Abstract
Blood-based adjunctive measures that can reliably predict abdominal aortic aneurysm (AAA)-related complications hold promise for mitigating the AAA disease burden. In this pilot study, we sought to evaluate the prognostic performance of complement factors in predicting AAA-related clinical outcomes. We recruited consecutive AAA patients (n = 75) and non-AAA patients (n = 75) presenting to St. Michael’s Hospital. Plasma levels of complement proteins were assessed at baseline, as well as prospectively measured regularly over a period of 2 years. The primary outcome was the incidence of rapidly progressing AAA (i.e. aortic expansion), defined as change in AAA diameter by either 0.5 cm in 6 months, or 1 cm in 12 months. Secondary outcomes included incidence of major adverse aortic events (MAAE) and major adverse cardiovascular events (MACE). All study outcomes (AAA diameter, MACE and MAAE) were obtained during follow-up. Multivariable adjusted Cox regression analyses were performed to assess the prognostic value of plasma C2 levels in patients with AAA regarding rapid aortic expansion and MAAE and MACE. Event-free survival rates of both groups were also compared. Compared to non-AAA patients, patients with AAA demonstrated significantly higher plasma concentrations of C1q, C4, Factor B, Factor H and Factor D, and significantly lower plasma concentrations of C2, C3, and C4b (p = 0.001). After a median of 24 months from initial baseline measurements, C2 was determined as the strongest predictor of rapid aortic expansion (HR 0.10, p = 0.040), MAAE (HR 0.09, p = 0.001) and MACE (HR 0.14, p = 0.011). Based on the data from the survival analysis, higher levels of C2 at admission in patients with AAA predicted greater risk for rapid aortic expansion and MAAE (not MACE). Plasma C2 has the potential to be a biomarker for predicting rapid aortic expansion, MAAE, and the eventual need for an aortic intervention in AAA patients.
Similar content being viewed by others
Introduction
Abdominal aortic aneurysm (AAA) is a progressive cardiovascular disease with exceedingly high rates of morbidity and mortality, resulting in up to 200,000 annual deaths worldwide. Clinically, an AAA is defined as a 50% or greater increase in the diameter of the aorta1. Various risk factors have been linked with increased aortic wall degeneration, including but not limited to, old age, male sex, smoking, family history, hypertension, dyslipidemia, cardiovascular disease, and peripheral vascular disease2. Currently, the management of patients with known AAA includes serial surveillance (using either computed tomography (CT) or ultrasound) to monitor the maximum transverse diameter of the aneurysm3. Due to the life-threatening risk of rupture, repair of the AAA is generally indicated once the maximal transverse diameter reaches 5.0 cm in females and 5.5 cm in males4,5.
The progressive development of AAA is a dynamic process with a complex pathogenesis, but hallmarks include vascular smooth muscle cell apoptosis, oxidative stress, elastin fragmentation, extracellular matrix degradation, and inflammation6,7. Various circulating screening biomarkers have been proposed for the screening of AAA presence and size2,8; however, validated studies investigating biomarkers for prognostication of AAA-related complications have been scarce.
Previous studies have demonstrated the pivotal role played by the innate immune system in the progression of aortic aneurysms9,10,11. The complement system, which is part of the innate immunity and consists of more than thirty proteins and three pathways (the Classical, Lectin, and the Alternative)12, has elements that are found in every strata of the aneurysmal aortic tissue13,14,15. Since an association between plasma complement factors and aneurysm progression has previously been established13,16, we conducted this pilot study to: (1) investigate whether complement factors can serve as an adjunct in the prognosis of AAA-related complications and (2) study the potential of complement factors in facilitating the stratification of AAA patients as either high/low-risk for major adverse cardiac events (MACE), rapid aortic expansion, and/or major adverse aortic events (MAAE).
Methods
Patient recruitment and assessment
The first consecutive 75 patients encountered with asymptomatic infrarenal AAA and 75 controls without AAA presenting to ambulatory clinics at St. Michaels Hospital (Toronto, Canada) between May 2017 to May 2018 were included in this study. The patient's clinical data, physical exam, and abdominal aortic ultrasound were recorded upon initial encounter. Clinical data captured from patients included baseline demographics, history of cardiovascular diseases, cardiovascular risk factors (hypertension, hypercholesterolemia, and diabetes), and smoking status, as described previously17. The presence of AAA was verified through an ultrasound, with the diagnosis established by a vascular physician as per AAA-related clinical guidelines set forth by the Society of Vascular Surgery3. In short, patients were diagnosed with AAA if the observed aortic diameter on imaging was ≥ 3 cm. The control group consisted of patients presenting with non-AAA, vascular-related pathologies (varicose veins, thoracic outlet syndrome etc.) in addition to having an aortic diameter of < 3 cm.
