Abstract
The aim of this study was to evaluate the association between serum testosterone and abdominal body composition based on abdominopelvic computed tomography (APCT) measurements after adjusting for individual metabolic syndrome components. We performed a cross-sectional study using male subjects (age range: 22–84 years) who underwent a general health examination with abdominopelvic computed tomography and testosterone measurements. Body composition was evaluated with APCT. To confirm an association between testosterone and abdominal body composition, we conducted linear regression analysis. The effect of abdominal body composition was adjusted for important clinical factors such as age, albumin, and metabolic components in the multivariable regression analysis. Overall, 1453 subjects were included in the primary analysis. After adjustment for age, individual metabolic components, albumin, hemoglobin A1c, and C-reactive protein, we found that subcutaneous fat area index (β = − 0.042, p < 0.001), total abdominal muscle area index (β = 0.115, p < 0.001), normal attenuation muscle area index (β = 0.070, p < 0.001), and loge-transformed lower attenuation muscle area index (β = 0.140, p = 0.002) had an association with loge-transformed testosterone level. After adjusting for individual metabolic syndrome components, testosterone was associated negatively with subcutaneous fat, but not visceral fat. In addition, testosterone was positively correlated with abdominal muscle regardless of qualitative features such as fat-rich and fat-free.
Similar content being viewed by others
Korea is rapidly becoming an aging society and aging itself is becoming a global health issue. Body composition can change with aging, which includes decreased skeletal muscle mass and increased abdominal fat mass1. The causal relationships between testosterone and the age-related changes have not yet been elucidated; however, some studies have established this relationship2. Epidemiological studies have found that lower testosterone levels are related to decreased muscle mass, central obesity, and the accumulation of abdominal fat3,4.
Metabolic syndrome (MetS) is characterized by several specific components, including increased waist circumference, dyslipidemia, hypertension, and impaired glucose tolerance. We previously showed that MetS and diabetes were associated with a change in abdominal body composition5,6. MetS also has a close relation with low testosterone7. Due to the strong correlation that exists among testosterone, MetS, and body composition, MetS should be considered and adjusted in order to evaluate whether testosterone directly affects body composition. However, few studies have evaluated the relationship between testosterone and abdominal body composition after adjusting for MetS. For this reason, the results of previous studies on testosterone and abdominal body composition differed8. Therefore, it is necessary to confirm how testosterone directly affects abdominal body composition without secondary effects by MetS.
Computed tomography (CT) can directly measure areas of fat and muscle; therefore, CT is a useful tool for assessing body fat and muscle distributions9. This study aimed to evaluate the association between serum testosterone and abdominal body composition based on abdominopelvic CT (APCT) measurements from a large cohort who voluntarily underwent health examinations after adjusting for individual MetS components.
We hypothesized that there are changes in the association between testosterone and abdominal body compositions before and after correction for individual MetS components.
Materials and methods
Study design
We used a cross sectional design that is appropriate to confirm the relationship between testosterone and abdominal body composition based on large data from Health Promotion Centre.
Study participants
We retrospectively obtained data from 1612 participants aged ≥ 20 years (range: 22–84 years) who underwent self-referral APCT and testosterone level measurement as a part of routine check-ups at the Health Promotion Centre, Ulsan University Hospital, between March 2014 and June 2019. Ulsan is an industrial city in Korea with many large companies, which provide health check-ups every 2 years for their employees. The exclusion criteria were as follows: (1) presence of chronic diseases affecting muscle mass, such as stroke, tuberculosis, chronic kidney disease, chronic liver disease, and cancer, and (2) insufficient medical records. Finally, 1453 subjects were included in the analysis (Fig. 1). Clinical and laboratory variables were collected using the clinical data warehouse platform in conjunction with electronic medical records at the Ulsan University Hospital. This study was approved by Institutional Review Board of Ulsan University Hospital (No. 2021-11-033); it conformed to the principles outlined in the Declaration of Helsinki. The need for informed consent was waived by Institutional Review Board of Ulsan University Hospital owing to the retrospective nature and the anonymization of the data included in the study.
