much less than those of the salt treatments with the same content. The increase in urea slowed the decreasing trend of nirK gene abundance caused by the salt-alkali treatment, and when the salt-alkali addition was greater than 3‰, urea had no significant effect on the nirK gene abundance (Fig. 2a). The urea level had no significant effect on the nirS gene abundance under salt-alkali conditions (Fig. 2b). For the nosZ gene, the gene abundance of N1 treatment decreased rapidly when the salt-alkali gradient increased, while that of N3 treatment decreased gradually. The decreased range of gene abundance of N2 treatment was between N1 and N3 treatment (Fig. 2c).
Relationship between denitrifying gene abundance and environmental factors
Pearson’s correlation analysis was performed to evaluate the correlation between denitrification rates and denitrifying gene abundance. The denitrification rates were significantly correlated with the abundance of the denitrifying genes nirK, nirS and nosZ under both saline and saline-alkali conditions (Fig. 3a,b).
Heatmap of the correlation among the denitrification rates, denitrifying gene abundances and environmental factors. (a) Saline environment; (b) Saline-alkali environment. DNF indicates the denitrification rate. The numbers on the lower left indicate the correlation coefficients of the denitrification rates, denitrifying gene abundances and environmental factors; a coefficient value > 0.31 indicates that the correlation between the two variables is significant at a 95% confidence level. The shaded ellipses on the upper right indicate the direction and magnitude of the interactions between two variables. The darker the red/blue shades are, the greater the positive/negative correlations between two variables. “W-” and “S-” indicate the overlying water and sediment, respectively. The figures were created using Origin 9.0 (OriginLab Corporation, USA).
Regardless of saline and saline-alkali conditions, the denitrification rates were positively correlated with N concentrations, including NH4+-N and NO3–N, but negatively correlated with variables related to salinity, including Na+ and EC, of the overlying water and sediment (Fig. 3a,b). Moreover, the denitrification rates were also negatively correlated with the DO of the overlying water. In the saline conditions, the pH of the overlying water and sediment did not change significantly when the salt gradient increased. However, the denitrification rates were negatively and significantly correlated with the pH of the sediment in the saline-alkali conditions (Fig. 3b). The pH of the overlying water–sediment system played different roles in the denitrification rates in the saline and saline-alkali conditions. The denitrification rates decreased with the increase of pH in the saline-alkali conditions, while pH had no significant effect on denitrification rates in the saline conditions.
Similar to the relationship between the denitrification rates and environmental factors, the denitrifying gene abundance was negatively correlated with salinity and DO in both the saline and saline-alkali conditions (Fig. 3a,b). Furthermore, in the saline-alkali condition, the abundances of the nirK, nirS and nosZ genes were significantly and negatively correlated with pH. Redundancy analysis (RDA) was performed to further evaluate the relationship among environmental factors and the abundance of denitrifying genes (Fig. 4). The RDA showed that the physico-chemical properties of the overlying water–sediment system explained 79.43% (RDA1, 77.31%; RDA2, 2.12%) and 75.33% (RDA1, 73.36%; RDA2, 1.97%) of the variance in the denitrifying gene abundance in the saline and saline-alkali conditions, respectively. As Fig. 4 shows, the denitrifying gene abundances were positively correlated with N concentration and negatively correlated with salinity and DO. Meanwhile, Fig. 4b indicates that the denitrifying gene abundances were also negatively correlated with the pH of the overlying water and sediment in the saline-alkali condition.
Redundancy analysis (RDA) plot of the relationship between the abundances of denitrifying functional genes and environmental factors (a, saline environment; b, saline-alkali environment). Solid blue lines indicate the denitrifying gene abundances, and solid red lines show environmental factors. “W-” and “S-” indicate the overlying water and sediment, respectively.
The determinants of denitrification in saline-alkali soils
To investigate the effect of environmental factors on denitrification rates and denitrifying gene abundances in more detail, we input those variables in a PLSR model. In this model, salinity, pH, DO, N concentrations and denitrifying gene abundances were considered predictors and denitrification rates were considered responding variables. We established two PLSR models based on salt and salt-alkali conditions according to the different the overlying water–sediment system environments. The two models explained 90.2% and 91.8% of the variance in the denitrification rates in the saline and saline-alkali environments, respectively (Tables S2, S3).