Patient selection criteria
Patients presenting with our study endpoints (see below), or one or more of the following indications were not eligible for inclusion: AAA diameter exceeding operative threshold (AAA diameter > 5 cm for females or > 5.5 cm for males), presenting with ruptured AAA, or presenting with symptomatic AAA (defined as symptoms that can be attributed to the aneurysm, such as abdominal pain or limb ischemia). Additionally, we excluded patients with a prior history of AAA repair, AAA secondary to mycotic or inflammatory etiology, sepsis (< 3 months) or malignancy. Lastly, patients with thoracic or thoracoabdominal aneurysms, as well as aortic dissections, were excluded.
Ethics approval and blood sampling
Informed consent was obtained from all participants, and ethical approval was granted by St. Michael’s Hospital. Blood samples were obtained by venipuncture during the initial ambulatory visit. After adequate centrifugation, plasma samples were aliquoted and stored at − 80 °C. Levels of complement proteins (described below) were quantified using the same blood sample. All methods were carried out in accordance with relevant guidelines and regulations.
Complement proteins and factors multiplex assay
Luminex MILLIPLEX MAP Kit Human Complement Magnetic Bead Panels 1 and 2 multiplex assay kits (EMD-Millipore; Billerica, MA, USA) were used to measure the plasma levels of the following proteins involved in the complement pathway: Complement C1q (C1q), Complement C2 (C2), Complement C3 (C3), Complement C4 (C4), Complement C4b (C4b), Complement C5 (C5), Adipsin Mannose-Binding Lectin (MBL), Complement Factor B (Factor B), Complement Factor D (Factor D), Complement Factor H (Factor H), and Complement Factor I (Factor I). The manufacturer's protocol was followed for the multiplex bead assays. Sample intra-assay Coefficients of Variability (CV) was < 10% while the inter-assay CV was 15%. Prior to any sample analysis, Fluidics Verification and Calibration bead kits (Luminex Corp) were used to calibrate the MagPix analyzer (Luminex Corp; Austin, Texas). At least 50 beads for each protein were acquired using Luminex xPonent software and analyzed using Milliplex Analyst software (v.5.1; EMD-Millipore).
Measured outcomes
The primary outcome of this study was the rapid expansion of the AAA diameter observed during the follow-up period and defined as AAA size > 1 cm over 12 months or 0.5 cm over 6 months 3. Secondary outcomes included the incidence of major adverse aortic events (MAAE) and major adverse cardiovascular events (MACE). MAAE was defined as the composite incidence of elective AAA repair (open or endovascular repair), emergent AAA repair, AAA-related deaths, and AAA-induced complication (arterial thrombosis due to emboli from AAA, primary aorto-enteric fistula or primary aortocaval fistulas). MACE was defined as the composite incidence of cardiovascular-related mortality, stroke, or myocardial infarction.
Two-year prospective follow-up
Over a period of 24 months after the initial baseline visit, patients were seen at 6-month or 12-month intervals (depending on their AAA size). This follow-up period was based on the AAA surveillance protocol recommended by the SVS guidelines3. During these follow-up visits, changes in clinical history or medications were recorded, AAA diameter was re-measured (via ultrasound), and the incidence of emergent AAA repair (secondary to the development of symptomatic AAA) or ruptured AAA were noted. Furthermore, the need for elective AAA repair (i.e. repair of AAA as per SVS guidelines—AAA diameter > 5 cm for females or > 5.5 cm for males) or repair of AAA due to rapid expansion were also recorded3.
Statistical analysis
Baseline demographic and clinical characteristics were summarized as means and standard deviations (SDs) or numbers and proportions. Baseline differences between groups were calculated using independent t-test for continuous variables and chi-square test for categorical variables. Normality of plasma complement factor levels were assessed by the Kolmogorov–Smirnov test, and summarized as medians and interquartile ranges (IQRs) accordingly. Event rates for rapid AAA diameter expansion, MAAE, and MACE at 2 years were reported for the overall cohort and compared between AAA and non-AAA patient groups using chi-square test. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) for events per one unit increase in plasma complement factors were calculated using univariable and multivariable models adjusted for age, sex, hypertension, dyslipidemia, smoking, and history of coronary artery disease. Receiver operator curve (ROC) analysis was conducted to identify a cut-off value for C2 that could facilitate stratification of AAA patients at-risk of adverse clinical outcomes into low versus high-risk groups. The cut-off value was chosen based on a high positive likelihood ratio (LR +) yielding a sensitivity above 90%. Overall event-free survival rates of both groups were displayed using Kaplan–Meier curves, and differences between curves were compared with a log-rank test. Significance was set at a two-tailed p < 0.05. All analyses were carried out using SPSS software version 23 (SPSS Inc., Chicago, Illinois, USA).