Clinical and laboratory measurements
Data on clinical factors (e.g., comorbidities, such as hypertension, diabetes, dyslipidemia, and cardiovascular disease) were obtained from systemized self-reported questionnaires issued to the participants prior to their check-up, as described previously5,6. Height and weight were collected while the participants wore light clothing without shoes. Body mass index (BMI) was calculated as the weight (kg) divided by the square of the height (m2). Waist circumference (cm) was measured midway between the costal margin and the iliac crest at the end of a normal expiration. Blood pressure was checked on the right arm after a 5 or more min rest using an automatic manometer with an appropriate cuff size. After overnight fasting, morning blood samples were collected and were subsequently analyzed at the central laboratory of our hospital. Fasting blood glucose, hemoglobin A1c (HbA1c), albumin, C-reactive protein (CRP), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides were measured. CRP was used for analysis as a categorical variable because it was impossible to measure less than 0.042 mg/dL, and the cutoff value was set at 2.0 mg/dL reported in the JUPITER trial10. Serum testosterone level was measured by competitive immunoassay using direct chemiluminescent technology on ADVIA Centaur® XP system (Siemens Healthcare Diagnostics, Malvern, PA, USA).
MetS was defined based on the revised National Cholesterol Education Program criteria proposed by the American Heart Association/National Heart, Lung, and Blood Institute11. MetS requires the presence of at least three of the following five components: (1) abdominal obesity (waist circumference ≥ 90 cm for Asian men and ≥ 80 cm for Asian women), (2) triglyceride level ≥ 150 mg/dL, (3) HDL cholesterol level < 40 mg/dL for males or < 50 mg/dL for females or those receiving drug treatment, (4) systolic/diastolic blood pressure ≥ 130/85 mmHg or receiving drug treatment, and (5) fasting plasma glucose concentration ≥ 100 mg/dL or receiving drug treatment.
CT image acquisition and body composition assessment
All CT images were obtained using the SOMATOM Definition Flash system (Siemens Healthcare, Erlangen, Germany), as described previously5,6. Enhanced images were obtained after a 80 s delay after contrast injection. The scanning parameters were as follows: beam collimation, 128 × 0.6 mm; beam pitch, 0.6; gantry rotation time, 0.5 s; field of view to fit, 100 kVp. An automatic exposure control system (CARE Dose 4D, Siemens Medical Solutions, Erlangen, Germany) was used.
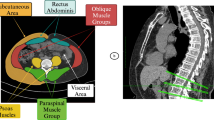
Body composition was evaluated with APCT using the Asan-J software, which was developed based on ImageJ (NIH, Bethesda, MD, USA), as described previously5,6,12. Two consecutive axial CT images at the inferior endplate of the L3 lumbar vertebra were captured for each patient (Appendix S1). Using the Asan-J software, we calculated the total abdominal muscle area (TAMA) (cm2), including all muscles in the field (psoas, paraspinal, transversus abdominis, rectus abdominis, quadratus lumborum, and internal/external obliques), with predetermined Hounsfield unit (HU) thresholds on CT. The TAMA was divided into a low-attenuation abdominal muscle area (LAMA) and a normal-attenuation abdominal muscle area (NAMA) based on HUs on CT (TAMA, − 29–150 HU; LAMA, − 29–29 HU; NAMA, 30–150 HU)13,14 LAMA implies lipid-rich skeletal muscle, which has more fat elements between and inside the muscle fibers. However, NAMA indicates lipid-poor skeletal muscle, which includes less fat between and inside the muscles15. Furthermore, the visceral fat area (VFA) (cm2) and the subcutaneous fat area (SFA) (cm2) were evaluated using adipose tissue thresholds on CT (− 190 to − 30 HU)16,17. We adjusted the cross-sectional areas of the abdominal fat and muscles by BMI based on the Foundation for the National Institutes of Health Sarcopenia Project recommendation18; these were named index such as the TAMA index (TAMAi) (TAMAi = TAMA [cm2]/BMI [kg/m2]), LAMA index (LAMAi) (LAMAi = LAMA [cm2]/BMI [kg/m2]), NAMA index (NAMAi) (NAMAi = NAMA [cm2]/BMI [kg/m2]), IMFA index (IMFAi) (IMFAi = IMFAi [cm2]/BMI [kg/m2]), VFA index (VFAi) (VFAi = VFA [cm2]/BMI [kg/m2]), and SFA index (SFAi) (SFAi = SFA [cm2]/BMI [kg/m2]).
