Abstract
The SARS-CoV-2 Omicron (B.1.1.529) Variant of Concern (VOC) and its sub-lineages (including BA.2, BA.4, BA.5, BA.2.12.1) contain spike mutations that confer high level resistance to neutralizing antibodies induced by vaccination with ancestral spike or infection with previously circulating variants. The NVX-CoV2373 vaccine, a protein nanoparticle vaccine containing the ancestral spike sequence, has value in countries with constrained cold-chain requirements. Here we report neutralizing titers following two or three doses of NVX-CoV2373. We show that after two doses, Omicron sub-lineages BA.1 and BA.4/BA.5 were resistant to neutralization by 72% (21/29) and 59% (17/29) of samples respectively. However, after a third dose of NVX-CoV2373, we observed high titers against Omicron BA.1 (GMT: 1,197) and BA.4/BA.5 (GMT: 582), with responses similar in magnitude to those triggered by three doses of an mRNA vaccine. These data are of particular relevance as BA.4/BA.5 is dominating in multiple locations, and highlight the potential utility of the NVX-CoV2373 vaccine as a booster in resource-limited environments.
Similar content being viewed by others
Introduction
The SARS-CoV-2 Omicron (B.1.1.529) Variant of Concern (VOC)1 and its sub-lineages2,3 (including BA.2, BA.4, BA.5, BA.2.12.1) contain changes to the spike driven by immune escape, and are relatively immune evasive compared with the ancestral-like virus to neutralizing antibodies elicited by coronavirus disease 2019 (COVID-19) vaccines4,5. Similarly, individuals infected with SARS-CoV-2 exhibit reduced neutralizing titers against multiple Omicron sub-lineages4, with BA.4 and BA.5 currently detected in over 95 countries and the latter dominating globally. While BA.4 and BA.5 have been classified as distinct sub-lineages, they share the same dominant spike mutations.
Neutralization escape by the Omicron VOC has also been observed following vaccination, regardless of the vaccine type and platform4,5,6,7,8,9, including with two doses of the NVX-CoV2373 vaccine where a 16-fold lower titer than the ancestral variant was observed10. However, booster doses, especially using mRNA vaccines, enhance neutralization capacity against Omicron5,8. The NVX-CoV2373 vaccine, which was tested in two phase 3 trials in the US, UK and Mexico demonstrated 90% efficacy against symptomatic and 100% efficacy against severe COVID-19, when ancestral-like and Alpha variants dominated the infections11,12. A Phase 2b trial in South Africa in 2020–2021 demonstrated 48% efficacy against symptomatic COVID-19, likely due to relatively antibody-evasive neutralization resistant Beta variant, despite 100% efficacy against severe disease13. The vaccine has received authorization for use by the European Medicines Agency, is listed on the World Health Organization’s emergency use listing for COVID-19 vaccines and has received emergency use authorization from the US FDA13,14,15,16. This protein-based vaccine is appealing in low-and middle-income countries (LMICs) because of its stability and reduced cold chain requirements. Here, we investigated the effect of a third dose on the neutralizing capacity of NVX-CoV2373 vaccinee sera.
Results
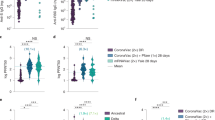
Using a spike-pseudotyped assay, we tested neutralization of the ancestral D614G, Beta, Omicron BA.1 and Omicron BA.4/BA.5 by NVX-CoV2373 vaccinee sera following a 2 dose (n = 29) and 3 dose (n = 48) regimen. These sera were collected from 52 individuals, of which 37% were female and 50% were under 29 years of age (Supplementary table 1). Fourteen days after two doses of NVX-CoV2373, geometric mean titers (GMT) were highest against the D614G variant (GMT: 1,401), with reductions in GMT to 173 (8.1-fold reduction), 34 (41-fold reduction) and 47 (30-fold reduction) against Beta, Omicron BA.1 and Omicron BA.4/BA.5 respectively. For the Omicron sub-lineages BA.1 and BA.4/BA.5, titers were lower than the limit of detection of the assay for 72% (21/29) and 59% (17/29) of samples, respectively, after the 2nd dose of vaccine (Fig. 1, grey).