Ethics statement
The studies involving human participants were reviewed and approved by Unity Health Toronto's Research Ethics Board. The patients/participants provided their written informed consent prior to participating in this study.
Results
Clinical characteristics
Baselines clinical characteristics of the recruited 75 AAA patients (50%) and 75 non-AAA patients (50%) are presented in Table 1. Overall, the mean age of the cohort was 67 (± 12) years. There were 101 (67%) male participants, 91 (61%) patients with hypertension, 94 (63%) with hypercholesterolemia, 23 (15%) with diabetes, 11 (7%) with renal insufficiency, 36 (24%) current smokers, 4 (3%) with history of congestive heart failure (CHF), 39 (26%) with history of coronary artery disease (CAD) and 13 (9%) with history of stroke. Patients with an AAA were significantly older than patients without an AAA (72 [± 8] vs. 61 [± 13], p = 0.001), had a higher percentage of active smokers (37% vs. 11%, p = 0.001), more likely to have hypercholesterolemia (80% vs. 47%, p = 0.001), renal insufficiency (13% vs. 1%, p = 0.004), CAD (39% vs. 13%, p = 0.01), and history of stroke (15% vs. 3%, p = 0.013). With regards to patient medical optimization, AAA patients were more likely to be on statins (81vs. 49%, p = 0.001), ACE inhibitors (62% vs. 31%, p = 0.001), and aspirin (60% vs. 28%, p = 0.001) when compared to non-AAA patients (Table 1).
Composition of circulating plasma complement factors in patients with and without and AAA
Compared to non-AAA patients, patients with an AAA had significantly higher median [IQR] levels of plasma factors C1q (59.5 ug/mL [32.8–67.4] vs. 41.1 ug/mL [30.6–57.9], p = 0.001), C4 (686 ug/mL [528–878] vs. 593 ug/mL [275–748], p = 0.001), Factor B (254 ug/mL [180–327] vs. 212 ug/mL [145–272], p = 0.001), Factor H (337 ug/mL [246–438] vs. 302 ug/mL [218–366], p = 0.002), and Factor D (3.59 ug/mL [2.14–5.68] vs. 2.24 ug/mL [1.32–3.34], p = 0.022). Conversely, compared to non-AAA patients, patients with AAA had a significantly lower median [IQR] levels of plasma factors C2 (0.26 ug/mL [0.19–0.41] vs. 0.33 ug/mL [0.28–0.42], p = 0.027), C3 (83.3 ug/mL [40.8–145] vs. 136 ug/mL [53–329], p = 0.014), and C4b (9.07 ug/mL [6.53–12.3] vs. 13.7 ug/mL [7.97–20.2], p = 0.006). No significant difference was noted in C5 (13.5 ug/mL [10.4–18.3] vs. 13.2 ug/mL [7.65–24.2], p = 0.666) and Factor I (25.9 ug/mL [16.6–52.8] vs. 22.4 ug/mL [14.7–41.3], p = 0.968) levels between both patient groups (Table 2).
Clinical outcomes
Complete, two-year follow-up data were available for 143 (95%) patients, with a mean duration of 22.0 (± 2.1) months. Over the follow-up period, 12 (8%) patients were observed to have rapid aortic expansion, 33 (22%) had a MAAE, and 30 (20%) had a MACE (Table 3).
Association between complement proteins and study endpoints
Among all the proteins investigated, C2 was singularly significantly predictive of all three clinical outcomes—rapid aortic expansion, MAAE and MACE. A decrease in plasma C2 (per ug/mL) was associated with significant increase in risk for rapid aortic expansion (adjusted HR 0.10 [95% CI 0.08–0.81], p = 0.040), MAAE (adjusted HR 0.09 [95% CI 0.03–0.26], p = 0.001) (Fig. 1B) and MACE (adjusted HR 0.14 [95% CI 0.03–0.63], p = 0.011) (Table 4). On the other hand, we noted that few of the investigated proteins were able to predict some but not all investigated outcomes. An increase in plasma Factor H (per ug/mL) was associated with an increase in the risk of MAAE (adjusted HR 1.12 [95% CI 1.05–6.70], p = 0.049) and risk of MACE (adjusted HR 0.51 [95% CI 0.30–0.87], p = 0.014) (Table 4). An increase in plasma C4b (per ug/mL) was associated with a decreased risk of MAAE (adjusted HR 0.35 [95% CI 0.16–0.76], p = 0.011) (Table 4). Lastly, an increase in plasma MBL (per ug/mL) was associated decrease in MACE (adjusted HR 0.54 [95% CI 0.30–0.87], p = 0.014) (Table 4). Since C2 was the only protein candidate that was significantly predictive of all primary and secondary study outcomes, it was selected for further analysis.
