Abstract
In this large cohort of healthcare workers, we aimed to estimate the rate of reinfections by SARS-CoV-2 over 2 years of the COVID-19 pandemic. We investigated the proportion of reinfections among all the cases of SARS-CoV-2 infection from March 10, 2020 until March 10, 2022. Reinfection was defined as the appearance of new symptoms that on medical evaluation were suggestive of COVID-19 and confirmed by a positive RT-PCR. Symptoms had to occur more than 90 days after the previous infection. These 2 years were divided into time periods based on the different variants of concern (VOC) in the city of São Paulo. There were 37,729 medical consultations due to COVID-19 at the hospital’s Health Workers Services; and 25,750 RT-PCR assays were performed, of which 23% (n = 5865) were positive. Reinfection by SARS-CoV-2 was identified in 5% (n = 284) of symptomatic cases. Most cases of reinfection occurred during the Omicron period (n = 251; 88%), representing a significant increase on the SARS-CoV-2 reinfection rate before and during the Omicron variant period (0.8% vs. 4.3%; p < 0.001). The mean interval between SARS-CoV-2 infections was 429 days (ranged from 122 to 674). The Omicron variant spread faster than Gamma and Delta variant. All SARS-CoV-2 reinfections were mild cases.
Similar content being viewed by others
Introduction
After over 2 years of COVID-19 pandemic, reinfections are still a debated topic1. Reinfection by SARS-CoV-2 means that a person was infected, recovered, and became infected again at least 90 days after the last infection2. Most individuals will develop some protection against reinfection after recovering from COVID-19; however, the evidence regarding duration and level of protection is still emerging1. Reinfections have been described although less severe and less lethal than primary infections3. The frequency with which reinfections occur, how quickly occur after a previous infection, and the severity in relation to initial infections are relevant information to understand the phenomenon and the impact of reinfections2. Reinfection cases were rare during the first year of pandemic4. However, a study coordinated by the Centers for Disease Control and Prevention (CDC) found an increase in reinfection cases during the Omicron period represented by 50% of reinfections5. Immunity acquired due to an infection by one variant of the virus may not be effective against a new variant6, and immunity acquired by vaccination may offer varying protection against different SARS-CoV-2 variants3.
The objective of this study is to estimate the rate of reinfections with SARS-CoV-2 in healthcare workers over 2 years of the COVID-19 pandemic.
Results
From March 10, 2020 until March 10, 2022 there were 37,729 medical consultations due to COVID-19 at the hospital’s Health Workers Services; 25,750 RT-PCR tests were done for suspected COVID-19 of which 23% (n = 5865) were positive. A total of 284 (5%) cases were considered SARS-CoV-2 reinfections. These cases belonged to 281 HW, of which three of them had a second episode of reinfection during the Omicron variant period. Our cohort was predominantly female (n = 202/281, 71%) with a median age of 39 years (30–47), 4% were unvaccinated, 32% had received two doses of a COVID-19 vaccine and 64% a booster dose. In addition, 22% (n = 62/281) had at least one risk factor for progression to severe COVID-19, mainly cardiovascular disease, diabetes, and older age. However, all cases were mild, there were no hospitalizations or deaths (Table 1).
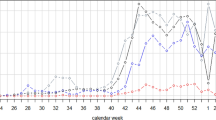
Table 2 displays a distribution of COVID-19 cases according to the emergence of VOCs in the city. Most cases of reinfection occurred during the Omicron VOC period (n = 251; 88%). The mean interval between infections was 429 (122–674) days (approximately 14 months); and was 507 (122–674) days (approximately 16 months) in the Omicron period.
There was a significant increase on the SARS-CoV-2 reinfection rate comparing the number of cases among before and during the Omicron variant period (0.8% vs. 4.3%; relative risk 5.45 [95% IC 3.80–7.81]; p < 0.001).
At the same time, the average number of new cases in our HW also increased drastically with an average of 24.5/day compared with 10.6/day in 2020 (before vaccination).
Discussion
HWs are considered to be at high risk of infection as they face the possibility of HW-to-HW transmission, patient-to-HW transmission, and community transmission7. Before the predominance of the Omicron variant in the city of Sao Paulo, reinfection represented less than 1% of symptomatic COVID-19 cases similar to previously described8,9. Moreover, higher reinfection rates have been described varying from 3.2 to 11.6% including asymptomatic cases10,11. However, in the Omicron period, this proportion increased more than five times. The Omicron variant spread faster than Gamma and Delta variants, evidenced by the progressively shorter duration of the transition periods. Nevertheless, during the Gamma and Delta periods, reinfection was rare, suggesting that previous infection and vaccination had an impact. This effect seems to be less important against the Omicron variant.
SARS-CoV-2 reinfections by natural immunity can last over a year but declines progressively3,12. In addition, it has been described that protection against reinfection varies between 77 and 98% across different SARS-CoV-2 variants, but few data are available regarding the Omicron variant3.
Furthermore, considering the long interval between the infections, it is possible that immunity, acquired through infection and vaccination, waned over time as previously shown3,12. Our data reinforced that the median interval between infections is approximately 14 months similar to previous studies3,12.