Statistical analyses
Clinical characteristics were summarized as frequency (percentage) for categorical variables and as mean ± standard deviation (SD) for continuous variables. The coefficient of variation was used to show the extent of variability of the continuous variables in this cohort. Before the main analysis, we examined distributions for all continuous variables and found some variables were severely skewed to the right; thus, we conducted log transformation to obtain more stable analysis results (Appendix S2). For example, the testosterone level underwent loge-transformation. To determine whether there was an association between testosterone and abdominal body compositions or metabolic factors, we conducted several linear regression model analyses. First, we fitted univariable and multivariable regression models. The effect of abdominal body compositions was then adjusted for important clinical factors such as age, albumin, and five metabolic syndrome components in the multivariable regression analysis. A p value less than 0.05 was considered statistically significant (two-tailed). All data analyses were performed using R software version 4.1.2.
Results
Participant characteristics and abdominal body composition of study subjects
A total of 1612 subjects were enrolled, of which 159 were excluded because 103 had comorbidities and 56 had insufficient medical records (Fig. 1). The remaining 1453 subjects were included in the primary analysis. The mean age of the study participants was 55.2 ± 8.4 years, and the mean serum testosterone level was 4.36 ± 1.73 ng/ml. Of the 1453 subjects, 450 (31.0%) were diagnosed with MetS. The participant characteristics included abdominal body composition are listed in Table 1.
Association between testosterone and abdominal body composition
In the univariable linear regression analyses, the loge-transformed testosterone had a negative correlation with HbA1c (unstandardized β (95% confidence interval [CI]) = − 0.060 (− 0.086 to − 0.034), p < 0.001) and CRP ≥ 2.0 mg/dL (unstandardized β (95% CI) = − 0.381 (− 0.572 to − 0.191), p < 0.001) (Table 2 and Fig. 2). All metabolic components had a negative correlation with the loge-transformed testosterone. Regarding abdominal body composition, SFAi (unstandardized β (95% CI) = − 0.048 (− 0.059 to − 0.031), p < 0.001), VFAi (unstandardized β (95% CI) = − 0.026 (− 0.037 to − 0.016), p < 0.001), TAMAi (unstandardized β (95% CI) = 0.110 (0.077–0.142), p < 0.001), and NAMAi (unstandardized β (95% CI) = 0.076 (0.049–0.104), p < 0.001) had an association with the loge-transformed testosterone.
Scatter plots to show the association between loge-transformed testosterone and individual body composition parameters. (a) subcutaneous fat index, (b) visceral fat index, (c) intermuscular fat index, (d) total abdominal muscle index, (e) normal-attenuation muscle index, and (f) loge (low-attenuation muscle index).
After adjustment for age, individual metabolic components, albumin, hemoglobin A1c, and CRP ≥ 2 mg/dL, SFAi (unstandardized β (95% CI) = − 0.042 (− 0.059 to − 0.024), p < 0.001), TAMAi (unstandardized β (95% CI) = 0.115 (0.076–0.153), p < 0.001), NAMAi (unstandardized β (95% CI) = 0.070 (0.035–0.104), p < 0.001), and loge-transformed LAMAi (unstandardized β (95% CI) = 0.140 (0.050–0.230), p = 0.002) had an association with the loge-transformed testosterone (Table 3 and appendix S3).
