Abstract
There have been limited studies on the association between prognosis and body weight change in patients with idiopathic pulmonary fibrosis (IPF). This single-center retrospective observational study evaluated the impact of weight loss on outcomes in Korean patients with IPF receiving pirfenidone at a tertiary medical institution. We analyzed 215 IPF patients prescribed pirfenidone from January 1st, 2015 to December 31st, 2019. The patients were categorized into maintained weight (MW; weight gain or loss < 5%/year) and reduced weight (RW; weight loss ≥ 5%/year) groups. The mean age was 71.8 years and 175 (81.4%) were male. There were 54 (25.1%) patients in the RW group. All patients showed a decrease in body weight (baseline vs. after 1 year; 64.1 kg vs. 62.8 kg, P < 0.001). Although baseline lung function showed a difference, there was no difference in the rate of change (forced vital capacity [% of predicted]; P = 0.221, diffusing capacity of the lung for carbon monoxide [% of predicted]; P = 0.973). The MW group had a lower risk of all-cause mortality (P < 0.001). Weight loss appeared to be a significant risk factor for mortality in patients with IPF. Not only disease control with antifibrotic agents, but also efforts to prevent weight loss may be necessary.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease characterized by progressive loss of lung function1. Although the prognosis of IPF is generally poor and the condition is often fatal, the course of progression is variable, with the median survival in untreated patients being approximately 3 years from diagnosis2. The identification of prognostic factors for disease progression is, therefore, an important and active area of interest3. Comorbidities, old age, smoking, decline in forced vital capacity (FVC), GAP score, and exercise capacity are established predictors of mortality in patients with IPF4,5.
Body weight and body mass index (BMI) could be easily measured in clinical practice, and previous studies have suggested that lower BMI6,7 or weight loss8 may be associated with a worse prognosis in patients with IPF. A recent study9 analyzing the degree of weight loss and prognosis according to BMI in fibrotic interstitial lung disease (ILD) including IPF also suggests that weight and BMI reduction are independent risk factors for 1-year mortality. However, studies of nintedanib, pirfenidone10, and those mentioned above6,7,8 have analyzed prognoses related to acute exacerbations and deterioration of lung function. In addition, a previous study11 reported the prevalence of malnutrition based on the fat-free mass index as 28%. In another recent study12, malnutrition and decreased food intake were associated with poor IPF outcomes. However, real-world data on long-term weight loss and clinical outcomes in Asians are still lacking. Thus, it remains unclear whether weight changes are associated with prognosis and changes in pulmonary function tests (PFTs) in patients with IPF being treated with pirfenidone.
We hypothesized that changes in body weight during treatment of IPF could be related to the prognosis of IPF and changes in PFTs, including FVC or diffusing capacity for carbon monoxide (DLCO). In our study, we assessed body weight change during treatment with pirfenidone and analyzed the associations between changes in body weight and clinical outcomes in Korean patients with IPF.
Results
Baseline characteristics
In total, 215 patients were included in the study population (Fig. 1). The mean (± standard deviation [SD]) age of the patients was 71.8 (± 7.4) years, and 175 (81.4%) patients were male. The baseline BMI of patients was 24.0 (± 2.9) kg/m2, and 28 (13.0%) patients were diagnosed with IPF by surgical lung biopsy. There were 169 (78.6%) smokers in the study population. The mean (± SD) ages of the maintained weight (MW) and reduced weight (RW) groups were 71.2 (± 7.2) and 73.8 (± 7.7) years, respectively, with a statistically significant difference (P = 0.032). The proportion of males was higher in the MW group than in the RW group: 134 (83.2%) and 41 (75.9%), respectively, but this difference did not have statistical significance (P = 0.233). In addition, the RW group was more likely to have lower BMI, baseline body weight, and lung function (Table 1).
Patients showed a statistically significant decrease in body weight and BMI during the follow-up (baseline vs. after 1 year [kg] 64.1 vs. 62.8, P < 0.001; BMI (kg/m2) 24.1 vs. 23.7, P < 0.001; Table S.1). There were 161 (74.9%) patients in the MW group and 54 (25.1%) patients in the RW group.