The relationship among environmental factors found by the regression analysis could be seen in the PLSR weight plot of component 1 versus component 2 (Fig. 5). For the saline environment, component 1 accounted for 50.7% of the variation in the independent variables (environmental factors) and 83.4% of the variation in the denitrification rates. Component 2 accounted for another 15.1% and 6.8%, resulting in a total of 65.8% and 90.2%, respectively (Table S2, Fig. 5a). The DO of the overlying water and the system salinity were clustered along component 1, and the negative relationship between these factors and the denitrification rates was obvious since they were found at opposite ends of component 1 (Fig. 5a). For the saline-alkali environment, components 1 and 2 accounted for 53.0% and 18.3% of the variation in the environmental factors and 67.7% and 24.1% of the variation in the denitrification rates, respectively (Table S3, Fig. 5b). Denitrification rates were negatively correlated with the overlying water DO, salinity and pH of the system because they were located at both ends of component 1. In contrast, NO3–N in the overlying water, NH4+-N in the sediment and the abundance of denitrifying genes positively correlated with the denitrification rates since they were in the same region as the denitrification rates (Fig. 5b).
Variable importance in prediction (VIP) generated by PLSR analysis reflects the contribution of the independent variables to the denitrification rates; that is, the greater the VIP value is, the greater the explanatory ability of the environmental factors on the denitrification rates (Fig. 6). It is generally considered that independent variables with VIP values > 1.0 are important, independent variables with VIP values between 0.5 and 1.0 are of secondary importance, and independent variables with VIP values less than 0.5 are not important. In the saline environments, the salinity (EC and Na+ concentration), DO, N concentration (NO3–N in the overlying water, NO3–N and NH4+-N in the sediment) and denitrifying gene abundance (nirK and nosZ) were identified as important predictors with VIP values > 1 (Fig. 6a). The VIP value of pH was < 1, which indicated that it was not an important indicator of the saline environment in the PLSR model (Fig. 6a). In the saline-alkali environment, NO3–N in the overlying water and the abundance of denitrifying genes (nirK, nirS and nosZ) were important predictors in the PLSR model, and salinity, DO, pH and N concentration (NO3–N and NH4+-N in the sediment) were the second most important predictors (Fig. 6b). In contrast to the saline environment, the pH and salinity showed similarly important contributions to the denitrification rates in the PLSR model of the saline-alkali environment (Fig. 6b). The geometric mean error ratio (GMER), which is calculated from the error ratio of measured vs. predicted values, is widely used in the literature to describe model biases. A GMER equal to 1 corresponds to an exact match between measured and predicted data, and therefore, the best model will have a GMER close to 139. The GMER of the two PLSR models that we established in the saline and saline-alkali environments were 1.004 and 1.002, respectively (Fig. 7).
Variable importance for the projections (bars) and regression coefficients (lines) for each predictor of denitrification rates in a saline environment (a) and a saline-alkali environment (b). VIP, variable importance in prediction. “W-” and “S-” indicate the overlying water and sediment, respectively.
Discussion
Effects of saline-alkali environments on denitrification
Coastal ecosystems are hotspots for N cycling21, especially for removal of Nr from farmland drainage ditches through denitrification process5. Salinity plays a critical role in the denitrification process of drainage ditch ecosystem. Elevated salinity has been reported to alter N concentration21, the substrate for denitrification, and decrease denitrification significantly (Fig. 1a). The results from this study have shown that the shifts in substrate availability and salinity were largely responsible for the variation in denitrification rates, and salinity also affected the availability of the substrate. High Na+ concentrations cause rapid NH4+ mobilization from the cation exchange sites of sediment in the short term18 and increase the NH4+ availability in soil porewater23, which promotes the volatilization of ammonia30,40 and reduces the substrate in the process of nitrification and denitrification41. Additionally, the decreased of NO3–N concentration in the overlying water with the increase of salinity directly reducing the availability of denitrification substrate (Table S1).