Neutralization of SARS-CoV-2 variants by NVX-CoV2373 vaccinee plasma. Neutralization of ancestral D164G, Beta, Omicron BA.1 and Omicron BA.4/BA.5 pseudoviruses by NVX-CoV2373 vaccinee plasma following 2 (grey) or 3 (teal) doses. Samples were collected 14 days after the second dose and 1 month after the third dose. Geometric mean titers (GMT) for each virus are shown above the individual points, and percent of specimens where no neutralization was observed (red) is indicated in the pie charts. Number of vaccinee specimens tested are indicated and p values were calculated using the Mann–Whitney t-test for non-parametric data with p < 0,001 for D614G, Beta, Omicron BA.1 and Omicron BA.4/BA.5. Samples were used at a starting dilution of 1 in 20 (limit of detection) with a seven threefold dilutions to create a titration series.
At 1 month after the third dose of the NVX-CoV2372 vaccine, neutralizing antibody activity was evident against the Beta and Omicron BA.1 variants in all samples, in contrast to 2 weeks post the two doses discussed above (Fig. 1, pie charts). The neutralizing antibody titers against the D614G variant were boosted to a GMT of 10,862. Furthermore, we observed a significant ten-, 35- and 12-fold increase in titers against Beta (GMT: 1733), Omicron BA.1 (GMT: 1197) and Omicron BA.4/BA.5 (GMT: 582) respectively (Fig. 1, teal), though boosted titers were six- to 18-fold lower than those against D614G.
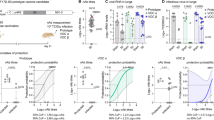
We next compared neutralization of Omicron BA.1 and BA.4/BA.5 following multi-dose regimens of adenoviral, mRNA and protein-based vaccines. We tested samples after 2 doses of the adenoviral or 3 doses of the mRNA vaccines, given that these are currently offered as booster regimens in South Africa. These sera were collected from 11 individuals who received the AD26.COV2S vaccine (73% were female and all were over 30 years of age) and nine individuals who received the BNT162b2 vaccine (100% female and 22% were under 29 years of age, Supplementary table 1). As expected, 2 doses of AD26.COV2.S elicited ten- and 14-fold lower GMT against BA.1 than 3 doses of the BNT162b2 and NVX-CoV2373 vaccines respectively (Fig. 2). Similarly the 2 dose AD26.COV2.S vaccine elicited 12- and 11-fold lower GMT against BA.4/BA.5 than 3 does of either the BNT162b2 and NVX-CoV2373 vaccines. All third dose BNT162b2 and NVX-CoV2373 plasma were able to neutralize Omicron BA.1 and BA.4/BA.5, while only 13–50% of the two dose AD26.COV2.S samples had neutralizing activity against these Omicron sub-lineages. The NVX-CoV2373 third dose plasma GMT against BA.1 and BA.4/BA.5 was comparable to the BNT162b2 titres.
Neutralization of Omicron BA.1 and BA.4/BA.5 by boosted vaccinee plasma. Neutralization of Omicron BA.1 and BA.4/BA.5 by vaccinee plasma following 2 doses of the AD26.COV2S or 3 doses of the BNT162b2 or NVX-CoV2373 vaccines. Number of doses, number of samples and date of sample collection after boost for each group are indicated. Geometric mean titers (GMT) for each virus are shown above the individual points, P values were calculated using two-way ANOVA with p < 0.001 for AD26CoV2.S versus NXV-CoV2373 and p = 0.0011 for NVX-CoV2373 BA.1 versus BA.4/BA.5). Samples were used at a starting dilution of 1 in 20 (limit of detection) with a seven threefold dilutions to create a titration series.
Discussion
In summary, we report enhanced neutralization of Omicron BA.1 and BA.4/BA.5 following three doses of the NVX-CoV2373 vaccine with responses comparing well to three doses of an mRNA vaccine. We note that 6 months after two doses of NVX-CoV2373, increased binding antibodies were reported, suggesting that responses after the third dose may mature further10. As durability of vaccine platforms varies, future studies should assess this for NVX-CoV2373 neutralization at later time-points10. The two dose NVX-CoV2372 vaccine regimen elicits robust memory CD4+ and CD8+ T cell responses in 100% and 65% of individuals respectively7,10. In addition, the two dose regimen induces antibodies with multiple Fc-mediated functions, which in non-human primate and human cohorts likely contribute to protection from infection10. This T cell and Fc effector function data, which is unlikely to differ following a third dose of the NVX-CoV2373 vaccine, coupled with neutralizing antibodies we have described here, suggests that this vaccine is likely to prevent severe disease after SARS-CoV-2 breakthrough infection with Omicron BA.4/5 sub-lineages (Supplementary Table 1).