A total of 75 patients required pirfenidone dose adjustment (56 in the MW group vs. 19 in the RW group). Among those who had a history of pirfenidone dose reduction, gastrointestinal side effects were the most common side effect in both the MW and RW groups (n = 17 [10.6%] and n = 14 [25.9%], respectively; Table S.2). However, there was no statistical relationship between weight loss and a dose reduction history of pirfenidone (P = 0.957; Table S.3).
Lung function decline
The mean annual changes in lung function were as follows: FVC (% of predicted), − 2.2%/year (95% confidence interval [CI] − 2.7, − 1.6); and DLCO (% of predicted), − 3.9%/year (95% CI − 4.5, − 3.2) (Table 2). When the interaction effects according to the two groups of body weight change (MW vs. RW) were analyzed, there was no difference in the rate of change (forced vital capacity [% of predicted]; Pinteraction = 0.221, diffusing capacity of the lung for carbon monoxide [% of predicted]; Pinteraction = 0.973] (Fig. 2).
Linear mixed-effect model of pulmonary function trends. (A) Predicted mean FVC (mL) of body weight group with 95% CI. (B) Predicted mean FVC (% of predicted) of body weight group with 95% CI. (C) Predicted mean DLCO (mL/mmHg/min) of body weight group with 95% CI. (D) Predicted mean DLCO (% of predicted) of body weight group with 95% CI. PInteraction analysis of interaction (type III) according to the two groups of body weight change (maintained weight vs. reduced weight) showed there was no interaction with time for each lung function change result. FVC (mL) Pinteraction = 0.214, FVC (% of predicted) Pinteraction = 0.221, DLCO (mL/mmHg/min) Pinteraction = 0.429, DLCO (% of predicted) Pinteraction = 0.973.
There was a statistically significant difference in lung function between the maintained and reduced weight groups. The estimated differences in lung function were as follows: FVC (% of predicted), − 11.7%, P < 0.001; DLCO (% of predicted), − 9.1%, P = 0.004 (Table 3, Fig. 2).
Survival Outcomes
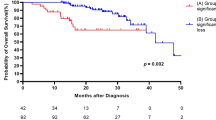
In time-to-event analysis, patients in the MW group had a significantly lower risk of experiencing all-cause mortality than patients who experienced a > 5%/year loss (P < 0.001) (Fig. 3). In univariate Cox proportional-hazards analysis, body weight loss was an independent risk factor for all-cause mortality (hazard ratio [HR] 3.270, 95% CI 2.254, 4.743, P < 0.001) along with age as well as lower baseline FVC and DLCO. In multivariate Cox proportional-hazards analysis, weight loss remained a significant factor (HR 2.358, 95% CI 1.572, 3.537, P < 0.001) along with age and lower baseline DLCO (Table 4). Weight loss was also analyzed as a risk factor for mortality in the time-dependent Cox regression analysis for the time point of 5% weight loss (HR 1.751, 95% CI 1.229, 2.494, P = 0.002) (Table S.4).
Discussion
In this study, significant body weight loss was observed in approximately 25% of patients treated with pirfenidone. Pirfenidone has many side effects and is frequently associated with gastrointestinal symptoms like nausea, anorexia, diarrhea, liver function test abnormalities, or cutaneous side effects13. During follow-up, IPF patients prescribed pirfenidone showed a tendency to lose weight and have decreased BMI during follow-up periods, but there was no statistically significant relationship between body weight loss and pirfenidone dose adjustment due to side effects and compliance. Moreover, body weight loss of ≥ 5% in patients with and without pirfenidone treatment (25.1% vs. 7.8%; data not shown) exhibited similar results as in the previous literature14. Although it was found that weight loss was not related to the rate of deterioration in lung function, patients experiencing significant weight loss during treatment had a higher risk of all-cause mortality compared to patients who maintained their weight. As in previous studies, age and baseline DLCO (% of predicted) were identified as risk factors for poor outcomes; in this study, significant weight loss was also confirmed to be a significant prognostic factor.