Cumulative event-free survival for in 75 patients with AAA divided into 2 groups, Low C2 group (n = 24) with C2 concentration < 0.202 ug/mL and High C2 group (n = 51) with C2 concentration > 0.202 ug/mL. (A) rapid AAA expansion (freedom from ΔAAA size > 1 cm/12 months or 0.5 cm/6 months) (B) MAAE and (C) MACE of all 75 patients diagnosed with AAA according to the levels of C2 levels (high versus low C2 levels), p-value = 0.001.
Correlation of plasma C2 levels and clinical variables
Among AAA and non-AAA patients, C2 plasma levels were not associated with any known established risk factor (age, sex, hypertension, hypercholesterolemia, diabetes, renal insufficiency, smoking, history of congestive heart failure, history of coronary artery disease, and history of stroke, p-value < 0.05) (Table 5). Similarly, C2 plasma levels were not associated with commonly used medications used to treat the listed risk factors (ACE inhibitors/ARBS, Aspirin, beta blockers, CCB, HCTZ, insulin, oral hypoglycemics, and statins, p-value < 0.05) (Table 5). However, median plasma C2 levels were significantly lower in patients with AAA (0.27 [0.21–0.45] vs. 0.39 [0.31–0.57], p-value = 0.002) relative to patients without AAA, (Table 5).
Prognostication of study outcomes based on C2 levels at presentation
Based on the ROC curve, we identified a C2 concentration of 0.202 ug/mL (AUC of 0.709 (p = 0.010, 95% CI 0.641–0.763), likelihood ratio (LR) + 5.34, 91% sensitive, and 62% specific) as the optimal cutoff value to facilitate stratification of AAA patients at-risk of clinical complications into low-risk vs high-risk groups. Utilizing this cutoff value, AAA patients (n = 75) were divided into 2 groups, (1) Low C2 group (n = 24) with C2 concentration < 0.202 ug/mL and (2) High C2 group (n = 51) with C2 concentration > 0.202 ug/mL. The clinical characteristics of both these groups are highlighted in Table 6.
Among AAA patients, a higher rate of rapid aortic expansion (33% vs. 4%, p = 0.001) and MAAE (67% vs. 33%, p = 0.007) was noted in patients with low C2 levels compared to the High C2 group. No significant difference was noted in the incidence of MACE (33% vs. 18%, p = 0.130) among AAA patients with high versus low C2 levels.
Kaplan–Meier analysis demonstrated that low plasma levels of C2 (< 0.202 ug/mL) can reliably stratify patients into those most likely to undergo rapid aortic expansion (p = 0.005; log-rank = 7.78) (Fig. 1A) as well as MAAE (p = 0.014; log-rank = 6.02) (Fig. 1B), but not MACE (p = 0.132; log-rank = 2.26) (Fig. 1C). Freedom from rapid expansion in AAA at 1 and 2 years were 94% and 92% in the high C2 group, respectively, and 71% and 67% in the low C2 group, respectively (Fig. 1A). MAAE-free survival rates at 1 and 2 years were 71% and 67% in the high C2 group, respectively, and 58% and 33% in the low C2 group, respectively (Fig. 1B). Finally, MACE-free survival rates at 1 and 2 years were 86% and 82% in the high C2 group, respectively, and 75% and 67% in the low C2 group, respectively (Fig. 1C).
Discussion
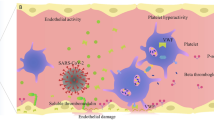
In this study, we demonstrated significant differences in plasma levels of component factors in AAA patients versus non-AAA patients. Our analysis demonstrated that baseline C2 was a reliable predictor of all three measured outcomes in this study, including rapid aortic expansion, MAAE and MACE over a two-year follow-up period. Based on Kaplan–Meier analysis data, measuring C2 levels at baseline may aid and serve as a potential biomarker for stratifying patients at risk of rapid aortic expansion or MAAE (Fig. 2).
As various elements of the complement system are found in different strata of the aortic tissue, a growing body of evidence demonstrates the active involvement of the complement system in acute cardiovascular events and aortic disease13,14,15. In murine animals, complement C3a and C5a depletion were protective against AAA formation13. In contrast, Zagrapan et al. found significantly increased levels of circulating C5a factor in the plasma levels of AAA patients compared to healthy patients16. Furthermore, Zagrapan et al. also linked plasma C5a levels with aneurysm progression, thereby conferring a potential role for complement factors as an adjunct for the prognosis of patients with AAA16. In this study, we demonstrated a significant difference in complement factors in the Classical pathway (C1q, C2, C4 and C4b), Lectin pathway (MBL) and Alternative pathway (C3, Factor B and Factor D) among patients with and without an AAA.