Discussion
This study provided further evidence of the very close relationship between testosterone and abdominal body composition, including abdominal fat and muscle. However, as shown in previous studies and our study, testosterone is also closely related to MetS7,19. In other words, low testosterone was highly correlated with the prevalence of MetS. In addition, men with decreased testosterone were closely related to increased insulin resistance and type 2 diabetes prevalence20. This was also reported in the Men Androgen Inflammation Lifestyle Environment and Stress study21. This is problematic because, as previous studies have shown, MetS and glycemic status are highly correlated with abdominal body composition5,12. The effect of testosterone on abdominal body composition could be secondary effects of MetS because of the relationship between testosterone and MetS. A previous study demonstrated this relationship between MetS and low testosterone in men, especially when visceral adiposity is high19. To date, there has been no study on the relationship between testosterone and abdominal body composition after adjusting for MetS as a confounding factor. Allan et al.4 reported the results of a randomized controlled trial (RCT) that confirmed a change in abdominal body composition after the administration of testosterone replacement therapy in a group matched with MetS. However, since this study did not evaluate the improvement in MetS due to testosterone replacement for 52 weeks, it was challenging to say that the analysis was done after adjusting MetS accurately.
In this study, after adjusting for MetS, testosterone only correlated with subcutaneous fat but not with visceral fat. The result that testosterone was related to subcutaneous fat but not to visceral fat was similarly reported in two RCTs22,23. These results had also been proven in animal experiments24. However, most previous studies performed the analysis without adjusting for MetS, showing a close relationship between testosterone and visceral fat, unlike the results in the present study4,25,26. MetS itself is not related to subcutaneous fat and is closely associated with visceral fat27,28,29. Therefore, the result could be different if the direct relationship between testosterone and abdominal body composition is analyzed after adjusting for MetS. Even in this study, before adjusting for MetS, testosterone was found to be closely related to both subcutaneous and visceral fat. These results can be explained using testosterone metabolites30. Testosterone is converted to estradiol by aromatase and dihydrotestosterone (DHT) by 5α-reductase. DHT specifically impedes subcutaneous fat growth, whereas estradiol specifically prevents the expansion of visceral fat. Therefore, the inhibition of estradiol was unable to prevent visceral fat growth31. Most circulating estradiol is aromatized from testosterone predominantly in adipose tissue. Therefore, if individual MetS components including obesity are adjusted, estradiol could also be adjusted, so that only the effect of DHT between testosterone metabolites would remain. Hence, it is considered that testosterone was related only to subcutaneous fat when individual MetS components were adjusted.
Testosterone is closely related to abdominal muscle mass. Our study also showed a positive correlation between testosterone and abdominal muscle mass, which was also observed in studies reporting changes in lean body mass or muscle size after testosterone replacement therapy32,33,34. Many studies have reported the mechanisms of the effect of testosterone on muscle; testosterone enhances an increase in muscle fiber size by increased protein synthesis, stimulates the mitotic activity of satellite cell in myoblast culture systems, and increases IGF-1 expression35. Most previous studies reported that only fat-free mass was associated with testosterone33,35. Similar to other studies, fat-rich muscle was not related to testosterone in the univariate analysis in this study. A previous our study showed that MetS was significantly associated with fat-rich muscle in males (OR 1.771, p < 0.001)12. Therefore, in order to analyze the relationship between testosterone and abdominal muscle mass, it was necessary to adjust for MetS. Interestingly, testosterone had a positive correlation with muscle regardless of qualitative features such as fat-rich and fat–free. This result could be because testosterone correlated positively with the fat-rich muscle, whereas MetS correlated negatively.
Our study had several limitations. First, since this study was designed as a cross-sectional study, we could not clarify the causal relationship between testosterone and abdominal body composition. Therefore, prospective randomized controlled trials are required to confirm the exact causal relationship. Second, the study was performed in a single health promotion center and most of the subjects were relatively healthy. Thus, there is a possibility of selection bias. To minimize the bias, we tried to include a relatively large sample size and to perform a multivariable linear regression analysis. Third, Ideally, sex hormone binding globulin should have been assessed and calculated free and bioavailable testosterone derived as two measurements reflect biologically active testosterone values as SHBG bound testosterone is considered inactive. However, Many subjects did not check SHBG and free testosterone because all subjects were tested for the health check.