Denitrification rates are generally considered to increase with increasing soil pH in acidic soils42,43, and this result was obtained in the saline environment in the present study, although this correlation was not significant (Fig. 3a). However, the denitrification rates were significantly and negatively related to sediment pH in the saline-alkali environment, and the pH of the sediment was far greater than that in the saline environment. Compared with the saline environment, the denitrification rates of the saline-alkali environment decreased even more (Fig. 1a), and besides salinity and DO (Fig. 6a), high pH was also a restrictive factor for denitrification in the saline-alkali soil (Fig. 6b). The presence of large amounts of alkaline compounds, such as bicarbonate ion (HCO3−), in the saline-alkali environment, which hydrolyze to produce hydroxide ions (OH−), causing an increase in system pH. The NO3–N concentration in the overlying water in the saline-alkali environment with high pH was further reduced, which decreasing the availability of denitrification substrate (Table S1). In summary, salinity and high pH negatively affected denitrification by reducing the availability of N in the short term. However, with the increase of salt-alkali gradients, the denitrification continued to decrease, while the availability of nitrate nitrogen was not decrease significantly. This indicated that there were other reasons that inhibited the denitrification rates, which will be discussed in the next section.
Microbial mechanisms behinds salt and alkali
The denitrification rate is essentially a nitrate reduction process mediated by microbial functional groups. In many cases, the denitrifying microorganisms are controlled by environmental factors and gradually adapts to the environment44. This study provides a detailed experimental comparison of denitrifying gene abundance in different saline and saline-alkali environments. Although this study is based on manipulated sediments recovered from dry soils, the restored microbial communities originally came from field soils and therefore represented in situ conditions to some extent21. While there is limited information on the relationship between salinity and community structure, this study provided strong evidence that high salinity concentrations significantly and negatively affect the gene abundances of denitrifiers. Dominant genera are substituted by salt-adaptable microbes as salinity increases45. A decline in the abundance of denitrifying genes was found with increasing salt or salt-alkali conditions (Fig. 2), which is similar to the findings of previous research21,46,47. However, salinity exhibited a limited influence on nirK and nirS abundances in this study, and low salinity concentrations had no significant effect on the abundance of nirK and nirS (Fig. 2a,b). The abundance of nosZ gene, encoding reductase for the last step of denitrification, was more sensitive to salinity than nirK and nirS, and played a dominant role in denitrification under low salt conditions as evidenced by the concurrent decrease of denitrification rates and nosZ gene abundances (Fig. 2c). As salinity continued to rise, the abundances of nirK and nirS decreased significantly. Nevertheless, the relative importance of nirK and nirS for denitrification in saline environments remains controversial20,21,48. A study in the San Francisco Bay estuary revealed that salinity had a significant negative correlation with nirK abundance and a significant positive correlation with nirS abundance49. Another study in a Jiulong River estuary mudflat revealed that an increase in salinity inhibited the abundances of nirK and nosZ, while nirS abundances remained stable21.
The identification of salinity as a strong driver of denitrifying gene abundance is consistent with several studies21,44,50. Moreover, salinity is a key regulator that decreases denitrification in coastal ecosystems, primarily as a result of its effect on denitrifiers51. There are several mechanisms by which salinity affects microbial communities. In laboratory incubation studies, an increase in salinity might directly limit the adaptability of microorganisms and simultaneously reduce soil respiration52, which could reduce oxygen consumption and increase the DO in the overlying water, thereby inhibiting the synthesis of nitrous oxide reductase53 and decreasing the abundance of nosZ gene30. Moreover, the increase of DO in the overlying water might inhibit the activity of anaerobic denitrifying bacteria54. Meanwhile, soil microbial populations usually adapt to freshwater or low salt environments, and the decrease in the gene abundances of denitrifying bacteria is potentially due to osmotic stress of microbial cells caused by elevated salinity levels55, which interrupts cellular function, growth and even cell lysis56. In addition, salinity has a direct inhibitory effect on denitrifying bacteria, with Cl- toxicity suggested as the possible reason23. Furthermore, the reduced activity of nitrifying bacteria and nitrifying enzymes by the chloride (Cl-) in saline environments further inhibits nitrification22,23 and decreases the substrate for denitrification.