Limitations of the study include the difference in timing of the sample collection following the second and third dose of the NVX-CoV2373 vaccine, with collection at 14 days and 1 month post vaccination respectively. Similarly, sample collection following administration of the AD26COV2.S and BNT162b2 booster doses varies between 1 and 3 months and includes relatively small sample numbers. Despite these limitations, this study highlights the neutralizing titres elicited by a third dose of the NVX-CoV2373 against currently circulating Omicron sub-lineages, which supports the use of this vaccine as a booster regimen17 in countries where mRNA cold chain requirements cannot be met due to limited infrastructure.
Methods
Samples and ethics approvals
Individuals vaccinated with two or three doses of the NVX-CoV2373 vaccine were sampled at 14 days after the second dose or 35 days after the third dose. The third NVX-CoV2373 dose was administered 6 months after the first dose. This trial is registered under the ClinicalTrials.gov number, NCT04533399 (registered 17/09/2020), and the protocol was approved by the South African Health Products Regulatory Authority and by the institutional review board at each trial centre as described in detail by Shinde and colleagues13. Health care workers vaccinated with two dose of AD26.COV2.S (5 × 1010 viral particles) as part of the Sisonke implementation trial were sampled at 2 months after vaccination. This trial is registered under the ClinicalTrials.gov number, NCT05148845, and the protocol was approved by the South African Health Products Regulatory Authority. These Sisonke individuals were recruited at the National Institute for Communicable Diseases (NICD), Johannesburg. Individuals vaccinated with two and three doses of the BNT162b22 vaccine were sampled at 2 months after the second dose or 1–3 months after the third dose and were recruited from Johannesburg. This study was given ethics approval by the University of the Witwatersrand Human Research Ethics Committee (Medical) M210465. All individuals provided written informed consent and all research was performed in accordance with the relevant guidelines/regulations and in accordance with the Declaration of Helsinki.
Lentiviral pseudovirus production and neutralization assay
The 293 T/ACE2. MF cells modified to overexpress human ACE2 were kindly provided by M. Farzan (Scripps Research). Cells were cultured in DMEM (Gibco BRL Life Technologies) containing 10% heat-inactivated fetal bovine serum (FBS) and 3 μg ml−1 puromycin at 37 °C, 5% CO2. Cell monolayers were disrupted at confluency by treatment with 0.25% trypsin in 1 mM EDTA (Gibco BRL Life Technologies). The SARS-CoV-2, Wuhan-1 spike, cloned into pCDNA3.1 was mutated using the QuikChange Lightning Site-Directed Mutagenesis kit (Agilent Technologies) to include D614G (ancestral D164G) or L18F,D80A, D215G, Δ242-244, K417N, E484K, N501Y, D614G, A701V (Beta) or Δ69-70, T915I, Δ143-145, Δ211, L212I, ins 214 EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F (Omicron BA.1) or T19I, L24S, Δ25–27, Δ69–70, G142D, V213G, G339D, S371F, S373P, S375F, T376A, D405N, R408S, K417N, N440K, L452R, S477N, T478K, E484A, F486V, Q498R, N501Y, Y505H, D614G, H655Y, N679K, P681H, N764K, D796Y, Q954H, N969K (Omicron BA.4/BA.5). Pseudoviruses were produced by co-transfection with a lentiviral backbone (HIV-1 pNL4.luc encoding the firefly luciferase gene) and either of the SARS-CoV-2 spike plasmids with PEIMAX (Polysciences). Culture supernatants were clarified of cells by a 0.45 μM filter and stored at − 80 °C. Plasma samples were heat-inactivated and clarified by centrifugation. Pseudovirus and serially diluted plasma/sera were incubated for 1 h at 37 °C, 5% CO2. Cells were added at 1 × 104 cells per well after 72 h of incubation at 37 °C, 5% CO2, luminescence was measured using PerkinElmer Life Sciences Model Victor X luminometer. Neutralization was measured as described by a reduction in luciferase gene expression after single-round infection of 293 T/ACE2.MF cells with spike-pseudotyped viruses. Titers were calculated as the reciprocal plasma dilution (ID50) or monoclonal antibody concentration (IC50) causing 50% reduction of relative light units. Equivalency was established through participation in the SARS-CoV-2 Neutralizing Assay Concordance Survey Concordance Survey 1 run by EQAPOL and VQU, Duke Human Vaccine Institute. Cell-based neutralization assays using live virus or pseudovirus have demonstrated high concordance, with highly correlated 50% neutralization titers (Pearson r = 0.81–0.89).