Conclusions
Our study identified the necessity of adjusting for MetS to confirm the association between testosterone level and abdominal body composition. After adjusting for individual MetS components, testosterone was significantly associated with subcutaneous fat, but not visceral fat. In addition, testosterone had a positive correlation with abdominal muscle regardless of qualitative features such as fat-rich and fat-free. These results suggest that testosterone has a close relationship with subcutaneous fat and abdominal muscle, regardless of MetS.
Data availability
Seong Cheol Kim had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- MetS:
-
Metabolic syndrome
- CT:
-
Computed tomography
- APCT:
-
Abdominopelvic computed tomography
- BMI:
-
Body mass index
- HbA1c:
-
Hemoglobin A1c
- CRP:
-
C-reactive protein
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HU:
-
Hounsfield unit
- TAMA:
-
Total abdominal muscle area
- TAMAi:
-
Total abdominal muscle area index
- LAMA:
-
Low-attenuation abdominal muscle area
- LAMAi:
-
Low-attenuation abdominal muscle area index
- NAMA:
-
Normal-attenuation abdominal muscle area
- NAMAi:
-
Low-attenuation abdominal muscle area index
- VFA:
-
Visceral fat area
- VFAi:
-
Visceral fat area index
- SFA:
-
Subcutaneous fat area
- SFAi:
-
Subcutaneous fat area index
- SD:
-
Standard deviation
- CV:
-
Coefficient of variation
- CI:
-
Confidence interval
- DHT:
-
Dihydrotestosterone
References
Chin, K. Y. et al. Testosterone is associated with age-related changes in bone health status, muscle strength and body composition in men. Aging Male 15, 240–245 (2012).
Araujo, A. B. et al. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J. Am. Geriatr. Soc. 56, 2000–2008 (2008).
Schaap, L. A. et al. Low testosterone levels and decline in physical performance and muscle strength in older men: Findings from two prospective cohort studies. Clin. Endocrinol. 68, 42–50 (2008).
Allan, C. A. & McLachlan, R. I. Androgens and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 17, 224–232 (2010).
Han, S. et al. Differences in abdominal body composition according to glycemic status: An inverse probability treatment weighting analysis. Endocrinol. Metab. 36, 855–864 (2021).
Jeon, Y. J. et al. Intramuscular and intermuscular abdominal fat infiltration in COPD: A propensity score matched study. Int. J. Chronic Obstruct. Pulm. Dis. 16, 1989–1999 (2021).
Zarotsky, V. et al. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology 2, 819–834 (2014).
Isidori, A. M. et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: A meta-analysis. Clin. Endocrinol. 63, 280–293 (2005).
Haggmark, T., Jansson, E. & Svane, B. Cross-sectional area of the thigh muscle in man measured by computed tomography. Scand. J. Clin. Lab. Investig. 38, 355–360 (1978).
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112, 2735–2752 (2005).
Lee, T. Y., Jeon, Y. J., Kim, C. R., Kang, B. J. & Park, G. M. Abdominal muscles and metabolic syndrome according to patient sex: A retrospective cross-sectional study. Healthcare 9, 1197 (2021).
Mitsiopoulos, N. et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 85, 115–122 (1998).
Aubrey, J. et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 210, 489–497 (2014).
Kim, D. et al. Correlation between midthigh low-density muscle and insulin resistance in obese nondiabetic patients in Korea. Diabetes Care 26, 1825–1830 (2003).
Park, H. J. et al. Development and validation of a deep learning system for segmentation of abdominal muscle and fat on computed tomography. Korean J. Radiol. 21, 88–100 (2020).
Maddocks, M. et al. Skeletal muscle adiposity is associated with physical activity, exercise capacity and fibre shift in COPD. Eur. Respir. J. 44, 1188–1198 (2014).
Studenski, S. A. et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 69, 547–558 (2014).
Wang, C. et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care 34, 1669–1675 (2011).