Additionally, we demonstrated an association between low circulating C2 levels and increased risk for rapid aortic expansion and MAAE in patients with AAA. In comparison, Hinterseher et al. demonstrated an increase in gene expression of C1Qa, C1Q and C2 and a decrease in expression of C2 inhibitor SERPING1 in human aortic aneurysmal tissue18. Furthermore, they noted an increase in complement protein C2 staining in cells of aortic aneurysmal tissue18.
The pathogenesis behind our findings (i.e. the association between low levels of C2 in plasma and an increased expression of C2) within aneurysmal aortic tissue still needs to be investigated further. Previously, the Classical pathway has been shown to be independently activated by pentraxins, such as C-reactive protein (CRP), which has also been linked to aortic expansion in patients with AAA19,20. Homozygous C2 deficiency, in addition to its association with severe infections and rheumatic disease, has also been linked with various forms of vasculitis with cutaneous and gastrointestinal manifestations21,22. However, a direct link between C2 deficiency and aneurysm formation is yet to be established. Thus, the biological role of C2 in aortic aneurysm progression would undoubtedly be an area of interest that would warrant further investigation.
To date, numerous circulating biomarkers have been investigated as potential predictive factors for AAA expansion and rupture. These markers can be categorized into those involved in the coagulation pathway23,24,25,26,27,28,29,30, extracellular matrix turnover and matrix degrading enzymes23,30,31,32,33,34,35,36,37,38,39,40,41,42 and lipids25,43,44,45,46. Furthermore, there have been various circulating biomarkers involved in the immune response system that have been investigated for an association with AAA expansion and/or rupture, which include, CRP24,25,35,46,47,48,49, interleukin-1β50, interleukin-250, interleukin-646,50,51, interleukin-850, interferon-gamma52, leukocytes24, macrophage inhibiting factor23,53, neutrophil gelatinase-associated lipocalin54, osteopontin55, osteoprotegerin56, peroxiredoxin57, tumour necrosis factor-α46,50, tumour necrosis factor-like weak inducer of apoptosis58 and C5a16. The lack of data on the role of the complement system in aortic expansion led to further analysis of the relationship between circulating complement factors and aortic expansion.
The clinical decision-making for AAA treatment can be complicated by intrapatient and interpatient variations59. Furthermore, recent studies have cast doubt over whether the maximum diameter alone should guide the treatment of patients with AAA60. Notably, circulating biomarkers have also been greatly emphasized due to their capacity to provide important prognostic information about subsequent aortic behaviour, thereby allowing for more patient-specific management8,48,61,62,63,64. At the time of writing this paper, more accurate prognostic predictors are needed to guide stratifying patients into those at risk for rupture rather than relying on diameter alone, as some small AAA are known to rupture, while some large AAAs can remain dormant for some time4,65. The current SVS guidelines suggest surveillance imaging for AAAs measuring 3.0–3.9 cm, 4.0–4.9 cm and 5.0–5.4 cm at 3-year, 12-month and 6-month intervals, respectively3. In contrast, our findings indicate that there may be a subgroup of AAA patients (those with low circulating plasma C2 at higher risk of rapid aortic expansion and MAAE) who may benefit from careful oversight and more frequent follow-up. Furthermore, circulating C2 levels may be utilized as a part of the clinical decision-making process to help reduce the risks associated with AAA treatment, particularly in high-operative-risk patients, until the risk of rupture is believed to outweigh the operative risk4. While our findings regarding plasma C2 levels may add to the potential biomarkers that can be used to prognosticate patients with AAA, further validation in a larger and more heterogeneous patient cohort is still required.
Limitations include the single-center nature of our study and the unaccounted study outcomes in patients lost to follow-up. A larger and more diverse sample size with prolonged follow-up may prove insightful in evaluating the true prognostication potential of C2 in patients with AAA, as this was a pilot study to determine whether the role of complement factors in AAA disease warrants further investigation. Future studies investigating the biological role of C2 in aneurysmal aortic tissue are also warranted. Not all complement factors and their associated activated forms and substrates were investigated in this trial. Lastly, there may have additional confounding factors aside from the ones measured in this study that may correlate with plasma C2 levels, which will surely need to be examined in future studies.