An important finding was that all reinfections were non-severe. However, most HW do not belong to high-risk groups and are mostly younger than 65. Another finding was that, in most cases of reinfection, the first infection occurred in the pre-VOC period as previously described3.
Our study has limitations. This is a single-center study; however, our number of tests was high. It was only possible to evaluate the number of tests performed at our hospital. We believe that most HW chose to test at our service, as testing was conveniently done at the workplace and free of charge. Furthermore, as HW were vaccinated during the evaluation period, it was not possible to evaluate separately the impact of infection and vaccination. We did not determine variants of concern by SARS-CoV-2 whole genome sequencing. Finally, only symptomatic infections were evaluated.
In summary, in our large 2-year cohort of HW, reinfection represented 5% of symptomatic COVID-19 cases. All reinfections were mild and occurred predominantly in the Omicron period.
Methods
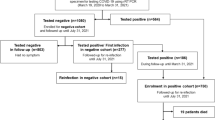
This is a prospective cohort study of HW with suspect COVID-19 symptoms. We evaluated all the HW who were diagnosed with COVID-19 with a positive RT-PCR test between March 10, 2020 and March 10, 2022. Demographic and clinical data were obtained from electronic health records.
Hospital das Clínicas (HC) is a 2200-bed tertiary care hospital affiliated with the University of São Paulo, Brazil, located in the city of São Paulo (> 12 million inhabitants)13. It has approximately 22,000 directly hired healthcare workers (HW), which who provide direct patient assistance and administration.
During the pandemic, symptomatic HW were evaluated by a physician at the hospital’s Health Worker Services and tested for SARS-CoV-2 by RT-PCR14 in accordance with relevant guidelines and regulations. HW received paid leave during symptomatic illness, and if positive for SARS-CoV-2 their leave was extended until the 10th day since the onset of symptoms. No further testing was required for returning to work.
The vaccination campaign with two doses of inactivated SARS-CoV-2 vaccine (CoronaVac/Sinovac, China) began in January, 2021. During the first months, 22,402 and 21,652 HWs have received the first dose and 2nd dose, respectively. In October, 2021 the government issued a recommendation that HWs take a 3rd dose of BNT162b2 (Pfizer-Biontech, USA). This was not organized and offered by the hospital and there are no data on adherence.
The 2 years of pandemic were divided into time periods based on the different variants of concern (VOC) in the city of São Paulo:
-
1.
Pre-VOC period from the first reported case of COVID-19 until the day before the first case report of the Gamma variant,
-
2.
Transition to Gamma from the day of the first case reported of the Gamma variant until the day before the report that 90% of cases were caused by the Gamma variant,
-
3.
Gamma period from the day of the report that 90% of cases were caused by the Gamma variant until the day before the first case report of the Delta variant,
-
4.
Transition Gamma–Delta from the day of the report of the first case of the Delta variant until the day before the report that 90% of cases were caused by the Delta variant,
-
5.
Delta period from the day of the report that 90% of cases were caused by the Delta variant until the day before the report of the first case of the Omicron variant,
-
6.
Transition Delta–Omicron from the day of the first case report of the Omicron variant until the day before the report that 90% of cases were caused by the Omicron variant,
-
7.
Omicron period from the day of the report that 90% of cases were caused by the Omicron variant until the end of the study period.
The main outcome was the proportion of reinfections among all the cases of COVID-19 in each period.
Reinfection was defined as the appearance of new symptoms that on medical evaluation were suggestive of COVID-19, confirmed by a positive RT-PCR test for SARS-CoV-2. Symptoms had to occur more than 90 days after the previous diagnosis of a RT-PCR-confirmed infection.
A mild COVID-19 case was considered as a HW who presented at least one of the following symptoms: fever or chills, cough, runny nose, sore throat, headache, nauseas or vomiting, myalgia, fatigue, diarrhea, anosmia, and ageusia. Moderate cases were defined as symptomatic cases who have shortness of breath, dyspnea, or an abnormal chest imaging with an oxygen saturation (SpO2) ≥ 94% on room air at sea level. Severe cases presented a SpO2 < 94%, respiratory rate > 30 breaths/min or a pulmonary infiltrate > 50% and required hospitalization. Finally, critical illness was considered as HW with respiratory failure, septic shock and/or multiple organ dysfunction15.
This study was analyzed and approved by the Ethics committee of the Hospital das Clínicas of the Faculty of Medicine of the University of São Paulo with a waiver of informed consent (CAAE number: 60253222.5.0000.0068).
SARS-CoV-2 RT-qPCR
RNA was extracted from saline solution 0.9% with an automated method using magnetic beads (Sample Preparation System RNA, Abbott, Illinois, USA). SARS-CoV-2 RT-qPCR was performed using an adapted protocol as previously described8. Gene E was detected as the first-line screening tool, followed by confirmatory testing with an assay detecting the N gene (Abbott, USA) and the commercial SARS-CoV-2 N1 + N2 RT-qPCR kit to detect N1 and N2 genes (Qiagen, USA). SARS-CoV-2 RT-qPCR result was considered positive with an amplification cycle threshold (Ct) ≤ 32 and Ct ≤ 33, respectively.