Caliber, M. & Saad, F. Testosterone therapy for prevention and reversal of type 2 diabetes in men with low testosterone. Curr. Opin. Pharmacol. 58, 83–89 (2021).
Atlantis, E. et al. Predictive value of serum testosterone for type 2 diabetes risk assessment in men. BMC Endocr. Disord. 16, 26 (2016).
Frederiksen, L. et al. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur. J. Endocrinol. 166, 469–476 (2012).
Munzer, T. et al. Effects of GH and/or sex steroid administration on abdominal subcutaneous and visceral fat in healthy aged women and men. J. Clin. Endocrinol. Metab. 86, 3604–3610 (2001).
Kelly, D. M. et al. Testosterone differentially regulates targets of lipid and glucose metabolism in liver, muscle and adipose tissues of the testicular feminised mouse. Endocrine 54, 504–515 (2016).
Abdelhamed, A. et al. Testosterone replacement alters the cell size in visceral fat but not in subcutaneous fat in hypogonadal aged male rats as a late-onset hypogonadism animal model. Res. Rep. Urol. 7, 35–40 (2015).
Ng Tang Fui, M. et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: A randomised controlled trial. BMC Med. 14, 153 (2016).
Park, H. S. & Lee, K. Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: A study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet. Med. 22, 266–272 (2005).
Tsukiyama, H. et al. Proposed cut-off values of the waist circumference for metabolic syndrome based on visceral fat volume in a Japanese population. J. Diabetes Investig. 7, 587–593 (2016).
Kim, Y. A., Kwak, S. G. & Cho, Y. J. Optimal cutoff values for visceral fat volume to predict metabolic syndrome in a Korean population. Medicine 100, e27114 (2021).
Sebo, Z. L. & Rodeheffer, M. S. Testosterone metabolites differentially regulate obesogenesis and fat distribution. Mol. Metab. 44, 101141 (2021).
Finkelstein, J. S. et al. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 369, 1011–1022 (2013).
Wittert, G. A. et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J. Gerontol. A Biol. Sci. Med. Sci. 58, 618–625 (2003).
Bhasin, S. et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J. Clin. Endocrinol. Metab. 90, 678–688 (2005).
Tapper, J. et al. Muscles of the trunk and pelvis are responsive to testosterone administration: Data from testosterone dose-response study in young healthy men. Andrology 6, 64–73 (2018).
Shin, M. J., Jeon, Y. K. & Kim, I. J. Testosterone and sarcopenia. World J. Mens Health 36, 192–198 (2018).
Funding
This research was supported by a grant of the Medical data-driven hospital support project through the Korea Health Information Service (KHIS), funded by the Ministry of Health and Welfare, Korea. Seungbong Han’s work was supported by a Korea University Medicine grant (Korea University Grant K2117421).
Author information
Authors and Affiliations
Contributions
S.C.K. participated in conception and design of the study. Y.J.J. and T.Y.K. performed the data collection. S.H. performed the data analysis. Y.J.J., S.P., K.H.M. and S.C.K. reviewed the literatures and interpreted the data. S.C.K. wrote the manuscript. S.H., G.M.P. and S.C.K. revised the manuscript and supervised the whole study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, S., Jeon, YJ., Lee, T.Y. et al. Testosterone is associated with abdominal body composition derived from computed tomography: a large cross sectional study. Sci Rep 12, 22528 (2022). https://doi.org/10.1038/s41598-022-27182-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27182-y
This article is cited by
-
The influence of body composition on the response to dynamic stimulation of the endocrine pituitary-testis axis
International Journal of Obesity (2024)
-
Levels of Sex Hormones and Abdominal Muscle Composition in Men from The Multi-Ethnic Study of Atherosclerosis
Scientific Reports (2024)
-
Association between visceral adipose tissue and total testosterone among the United States male adults: a cross-sectional study
International Journal of Impotence Research (2024)
-
Relationships of rapid eating with visceral and subcutaneous fat mass and plasma adiponectin concentration
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.