In conclusion, we demonstrated that C2 has a strong predictive potential for AAA-related complications despite adjusting for confounding factors. Provided our findings are validated, circulating plasma C2 may be used in the future as a viable adjunct blood-based biomarker for the identification of AAA patients at high risk of rapid expansion and MAAE.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Sampson, U. K. A. et al. Global and regional burden of aortic dissection and aneurysms: mortality trends in 21 world regions, 1990 to 2010. Glob Heart. 9(1), 171-180.e10 (2014).
Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 16(4), 225–242 (2019).
Chaikof, E. L. et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 67(1), 2-77.e2 (2018).
Lederle, F. A. et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 287(22), 2968–2972 (2002).
Brown, P. M., Zelt, D. T., Sobolev, B., Hallett, J. W. & Sternbach, Y. The risk of rupture in untreated aneurysms: The impact of size, gender, and expansion rate. J Vasc Surg. 37(2), 280–284 (2003).
Boddy, A., Lenk, G., Lillvis, J., Nischan, J., Kyo. Y. & Kuivaniemi, H. Basic research studies to understand aneurysm disease —PubMed. Drug News Perspect. 142–148. (2008).
Wassef, M. et al. Pathogenesis of abdominal aortic aneurysms: A multidisciplinary research program supported by the National Heart, Lung, and Blood Institute. J. Vasc. Surg. 34(4), 730–738 (2001).
Stather, P. W. et al. Meta-analysis and meta-regression analysis of biomarkers for abdominal aortic aneurysm. J. Br. Surg. 101(11), 1358–1372 (2014).
Brophy, C. M., Reilly, J. M., Smith, G. J. W. & Tilson, M. D. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann. Vasc. Surg. 5(3), 229–233 (1991).
Gregory, A. K. et al. Features of autoimmunity in the abdominal aortic aneurysm. Arch. Surg. 131(1), 85–88 (1996).
Xia, S., Ozsvath, K., Hirose, H. & Tilson, M. D. Partial amino acid sequence of a novel 40-kDa human aortic protein, with vitronectin-like, fibrinogen-like, and calcium binding domains: Aortic aneurysm-associated protein-40 (AAAP-40) [human MAGP-3, proposed]. Biochem. Biophys. Res. Commun. 219(1), 36–39 (1996).
Gros, P., Milder, F. J. & Janssen, B. J. C. Complement driven by conformational changes. Nat. Rev. Immunol. 8(1), 48–58 (2008).
Pagano, M. B. et al. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation 119(13), 1805–1813 (2009).
Martinez-Pinna, R. et al. Proteomic analysis of intraluminal thrombus highlights complement activation in human abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 33(8), 2013–2020 (2013).
Capella, J. F., Paik, D. C., Yin, N. X., Gervasoni, J. E. & Tilson, M. D. Complement activation and subclassification of tissue immunoglobulin G in the abdominal aortic aneurysm. J. Surg. Res. 65(1), 31–33 (1996).
Zagrapan, B., Eilenberg, W., Scheuba, A., Klopf, J., Brandau, A. & Story, J., et al. Complement factor C5a is increased in blood of patients with abdominal aortic aneurysm and has prognostic potential for aneurysm growth. J. Cardiovasc. Transl. Res. [Internet]. 2021 Aug 1 [cited 2022 Feb 27];14(4):761–9. Available from: https://pubmed.ncbi.nlm.nih.gov/33332020/
Khan, H. et al. Aspirin nonsensitivity in patients with vascular disease: Assessment by light transmission aggregometry (aspirin nonsensitivity in vascular patients). Res. Pract. Thromb. Haemost. 5(8), e12618 (2021).
Hinterseher, I. et al. Role of complement cascade in abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 31(7), 1653–1660 (2011).
Badger, S. A. et al. C-reactive protein (CRP) elevation in patients with abdominal aortic aneurysm is independent of the most important CRP genetic polymorphism. J. Vasc. Surg. 49(1), 178–184 (2009).
Shangwei, Z. et al. Serum high-sensitive C-reactive protein level and CRP genetic polymorphisms are associated with abdominal aortic aneurysm. Ann. Vasc. Surg. 1(45), 186–192 (2017).
Jönsson, G. B. et al. Rheumatological manifestations, organ damage and autoimmunity in hereditary C2 deficiency. Rheumatology (Oxford) 46(7), 1133–1139 (2007).
Her, M. Y., Song, J. Y. & Kim, D. Y. Hypocomplementemic urticarial vasculitis in systemic lupus erythematosus. J. Korean Med. Sci. 24(1), 184–186 (2009).
Lindholt, J. S., Jørgensen, B., Shi, G. P. & Henneberg, E. W. Relationships between activators and inhibitors of plasminogen, and the progression of small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 25(6), 546–551 (2003).