It has been reported that the optimal pH range for denitrifiers is 7–957; values beyond this range may hinder the denitrification process and lead to the accumulation of intermediates: NO2-, NO2 and N2O57. However, the pH of the sediment in the saline-alkali environment exceeding the suitable range of denitrifiers. High pH in the saline-alkali soils further decreased denitrifying gene abundance and denitrification rates (Figs. 1a, 2). Furthermore, great quantities of denitrifying bacteria attach to overlying water–sediment interfaces58, and the high pH may inhibit microbial processing of N by reducing the efficiency of enzymatic attacks59. The denitrification rates were significantly and positively correlated with NO3–N in the overlying water and sediment in the saline environment (Fig. 3a; Fig. S2). However, there was no significant correlation between the denitrification rates and sediment NO3–N in the saline-alkali environment (Fig. 3b; Fig. S3), although the NO3–N concentration of the sediment in the saline-alkali environment was higher than that in the saline environment. This indicated that the utilization of NO3–N in the sediment by denitrifiers may be inhibited in saline-alkali environments because the high pH possibly affects microbial access to N resources59. The NO3–N in the overlying water directly provided a substrate for denitrifiers and may have played a more vital role in the denitrification rates than did the NO3–N of the sediment in the saline-alkali environments (Fig. 6)22. This study suggested that the denitrification process became inactive or inhibited in a saline-alkali environment with a high pH, which is in accordance with Anderson et al.59. As confirmed by the present study, salinity and high pH had a great impact on N cycling by affecting the abundance of functional genes in the short term.
Implications for non-point N pollution management
Efforts to reduce non-point source N pollution from watersheds to coastal water are underway around the world60,61, and decreasing the output load of non-point source N pollution from ditches to receiving water bodies is an important way to achieve this goal. Improving the removal efficiency of Nr in ditches can effectively reduce the output load of non-point source N pollution. The N removal efficiency in the short term is related to the original N content and denitrification rate. Although the high original N concentration promoted the denitrification rate (Fig. 1a), the N removal efficiency in the short term of the treatments with low urea applications was higher than that of the treatments with high urea applications under the same salinity (Fig. 1b). In farmland drainage ditches with more exogenous N input, although the denitrification rate is high in the short term, it still takes a long time to eliminate non-point source N through denitrification. Therefore, the denitrification time can be prolonged by increasing the hydraulic retention time of the ditches to achieve a high N removal efficiency62. However, the relationship between the time required to fully remove non-point source N by denitrification and the initial N concentration in saline-alkali soils needs to be further studied.
Non-point source N output load management plays a critical role in achieving water quality goals63. At this stage, China’s agricultural non-point source N pollution management technology mainly focuses on three fields, namely, pollution source reduction, pollutant migration interception, and nutrient recycling64. Among them, pollutant migration interception refers to the interception, removal and utilization of non-point source N pollutants by physical, chemical or biological means during their migration into and accumulation in rivers and lakes in order to reduce the pollutant load of ditches and the concentration of pollutants in the lakes65. The denitrification process in the ditches is an effective way to intercept the migration of Nr into the rivers. A full understanding of the impact of saline-alkali environments on denitrification rates is conducive to formulating reasonable pollutant migration interception strategies for ditch pollutants and effectively improving the N removal efficiency of ditches in coastal areas. In coastal areas, the high salinity and pH of the soil in ditches inhibit the denitrification rates and hinder the reduction of nitrate, thus reducing the interception by ditches on pollutant migration. The combination between non-point source N pollution control and basin ditch management is highly recommended59. In the daily management of ditches, corresponding measures such as chemical improvements and irrigation leaching technologies should be adopted to reduce the salt and alkali contents of ditches and improve denitrification potential. In addition, the removal effect of denitrification on reactive nitrogen in ditches can be further increased by prolonging the hydraulic retention time of ditches. Furthermore, when estimating non-point source N pollution loads, the inhibition effects of saline-alkali environments on denitrification in ditches should be considered.