Data analysis
Demographic and clinical characteristics were presented as frequencies (percentages) for categorical variables and means (range) or medians (interquartile range [IQR]) for continuous variables according to the type of distribution. The comparison of SARS-CoV-2 reinfection rates was performed using the Chi square test (p < 0.05, 95% confidence intervals) and were calculated as number of reinfection cases before and after the Omicron variant considering the total accumulated number of SARS-CoV-2 infections in both periods. Epi Info™ version 7.2.4.0 was used for statistical analyses.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zuiani, A. & Wesemann, D. R. Antibody dynamics and durability in coronavirus disease-19. Clin. Lab. Med. 42, 85–96. https://doi.org/10.1016/j.cll.2021.10.004 (2022).
Centers for Disease Control and Prevention. Reinfections and COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html. Accessed 5 September 2022.
Pilz, S., Theiler-Schwetz, V., Trummer, C., Krause, R. & Ioannidis, J. P. A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 8(209), 112911. https://doi.org/10.1016/j.envres.2022.112911 (2022).
Akinbami, L. J. et al. Reinfection with severe acute respiratory syndrome coronavirus 2 among previously infected healthcare personnel and first responders. Clin. Infect. Dis. 75(1), e201–e207. https://doi.org/10.1093/cid/ciab952 (2022).
Plumb, I. D. et al. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19-associated hospitalization among adults with previous SARS-CoV-2 infection—United States, June 2021–February 2022. MMWR Morb. Mortal. Wkly. Rep. 71(15), 549–555. https://doi.org/10.15585/mmwr.mm7115e2 (2022).
Sabino, E. C. et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet 397, 452–455. https://doi.org/10.1016/S0140-6736(21)00183-5 (2021).
Costa, S. F. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seroprevalence and risk factors among oligo/asymptomatic healthcare workers: Estimating the impact of community transmission. Clin. Infect. Dis. 73(5), e1214–e1218 (2021).
Slezak, J. et al. Rate and severity of suspected SARS-Cov-2 reinfection in a cohort of PCR-positive COVID-19 patients. Clin. Microbiol. Infect. 27(12), 1860.e7-1860.e10. https://doi.org/10.1016/j.cmi.2021.07.030 (2021).
Pilz, S. et al. SARS-CoV-2 re-infection risk in Austria. Eur. J. Clin. Investig. 51(4), e13520. https://doi.org/10.1111/eci.13520 (2021).
Cohen, C. et al. SARS-CoV-2 incidence, transmission, and reinfection in a rural and an urban setting: Results of the PHIRST-C cohort study, South Africa, 2020–21. Lancet Infect. Dis. 22(6), 821–834. https://doi.org/10.1016/S1473-3099(22)00069-X (2022).
Hansen, C. H., Michlmayr, D., Gubbels, S. M., Mølbak, K. & Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 397(10280), 1204–1212. https://doi.org/10.1016/S0140-6736(21)00575-4 (2021).
Goldberg, Y. et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N. Engl. J. Med. 386(23), 2201–2212. https://doi.org/10.1056/NEJMoa2118946 (2022).
IBGE-Instituto Brasileiro de Geografia e Estatística. Panorama São Paulo. Available at: https://cidades.ibge.gov.br/brasil/sp/sao-paulo/panorama. Accessed 18 March 2022.
Corman, V. M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. [Published correction appears in Euro Surveill. 2021 Feb;26(5)]. Euro Surveill. 25(3), 2000045. https://doi.org/10.2807/1560-7917.ES.2020.25.3.20000459- (2020).
NIH COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed 18 September 2022.
Funding
This study was supported by the Itaú Unibanco “Todos pela saúde” program.
Author information
Authors and Affiliations
Contributions
A.R.G.: collected and interpreted data, wrote the first draft of the manuscript; M.S.O.: collected and interpreted data, wrote the first draft of the manuscript; B.M.T.: collected and interpreted data; A.L.M.: collected and interpreted data, and performed the statistical analysis; C.S.L.: collected data; A.C.M.: collected data; E.F.: collected data; F.L.M.: collected data; A.S.B.: collected data; M.D.L.: collected data; F.M.T.: collected data; S.F.C.: interpreted data and did the critical revision of the manuscript; A.S.L.: interpreted data and did the critical revision of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guedes, A.R., Oliveira, M.S., Tavares, B.M. et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci Rep 13, 712 (2023). https://doi.org/10.1038/s41598-022-25908-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25908-6
This article is cited by
-
How does the SARS-CoV-2 reinfection rate change over time? The global evidence from systematic review and meta-analysis
BMC Infectious Diseases (2024)
-
The symptoms and interval of Omicron SARS-CoV-2 reinfection among healthcare workers in a hospital of Southern China: a cross-sectional study
BMC Infectious Diseases (2024)
-
Are repeat COVID infections dangerous? What the science says
Nature (2023)
-
Endemic characteristics of SARS-CoV-2 infection
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.