Domanovits, H. et al. Acute phase reactants in patients with abdominal aortic aneurysm. Atherosclerosis 163(2), 297–302 (2002).
Tambyraja, A. L., Dawson, R., Valenti, D., Murie, J. A. & Chalmers, R. T. Systemic inflammation and repair of abdominal aortic aneurysm. World J. Surg. 31(6), 1210–1214 (2007).
Adam, D. J., Haggart, P. C., Ludlam, C. A. & Bradbury, A. W. Hemostatic markers before operation in patients with acutely symptomatic nonruptured and ruptured infrarenal abdominal aortic aneurysm. J. Vasc. Surg. 35(4), 661–665 (2002).
Skagius, E., Siegbahn, A., Bergqvist, D. & Henriksson, A. E. Fibrinolysis in patients with an abdominal aortic aneurysm with special emphasis on rupture and shock. J. Thromb. Haemost. 6(1), 147–150 (2008).
Flondell-Sité, D., Lindblad, B., Kölbel, T. & Gottsäter, A. Markers of proteolysis, fibrinolysis, and coagulation in relation to size and growth rate of abdominal aortic aneurysms. Vasc. Endovascular. Surg. 44(4), 262–268 (2010).
Hobbs, S. D., Haggart, P., Fegan, C., Bradbury, A. W. & Adam, D. J. The role of tissue factor in patients undergoing open repair of ruptured and nonruptured abdominal aortic aneurysms. J. Vasc. Surg. 46(4), 682–686 (2007).
Lindholt, J. S., Jørgensen, B., Fasting, H. & Henneberg, E. W. Plasma levels of plasmin-antiplasmin-complexes are predictive for small abdominal aortic aneurysms expanding to operation-recommendable sizes. J. Vasc. Surg. 34(4), 611–615 (2001).
Lindholt, J. S., Heickendorff, L., Vammen, S., Fasting, H. & Henneberg, E. W. Five-year results of elastin and collagen markers as predictive tools in the management of small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 21(3), 235–240 (2001).
Satta, J., Haukipuro, K., Kairaluoma, M. I. & Juvonen, T. Aminoterminal propeptide of type III procollagen in the follow-up of patients with abdominal aortic aneurysms. J. Vasc. Surg. 25(5), 909–915 (1997).
Lindholt, J. S., Vammen, S., Fasting, H., Henneberg, E. W. & Heickendorff, L. The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study. Eur. J. Vasc. Endovasc. Surg. 20(3), 281–285 (2000).
Lindholt, J. S., Ashton, H. A. & Scott, R. A. Indicators of infection with Chlamydia pneumoniae are associated with expansion of abdominal aortic aneurysms. J. Vasc. Surg. 34(2), 212–215 (2001).
Speelman, L. et al. The influence of wall stress on AAA growth and biomarkers. Eur. J. Vasc. Endovasc. Surg. 39(4), 410–416 (2010).
Lindholt, J. S., Jørgensen, B., Klitgaard, N. A. & Henneberg, E. W. Systemic levels of cotinine and elastase, but not pulmonary function, are associated with the progression of small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 26(4), 418–422 (2003).
Wilson, W. R. W. et al. Elevated plasma MMP1 and MMP9 are associated with abdominal aortic aneurysm rupture. Eur. J. Vasc. Endovasc. Surg. 35(5), 580–584 (2008).
Lindholt, J. S., Ashton, H. A., Heickendorff, L. & Scott, R. A. Serum elastin peptides in the preoperative evaluation of abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 22(6), 546–550 (2001).
Petersen, E., Gineitis, A., Wågberg, F. & Angquist, K. A. Serum levels of elastin-derived peptides in patients with ruptured and asymptomatic abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 22(1), 48–52 (2001).
de Céniga, M. V. et al. Search for serum biomarkers associated with abdominal aortic aneurysm growth–a pilot study. Eur. J. Vasc. Endovasc. Surg. 37(3), 297–299 (2009).
Pulinx, B. et al. Differential protein expression in serum of abdominal aortic aneurysm patients-a proteomic approach. Eur. J. Vasc. Endovasc. Surg. 42(5), 563–570 (2011).
Treska, V. & Topolcan, O. Plasma and tissue levels of collagen types I and III markers in patients with abdominal aortic aneurysms. Int. Angiol. 19(1), 64–68 (2000).
Watt, H. C. et al. Serum triglyceride: A possible risk factor for ruptured abdominal aortic aneurysm. Int. J. Epidemiol. 27(6), 949–952 (1998).