Conclusion
Based on flooded incubation experiments of farmland ditches under various salt and salt-alkali gradients, we examined the changes in denitrification rates and denitrifying gene abundance in saline and saline-alkali environments. With an increase in the salt gradient from 1‰ to 3‰, 8‰, and 15‰, the denitrification rates significantly decreased by 23.83–50.08%, 20.64–57.31% and 6.12–54.61% under the addition of 0.05‰, 0.10‰ and 0.15‰ urea conditions, respectively. The inhibition of denitrification rates in a saline-alkali environment with a high pH was stronger than that in a saline environment. With the increase in the salt-alkali gradient from 0.5‰ to 1‰, 3‰, and 8‰, the denitrification rates significantly decreased by 44.57–59.44%, 54.88–65.19% and 57.84–63.24% under the addition of 0.05‰, 0.10‰ and 0.15‰ urea conditions, respectively. The abundance of nitrite reductase genes (nirK and nirS) slightly decreased under a low salt gradient but decreased significantly under high salt conditions, and the abundance of the nitrous oxide reductase gene (nosZ) sharply decreased in the saline environment. Compared with the saline environment, the saline-alkali environment decreased the abundance of denitrifying genes more significantly. In addition, the increase in N concentration could limit the reduction in denitrifying gene abundance. The salinity, DO in the overlying water, N concentration and denitrifying gene abundance were key determinants of the denitrification rate. In the saline environment, the salinity and DO were the main limiting factors of the denitrification rate, and in the saline-alkali environment, high pH was also a determinant. Salinity and high pH decreased the denitrification rates by both inhibiting the abundance of denitrifying genes nirK, nirS, and nosZ and reducing the substrate availability for denitrification process. In addition, the decrease of substrate availability would also affect the abundance of denitrifying genes.
References
Cui, Z. L. et al. Closing the N-use efficiency gap to achieve food and environmental security. Environ. Sci. Technol. 48(10), 5780–5787 (2014).
Chen, F. et al. Net anthropogenic nitrogen inputs (NANI) into the Yangtze River basin and the relationship with riverine nitrogen export. J. Geophys. Res. Biogeo. 121(2), 451–465 (2016).
Huang, J., Xu, C.-C., Ridoutt, B. G., Wang, X.-C. & Ren, P.-A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 159, 171–179. https://doi.org/10.1016/j.jclepro.2017.05.008 (2017).
Ju, X. T. et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 106(9), 3041–3046 (2009).
She, D. et al. Limited N removal by denitrification in agricultural drainage ditches in the Taihu Lake region of China. J. Soil. Sediment. 18(3), 1110–1119 (2018).
Xia, Y. et al. Improving denitrification models by including bacterial and periphytic biofilm in a shallow water-sediment system. Water Resour. Res. 54(10), 8146–8159 (2018).
Álvarez-Rogel, J. et al. Nitrate removal and potential soil N2O emissions in eutrophic salt marshes with and without Phragmites australis. Geoderma 282, 49–58 (2016).
Audet, J., Olsen, T. M., Elsborg, T., Baattrup-Pedersen, A. & Riis, T. Influence of plant habitats on denitrification in lowland agricultural streams. J. Environ. Manage. 286, 112193. https://doi.org/10.1016/j.jenvman.2021.112193 (2021).
Li, L. et al. Potential denitrification activity response to long-term nitrogen fertilization: A global meta-analysis. J. Clean. Prod. https://doi.org/10.1016/j.jclepro.2022.130451 (2022).
Zhong, J. et al. Seasonal variation of potential denitrification rates of surface sediment from Meiliang Bay, Taihu Lake, China. J. Environ. Sci. 22(7), 961–967 (2010).
Sun, X., She, D., Fei, Y., Wang, H. & Gao, L. Three-dimensional fractal characteristics of soil pore structure and their relationships with hydraulic parameters in biochar-amended saline soil. Soil Till. Res. 205, 104809. https://doi.org/10.1016/j.still.2020.104809 (2021).
Wang, H., She, D., Sun, X., Tang, S. & Zheng, Y. Analysis of unsaturated shear strength and slope stability considering soil desalinization in a reclamation area in China. CATENA 196, 104949. https://doi.org/10.1016/j.catena.2020.104949 (2021).