Data availability
All data reported in this paper will be shared by the lead contacts, Penny L. Moore (pennym@nicd.ac.za) and Shabir Madhi (Shabir.Madhi@wits.ac.za) upon request. This paper does not report original code.
References
Viana, R. et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 603, 679–686 (2022).
Rambaut, A. et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 5, 1403–1407 (2020).
Tegally, H. et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 28(9), 1785–1790 (2022).
Cele, S. et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602, 654–656 (2022).
Garcia-Beltran, W. F. et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185, 457–466 (2022).
Richardson, S. I. et al. SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals. Cell Host Microbe 30, 880–886 (2022).
Zhang, Z. et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell (2022).
Bowen, J. E. et al. Omicron BA.1 and BA.2 neutralizing activity elicited by a comprehensive panel of human vaccines. bioRxiv (2022).
Cao, Y. et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 608(7923), 593–602 (2022).
Mallory, R. M. et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect. Dis. (2022).
Heath, P. T. et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 385, 1172–1183 (2021).
Dunkle, L. M. et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N. Engl. J. Med. 386, 531–543 (2022).
Shinde, V. et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 384, 1899–1909 (2021).
World Health Organization. WHO lists 9th COVID-19 vaccine for emergency use with aim to increase access to vaccination in lower-income countries. Vol 2022 (2021).
European Medicines Agency. Nuvaxovid. Vol 2022 (2021).
U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA authorizes emergency use of Novavax COVID-19 Vaccine, Vol 2022 (Adjuvanted, 2022).
Munro, A. P. S. et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 398, 2258–2276 (2021).
Acknowledgements
We thank Dr Thandeka Moyo-Gwete, Brent Oosthuysen, Donald Mhlanga, Frances Ayres, Haajira Kaldine, Nelia P. Manamela, Sebotsana Rasebotsa, Sharon Madzorera, Thanusha Naidoo, Thopisang Motlou for technical assistance in generating plasmids and / or proteins for this study.
Funding
PLM is supported by the South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa, the SA Medical Research Council SHIP program, and the Centre for the AIDS Programme of Research in South Africa (CAPRISA). We acknowledge funding from the Bill and Melinda Gates Foundation, through the Global Immunology and Immune Sequencing for Epidemic Response (GIISER) program. The phase II clinical trial was funded by Novavax and the Bill and Melinda Gates Foundation. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill and Melinda Gates Foundation.
Author information
Authors and Affiliations
Consortia
Contributions
Designed the study, performed analyses and wrote the manuscript: J.N.B., P.L.M. Performed experiments and analysed data: S.I.R., B.E.L., P.K., N.M., H.K., Data curation and project management: C.C. PIs for Sisonke (AD26CoV2.S) Trial: G.G., L.G.B. Site PIs for Novavax Trial: A.K., L.F., L.F., Q.B., K.D., M.T., M.M., Z.H., N.S., S.H., M.A., C.L., C.G., U.L., N.J., G.K. PIs for Novavax Trial: V.S., C.B., G.M.G., S.M.
Corresponding authors
Ethics declarations
Competing interests
Dr. Shinde reports being employed by and owning shares in Novavax; Dr. Q. Bhorat, receiving grant support from Wits Health Consortium, Regeneron Pharmaceuticals, GSK, Avillion, Sanofi, Novo Nordisk, and Novavax; Dr. Fouche, receiving grant support from BioNTech; Dr Bennet reports being employed by Novavax; Dr. Glenn, being employed by and owning stock in Novavax and owning stock in RA Capital; and Dr. Madhi, receiving grant support, paid to his institution, from Pfizer and GSK. All remaining authors have not reported any conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhiman, J.N., Richardson, S.I., Lambson, B.E. et al. Novavax NVX-COV2373 triggers neutralization of Omicron sub-lineages. Sci Rep 13, 1222 (2023). https://doi.org/10.1038/s41598-023-27698-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27698-x
This article is cited by
-
Current Progress, Challenges and Prospects in the Development of COVID-19 Vaccines
Drugs (2024)
-
Safety and Immunogenicity of a Recombinant Two-Component SARS-CoV-2 Protein Vaccine: Randomized, Double-Blind, Placebo-Controlled Phase I and Phase II Studies
Infectious Diseases and Therapy (2024)
-
Immunogenicity and efficacy of vaccine boosters against SARS-CoV-2 Omicron subvariant BA.5 in male Syrian hamsters
Nature Communications (2023)
-
Preclinical evaluation of manufacturable SARS-CoV-2 spike virus-like particles produced in Chinese Hamster Ovary cells
Communications Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.