Lindqvist, M., Wallinder, J., Bergström, J. & Henriksson, A. E. Plasma glycosylphosphatidylinositol phospholipase D (GPI-PLD) and abdominal aortic aneurysm. Int. J. Clin. Exp. Med. 5(4), 306–309 (2012).
Lindholt, J. S. et al. Smoking, but not lipids, lipoprotein(a) and antibodies against oxidised LDL, is correlated to the expansion of abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 21(1), 51–56 (2001).
Flondell-Sité, D., Lindblad, B. & Gottsäter, A. High levels of endothelin (ET)-1 and aneurysm diameter independently predict growth of stable abdominal aortic aneurysms. Angiology 61(4), 324–328 (2010).
de Haro, J. et al. Prediction of asymptomatic abdominal aortic aneurysm expansion by means of rate of variation of C-reactive protein plasma levels. J. Vasc. Surg. 56(1), 45–52 (2012).
Norman, P., Spencer, C. A., Lawrence-Brown, M. M. & Jamrozik, K. C-reactive protein levels and the expansion of screen-detected abdominal aortic aneurysms in men. Circulation 110(7), 862–866 (2004).
Wiernicki, I., Safranow, K., Baranowska-Bosiacka, I., Piatek, J. & Gutowski, P. Haptoglobin 2–1 phenotype predicts rapid growth of abdominal aortic aneurysms. J. Vasc. Surg. 52(3), 691–696 (2010).
Treska, V., Topolcan, O. & Pecen, L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin. Chem. Lab. Med. 38(11), 1161–1164 (2000).
Jones, K. G. et al. Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation 103(18), 2260–2265 (2001).
Juvonen, J. et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 17(11), 2843–2847 (1997).
Pan, J. H. et al. Macrophage migration inhibitory factor is associated with aneurysmal expansion. J. Vasc. Surg. 37(3), 628–635 (2003).
Ramos-Mozo, P. et al. Increased plasma levels of NGAL, a marker of neutrophil activation, in patients with abdominal aortic aneurysm. Atherosclerosis 220(2), 552–556 (2012).
Golledge, J. et al. Association between osteopontin and human abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 27(3), 655–660 (2007).
Moran, C. S. et al. Association of osteoprotegerin with human abdominal aortic aneurysm progression. Circulation 111(23), 3119–3125 (2005).
Martinez-Pinna, R. et al. Identification of peroxiredoxin-1 as a novel biomarker of abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 31(4), 935–943 (2011).
Martín-Ventura, J. L. et al. Soluble TWEAK plasma levels predict expansion of human abdominal aortic aneurysms. Atherosclerosis 214(2), 486–489 (2011).
Brady, A. R., Thompson, S. G., Fowkes, F. G. R., Greenhalgh, R. M. & Powell, J. T. Abdominal aortic aneurysm expansion: Risk factors and time intervals for surveillance. Circulation 110(1), 16–21 (2004).
McGloughlin, T. M. & Doyle, B. J. New approaches to abdominal aortic aneurysm rupture risk assessment: Engineering insights with clinical gain. Arterioscler. Thromb. Vasc. Biol. 30(9), 1687–1694 (2010).
Golledge, J., Tsao, P. S., Dalman, R. L. & Norman, P. E. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation 118(23), 2382–2392 (2008).
Maegdefessel, L. et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Invest. 122(2), 497–506 (2012).
Maegdefessel, L., Dalman, R. L. & Tsao, P. S. Pathogenesis of abdominal aortic aneurysms: microRNAs, proteases, genetic associations. Annu. Rev. Med. 65, 49–62 (2014).
Zhang, W. et al. Plasma microRNAs serve as potential biomarkers for abdominal aortic aneurysm. Clin. Biochem. 48(15), 988–992 (2015).
Nicholls, S. C., Gardner, J. B., Meissner, M. H. & Johansen, K. H. Rupture in small abdominal aortic aneurysms. J. Vasc. Surg. 28(5), 884–888 (1998).
Funding
The study was funded by the Bill and Vicky Blair Foundation.
Author information
Authors and Affiliations
Contributions
T.F. and M.Q. performed study concept and design; T.F., M. H. S., M.P., N. J., M. W., R. A., and M.Q. performed development of methodology and writing, review and revision of the paper; T.F., A. Z., and M. Q., provided acquisition, analysis and interpretation of data, and statistical analysis; M.Q. provided technical and material support. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feridooni, T., Zamzam, A., Popkov, M. et al. Plasma complement component C2: a potential biomarker for predicting abdominal aortic aneurysm related complications. Sci Rep 12, 21252 (2022). https://doi.org/10.1038/s41598-022-24698-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24698-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.