Wu, Y., Li, Y., Zheng, C., Zhang, Y. & Sun, Z. Organic amendment application influence soil organism abundance in saline alkali soil. Eur. J. Soil Biol. 54, 32–40 (2013).
Zhang, H. S., Zai, X. M., Wu, X. H., Qin, P. & Zhang, W. M. An ecological technology of coastal saline soil amelioration. Ecol. Eng. 67, 80–88 (2014).
Wang, X. et al. Effect of Trichoderma viride biofertilizer on ammonia volatilization from an alkaline soil in Northern China. J. Environ. Sci. 66, 199–207 (2018).
Yu, F. & Shi, W. Nitrogen use efficiencies of major grain crops in China in recent 10 years. Acta Pedol. Sin. 52(6), 1311–1324 (2015) (in Chinese).
Herbert, E. R. et al. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6, 10. https://doi.org/10.1890/ES14-00534.1 (2015).
van Dijk, G. et al. Salinization lowers nutrient availability in formerly brackish freshwater wetlands; unexpected results from a long-term field experiment. Biogeochemistry 143(1), 67–83 (2019).
Hu, Y. et al. Variability in soil microbial community and activity between coastal and riparian wetlands in the Yangtze River estuary: Potential impacts on carbon sequestration. Soil Biol. Biochem. 70, 221–228 (2014).
Franklin, R. B., Morrissey, E. M. & Morina, J. C. Changes in abundance and community structure of nitrate-reducing bacteria along a salinity gradient in tidal wetlands. Pedobiologia 60, 21–26 (2017).
Wang, H., Gilbert, J. A., Zhu, Y. & Yang, X. Salinity is a key factor driving the nitrogen cycling in the mangrove sediment. Sci. Total Environ. 631–632, 1342–1349 (2018).
Giblin, A. E., Weston, N. B., Banta, G. T., Tucker, J. & Hopkinson, C. S. The effects of salinity on nitrogen losses from an oligohaline estuarine sediment. Estuar. Coast. 33(5), 1054–1068 (2010).
Marks, B. M., Chambers, L. & White, J. R. Effect of fluctuating salinity on potential denitrification in coastal wetland soil and sediments. Soil Sci. Soc. Am. J. 80(2), 516–526 (2016).
Chen, J. H. et al. Start-up and microbial communities of a simultaneous nitrogen removal system for high salinity and high nitrogen organic wastewater via heterotrophic nitrification. Bioresour. Technol. 216, 196–202 (2016).
Zhao, Y. Y. et al. Effect of different salinity adaptation on the performance and microbial community in a sequencing batch reactor. Bioresour. Technol. 216, 808–816 (2016).
Jiang, Y. et al. Response and recovery of aerobic granular sludge to pH shock for simultaneous removal of aniline and nitrogen. Chemosphere 221, 366–374 (2019).
Jha, N. et al. Influence of liming-induced pH changes on nitrous oxide emission, nirS, nirK and nosZ gene abundance from applied cattle urine in allophanic and fluvial grazed pasture soils. Biol. Fert. Soils 56(6), 811–824 (2020).
Vega-Jarquin, C., Garcia-Mendoza, M., Jablonowski, N., Luna-Guido, M. & Dendooven, L. Rapid immobilization of applied nitrogen in saline-alkaline soils. Plant Soil 256(2), 379–388 (2003).
Qin, H. et al. A few key nirK- and nosZ-denitrifier taxa play a dominant role in moisture-enhanced N2O emissions in acidic paddy soil. Geoderma 385, 114917. https://doi.org/10.1016/j.geoderma.2020.114917 (2021).
Pan, Y. C., She, D. L., Chen, X. Y., Xia, Y. Q. & Timm, L. C. Elevation of biochar application as regulator on denitrification/NH3 volatilization in saline soils. Environ. Sci. Pollut. R. 28(31), 41712–41725 (2021).
Gao, S., He, Q. & Wang, H. Research on the aerobic granular sludge under alkalinity in sequencing batch reactors: Removal efficiency, metagenomic and key microbes. Bioresour. Technol. 296, 122280. https://doi.org/10.1016/j.biortech.2019.122280 (2020).
Kana, T. M. et al. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal. Chem. 66(23), 4166–4170 (1994).