The results of this study are similar to the results of other studies that assessed the relationship between BMI, weight loss, and clinical outcomes of IPF. The studies reported that weight loss and BMI are associated with an increased risk of mortality in IPF patients, which is regarded as a marker of disease progression8,14,15,16. A study in patients with a placebo arm reported the proportion of patients with a decline in FVC by more than 10% or death up to 1 year was higher in patients with an annualized weight loss ≥ 5% compared to the patients not experiencing ≥ 5% weight loss10. The results from our analysis and the results from previous studies suggest that weight loss during pirfenidone treatment may be associated with worse clinical outcomes, including a decline in PFTs, in patients with IPF8,14,15,16.
In a study of IPF patients treated with nintedanib, the converse results were reported for weight loss during a 52-week period; they reported a greater rate of decline in lung function in patients who maintained weight compared to those who experienced reduced body weight by over 5%14. In the study, the mean BMIs in the maintained weight and weight loss groups were 27.9 kg/m2 and 28.4 kg/m2, respectively, both meeting the criteria for ‘overweight’ according to the world health organization (WHO) classification17. Changes in lung function due to weight loss may show different trends depending on the initial degree of obesity or race. The nintedanib study was different from ours in that the majority of patients were white and the BMI of the patients in our study was normal. There were many intersections during the follow-up period; an observational study on long-term changes after 52 weeks is necessary for nintedanib as well. Given the lack of research about the risk factors for IPF patients treated with pirfenidone, this study is meaningful as it provides real-world data analyzing the long-term outcomes from over 5 years.
This study had several limitations. First, it was a retrospective observational study from a single center; prospective datasets are required to evaluate whether weight changes are independently associated with clinical outcomes. Nevertheless, this study showed consistent results in terms of weight change and prognosis. Second, we included patients who received a follow-up PFT, which means patients who were unable to receive a follow-up PFT due to death or rapid worsening of IPF were excluded. We may thus have focused on patients with a better condition and prognosis. However, the median survival of all enrolled patients in this study was 60 months, and considering that the median survival of patients using pirfenidone in another study was 6.87 years18, it cannot be said that the initial condition of our patients was good. Third, we could not obtain information on nutrition status or socioeconomic status, and intensity of daily activity. Fourth, we could not obtain enough data to evaluate cardiac function; heart failure may affect sodium retention and thus may have a potential effect on weight. Fifth, pirfenidone can result in weight loss in patients with IPF19, but we could not evaluate an association between the cumulative dose of pirfenidone and intensity of weight loss. Sixth, in South Korea, nintedanib for the treatment of IPF is not covered by insurance yet, so we could not make a comparison with the nintedanib group. Finally, looking at the baseline characteristics of patients with significant weight loss, although baseline BMI was not analyzed as a significant factor in survival in the study, compared to the patients who maintained their weight, patients with significant weight loss had lower baseline BMI and body weight; in addition, they tended to have poorer baseline lung function and tended to be older. Given the baseline characteristics of the reduced weight group, there is a limitation relating to whether significant weight loss was a result of disease progression or a treatable trait.
In this study, 25.1% of patients experienced an average annual weight loss of 5% or more, and they had poor lung function and prognosis. In daily practice, body weight is noninvasively measured and is a relatively easy, measurable indicator of prognosis in IPF patients. For this reason, it may be necessary not only to control the disease with antifibrotic agents but also to provide efforts to prevent weight loss and maintain physical status with nutritional support and the maintenance of muscle mass with rehabilitation20. The nutritional status of patients with IPF requires further study, including the impact of nutrition support teams or pulmonary rehabilitation. Further well-designed prospective studies on the traits requiring treatment are needed.
Conclusions
In this study, approximately 25% of the IPF patients on pirfenidone treatment experienced significant weight loss of 5% or more per year, and these patients had a poor prognosis. Although well-designed studies of treatable factors are needed to improve the outcomes of patients with IPF and continue treatment, it may be helpful to evaluate weight trends during follow-up, as well as adjust the dose of antifibrotic agents and track lung function. In addition, efforts to prevent weight loss may also be necessary.