Pan, Y. C., She, D. L., Shi, Z. Q., Chen, X. Y. & Xia, Y. Q. Do biochar and polyacrylamide have synergistic effect on net denitrification and ammonia volatilization in saline soils?. Environ. Sci. Pollut. R. 28(42), 59974–59987 (2021).
Schmidt, J. E., Kent, A. D., Brisson, V. L. & Gaudin, A. C. M. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 7(1), 756 (2019).
Henry, S. et al. Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J Microbiol. Methods. 59, 327–335 (2004).
Kandeler, E., Deiglmayr, K., Tscherko, D., Bru, D. & Philippot, L. Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl. Environ. Microbiol. 72, 5957–5962 (2006).
Henry, S., Bru, D., Stres, B., Hallet, S. & Philippot, L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 72(8), 5181–5189 (2006).
Carrascal, L. M., Galvan, I. & Gordo, O. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 118(5), 681–690 (2009).
Wagner, B. et al. Evaluation of pedo-transfer functions for unsaturated soil hydraulic conductivity using an independent data set. Geoderma 102(3–4), 275–297 (2001).
Li, Y. et al. Salinity-induced concomitant increases in soil ammonia volatilization and nitrous oxide emission. Geoderma 361, 114053. https://doi.org/10.1016/j.geoderma.2019.114053 (2020).
Ardon, M., Morse, J. L., Colman, B. P. & Bernhardt, E. S. Drought-induced saltwater incursion leads to increased wetland nitrogen export. Glob. Chang. Biol. 19(10), 2976–2985 (2013).
Cuhel, J. et al. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microb. 76(6), 1870–1878 (2010).
Qian, W., Ma, B., Li, X., Zhang, Q. & Peng, Y. Long-term effect of pH on denitrification: High pH benefits achieving partial-denitrification. Bioresour. Technol. 278, 444–449 (2019).
Fu, G., Han, J., Yu, T., Huangshen, L. & Zhao, L. The structure of denitrifying microbial communities in constructed mangrove wetlands in response to fluctuating salinities. J. Environ. Manage. 238, 1–9 (2019).
Xia, Z. et al. Nitrogen removal pathway and dynamics of microbial community with the increase of salinity in simultaneous nitrification and denitrification process. Sci. Total Environ. 697, 134047. https://doi.org/10.1016/j.scitotenv.2019.134047 (2019).
Wang, J. et al. Denitrification and anammox: Understanding nitrogen loss from Yangtze Estuary to the east China sea (ECS). Environ. Pollut. 252, 1659–1670 (2019).
Zheng, Y. et al. Diversity, abundance, and distribution of nirS-harboring denitrifiers in intertidal sediments of the Yangtze Estuary. Microb. Ecol. 70(1), 30–40 (2015).
Ma, L. et al. Quantitative response of nitrogen dynamic processes to functional gene abundances in a pond-ditch circulation system for rural wastewater treatment. Ecol. Eng. 134, 101–111 (2019).
Mosier, A. C. & Francis, C. A. Denitrifier abundance and activity across the San Francisco Bay estuary. Environ. Microbiol. Rep. 2(5), 667–676 (2010).
Zaghmouri, I., Michotey, V. D., Armougom, F., Guasco, S. & Bonin, P. C. Salinity shifts in marine sediment: Importance of number of fluctuation rather than their intensities on bacterial denitrifying community. Mar. Pollut. Bull. 130, 76–83 (2018).
Zhou, M., Butterbach-Bahl, K., Vereecken, H. & Brueggemann, N. A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob. Chang. Biol. 23(3), 1338–1352 (2017).
Wong, V. N. L., Dalal, R. C. & Greene, R. S. B. Salinity and sodicity effects on respiration and microbial biomass of soil. Biol. Fert. Soils 44(7), 943–953 (2008).
Sun, X., Jayakumar, A. & Ward, B. B. Community composition of nitrous oxide consuming bacteria in the oxygen minimum zone of the eastern tropical South Pacific. Front. Microbiol. 8, 1183. https://doi.org/10.3389/fmicb.2017.01183 (2017).