Methods
Study design and participants
This study was a single-center retrospective observational study conducted in a tertiary referral hospital in South Korea from January 1st, 2015 to December 31st, 2019. The diagnosis of IPF met the Society consensus definition of the official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/and Latin American Thoracic Society statement1. In total, 315 patients were identified as meeting the following criteria: (1) a diagnosis of IPF on the basis of high-resolution computed tomography (HRCT) and lung biopsy if available, and (2) being prescribed pirfenidone during follow-up. We excluded patients who: (1) were lost to follow-up without additional PFT data, (2) were transferred to other centers resulting in insufficient clinical information, (3) received pirfenidone for less than 1 month, and (4) had a confirmed connective tissue disease related to ILD during follow-up while receiving pirfenidone. The HRCT imaging was reviewed by an experienced chest radiologist. Among the above IPF patients, we reviewed 215 patients after applying the above exclusion criteria.
Data collection
Clinical data were collected from the patients’ electronic medical records. The following data were recorded: (1) demographics and comorbidities, (2) dose of prescribed pirfenidone (3) patients’ height and body weight obtained from the serial PFT, and (4) survival records obtained from the Ministry of the Interior and Safety. Each time a PFT was performed, the body weight and height were measured and recorded in the laboratory, and this was used for analysis. The annualized percentage change in body weight was categorized based on the US Food and Drug Administration (FDA) guidance for developing products for weight management, which recommends an annualized weight loss of ≥ 5% loss of reference value for weight loss17.
The ratio of weight change was calculated by dividing the difference between the latest and baseline weight (body weight at the time of first PFT performed at diagnosis) by baseline body weight. The annualized percentage of weight change was calculated by multiplying the ratio of weight change by 100 and dividing by the periods (12 months) [(latest body weight-baseline body weight)×100/baseline body weight/12 months]. We categorized the annualized percentage of weight change in body weight into two groups: MW group (weight gain or weight loss < 5%/year during follow-up periods) and RW group (weight loss ≥ 5%/year during follow-up periods). Annual changes in lung function were also calculated as changes over time in the PFT results. We performed followed up PFT every 6–12 months from the baseline study.
Statistical analysis
Baseline demographics and characteristics were reported descriptively. A paired t test was used to compare changes in body weight and BMI during follow-up. The correlation between weight loss and dose reduction events of pirfenidone due to side effects was evaluated using chi-square testing. Student’s t test was used for the descriptive analysis comparisons. We used a linear mixed-effect model to analyze trends of indices of pulmonary function tests such as FVC and DLCO. Time-to-event analyses of survival are presented according to the presence or absence of significant weight loss. The log-rank test was used to evaluate the Kaplan–Meier curve for survival. The Cox proportional hazards test was used for the analysis of mortality. Variables with a P-value < 0.2 in the univariate analysis were entered into the multivariable analysis and selected by the backward log-likelihood ratio method. Since the weight change is a time-dependent variable, the time-dependent Cox proportional hazard model was used for the analysis considering the time of weight loss of 5% or more. Variables that did not satisfy the proportional hazards assumption were categorized, and stratification was applied even after the categorization did not meet the assumption. In this case, although P-value < 0.05 in univariable analysis, hazard ratio results did not appear in multivariable analysis. A P-value of < 0.05 was considered statistically significant. Statistical analyses were performed using IBM® SPSS® Statistics version 25 (IBM Corp., Armonk, NY, USA) and R version 4.1.1.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. B-2002-592-103), and is consistent with the principles of the Declaration of Helsinki. The requirement for informed consent was waived by IRB of Seoul National University Bundang Hospital because of the retrospective nature of the study.
Data availability
The data that support the findings of this study are not openly available due to the fact that consent to share data was not obtained from participants. However, the datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
References
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, e44–e68. https://doi.org/10.1164/rccm.201807-1255ST (2018).
Strongman, H., Kausar, I. & Maher, T. M. Incidence, prevalence and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv. Ther. 35, 724–736. https://doi.org/10.1007/s12325-018-0693-1 (2018).
Collard, H. R. et al. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 168, 538–542. https://doi.org/10.1164/rccm.200211-1311OC (2003).
Paterniti, M. O. et al. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 14, 1395–1402. https://doi.org/10.1513/AnnalsATS.201606-458OC (2017).
Tran, T. et al. The European multipartner IPF registry (EMPIRE): Validating long-term prognostic factors in idiopathic pulmonary fibrosis. Respir. Res. 21, 11. https://doi.org/10.1186/s12931-019-1271-z (2020).
Alakhras, M. et al. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 131, 1448–1453. https://doi.org/10.1378/chest.06-2784 (2007).
Kishaba, T. et al. Body mass index-percent forced vital capacity-respiratory hospitalization: New staging for idiopathic pulmonary fibrosis patients. J. Thorac. Dis. 8, 3596–3604. https://doi.org/10.21037/jtd.2016.12.49 (2016).
Nakatsuka, Y. et al. The clinical significance of body weight loss in idiopathic pulmonary fibrosis patients. Respiration 96, 338–347. https://doi.org/10.1159/000490355 (2018).
Comes, A. et al. Association of BMI and change in weight with mortality in patients with fibrotic interstitial lung disease. Chest 161, 1320–1329. https://doi.org/10.1016/j.chest.2021.11.008 (2022).
Jouneau, S. et al. Post hoc analysis of clinical outcomes in placebo- and pirfenidone-treated patients with ipf stratified by BMI and weight loss. Respiration 101, 142–154. https://doi.org/10.1159/000518855 (2022).
Jouneau, S. et al. What are the best indicators to assess malnutrition in idiopathic pulmonary fibrosis patients? A cross-sectional study in a referral center. Nutrition 62, 115–121. https://doi.org/10.1016/j.nut.2018.12.008 (2019).
Jouneau, S. et al. Malnutrition and decreased food intake at diagnosis are associated with hospitalization and mortality of idiopathic pulmonary fibrosis patients. Clin. Nutr. 41, 1335–1342. https://doi.org/10.1016/j.clnu.2022.05.001 (2022).
Lancaster, L. H. et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 26, 170057. https://doi.org/10.1183/16000617.0057-2017 (2017).
Jouneau, S. et al. Analysis of body mass index, weight loss and progression of idiopathic pulmonary fibrosis. Respir. Res. 21, 312. https://doi.org/10.1186/s12931-020-01528-4 (2020).
Kulkarni, T. et al. Decrements of body mass index are associated with poor outcomes of idiopathic pulmonary fibrosis patients. PLoS ONE 14, e0221905. https://doi.org/10.1371/journal.pone.0221905 (2019).
Pugashetti, J. et al. Weight loss as a predictor of mortality in patients with interstitial lung disease. Eur. Respir. J. 52, 1801289. https://doi.org/10.1183/13993003.01289-2018 (2018).
Food and Drug Administration. Developing products for weight management, guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/developing-products-weight-management-revision-1 (2007).
Roskell, N. et al. Long-term survival analysis: Pirfenidone compared to standard care for the treatment of patients with idiopathic pulmonary fibrosis. Eur. Clin. Respir. J. 44, 1905 (2014).
Proesmans, V. L. J. et al. Self-reported gastrointestinal side effects of antifibrotic drugs in Dutch idiopathic pulmonary fibrosis patients. Lung 197, 551–558. https://doi.org/10.1007/s00408-019-00260-1 (2019).
Faverio, P. et al. Nutrition in patients with idiopathic pulmonary fibrosis: Critical issues analysis and future research directions. Nutrients 12, 1131. https://doi.org/10.3390/nu12041131 (2020).
Acknowledgements
We express our gratitude to pf. Eunjeong Ji in the Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital, for valuable advice regarding the statistical analysis.
Funding
This study was supported by Seoul National University Bundang Hospital grant number 06-2020-0046.
Author information
Authors and Affiliations
Contributions
T.H.K. and B.S.K. drafted the manuscript and participated in data analysis. All authors were involved in the conception and/or design of the study, and interpretation of results, contributed to the manuscript from the outset, and read and approved the final draft. All authors revised the work for important content and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, T.H., Shin, YY., Kim, HJ. et al. Impact of body weight change on clinical outcomes in patients with idiopathic pulmonary fibrosis receiving pirfenidone. Sci Rep 12, 17397 (2022). https://doi.org/10.1038/s41598-022-22449-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22449-w
This article is cited by
-
Association between body fat decrease during the first year after diagnosis and the prognosis of idiopathic pulmonary fibrosis: CT-based body composition analysis
Respiratory Research (2024)
-
Weight loss and outcomes in subjects with progressive pulmonary fibrosis: data from the INBUILD trial
Respiratory Research (2023)
-
Circadian clock molecule REV-ERBα regulates lung fibrotic progression through collagen stabilization
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.