Penn, J., Weber, T. & Deutsch, C. Microbial functional diversity alters the structure and sensitivity of oxygen deficient zones. Geophys. Res. Lett. 43(18), 9773–9780 (2016).
Panswad, T. & Anan, C. Impact of high chloride wastewater on an anaerobic/anoxic/aerobic process with and without inoculation of chloride acclimated seeds. Water Res. 33(5), 1165–1172 (1999).
Rath, K. M. & Rousk, J. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 81, 108–123 (2015).
Tang, Y., Zhou, C., Ziv-El, M. & Rittmann, B. E. A pH-control model for heterotrophic and hydrogen-based autotrophic denitrification. Water Res. 45(1), 232–240 (2011).
Chen, D. et al. Controlling denitrification accompanied with nitrite accumulation at the sediment–water interface. Ecol. Eng. 100, 194–198 (2017).
Anderson, C., Peterson, M. & Curtin, D. Base cations, K+ and Ca2+, have contrasting effects on soil carbon, nitrogen and denitrification dynamics as pH rises. Soil Biol. Biochem. 113, 99–107 (2017).
Hou, X. Y., Ying, L. L., Chang, Y. Y., Qian, S. S. & Zhang, Y. Modeling of non-point source nitrogen pollution from 1979 to 2008 in Jiaodong Peninsula, China. Hydrol. Process. 28(8), 3264–3275 (2014).
Yang, H. et al. Nitrogen removal by eutrophic coastal wetlands accomplished with CH4 emission reduction. J. Clean. Prod. https://doi.org/10.1016/j.jclepro.2021.130082 (2022).
Shields, C. A. et al. Streamflow distribution of non-point source nitrogen export from urban-rural catchments in the Chesapeake Bay watershed. Water Resour. Res. 44, 9. https://doi.org/10.1029/2007WR006360 (2008).
Pan, S. F. et al. Impacts of multiple environmental changes on long-term nitrogen loading from the Chesapeake Bay Watershed. J. Geophys. Res. Biogeo. 126, 5. https://doi.org/10.1029/2020JG005826 (2021).
Yu, Y. L. et al. Situation analysis and trend prediction of the prevention and control technologies for planting non-point source pollution. Environ. Sci. 41(8), 3870–3878 (2020) (in Chinese).
Wang, X., Qiao, B., Li, S., Li, J. & Ren, B. Studies on the interception effects of ecological ditch on nitrogen and phosphorus in the rainfall runoff of different rice growth period. J. Hydraul. Eng. 46(12), 1406–1413 (2015) (in Chinese).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (U20A20113 and 42177393), the Postgraduate Research and Practice Innovation Program of Jiangsu Province (Grant Number KYCX22_0671), the Water Science and Technology Project of Jiangsu Province (Grant Number 2021054), the Natural Resources Science and Technology Project of Jiangsu Province (Grant Number 2022046), and the Natural Resources and Planning Science and Technology Innovation Project of Nantong (Grant Number 2022005).
Author information
Authors and Affiliations
Contributions
Y.P.: Investigation, Formal analysis, Visualization, Writing- Original draft, and Writing—Reviewing and Editing; D.S.: Supervision, Conceptualization, Resources, Funding acquisition, and Writing—Reviewing and Editing; Zhenqi Shi: Investigation, Methodology; T.C.: Investigation; Formal analysis; Y.X.: Methodology, Supervision; J.S.: Supervision, Funding acquisition, Writing—Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, Y., She, D., Shi, Z. et al. Salinity and high pH reduce denitrification rates by inhibiting denitrifying gene abundance in a saline-alkali soil. Sci Rep 13, 2155 (2023). https://doi.org/10.1038/s41598-023-29311-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29311-7
This article is cited by
-
Grazing intensity changes root traits and resource utilization strategies of Stipa breviflora in a desert steppe
Plant and Soil (2024)
-
Field application of hydroxyapatite and humic acid for remediation of metal-contaminated alkaline soil
Environmental Science and Pollution Research (2024)
-
Effect of salinity on biological nitrogen removal from wastewater and its mechanism
Environmental Science and Pollution Research (2024)
-
Spatial distribution characteristics of denitrification functional genes and the environmental drivers in Liaohe estuary wetland
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.