Abstract
Hydrogen peroxide (H2O2) has attracted considerable attention for use as a disinfectant ingredient for various applications over the decades. The use of H2O2 within the safety regulations can avoid its toxicity to human health and the environment. In this study, a paper-based sensor containing green-synthesized silver nanoparticles (P-AgNPs) was developed for use in a smartphone in the determination of the H2O2 concentration. In the synthesis process, an extract of spent coffee grounds was used as a bioreducing agent. The effects of reaction time and silver nitrate (AgNO3) concentration on the green synthesis of silver nanoparticles (AgNPs) were investigated. The optimum conditions for the preparation of P-AgNPs were determined to be 100 mM AgNO3 (P-AgNPs-100) and 15 h synthesis time. The P-AgNPs-100 sensor exhibited high sensitivity with a detection limit of 1.26 mM H2O2, which might be suitable for the detection of H2O2-based household and beverage sanitizers. The H2O2 detection capability of P-AgNPs-100 was comparable to that of a commercial strip sensor. Furthermore, P-AgNPs-100 had a detection efficiency of more than 95% after long-term storage for 100 days.
Similar content being viewed by others
Introduction
Hydrogen peroxide (H2O2) has become more crucial to humanity since the breakout of COVID-19. H2O2 can be used as a disinfectant ingredient in nasal spray1, beverages2, and household cleansers3. Apart from these applications, H2O2 has also been widely used in textile, water treatment, and food processes4. However, the misuse of H2O2 preparations may be toxic to human health and the environment. At low concentrations (i.e., approximately 3% solution), H2O2 can cause mild gastrointestinal irritation, mild mucosal irritation, and vomiting5. Therefore, the use of H2O2 for various applications needs to be monitored. Among several analytical methods for H2O2 detection, optical sensors based on the surface of noble metal nanoparticles (NPs) known as localized surface plasmon resonance (SPR) have gained attention from researchers because NPs, such as gold, platinum, and silver, exhibit high absorption coefficients and high surface-to-volume ratios6,7,8,9. Silver nanoparticles (AgNPs) are one of the NPs that have attracted considerable interest for optical sensor applications because of their superior plasmonic characteristic8. Recently, a functionalized paper containing AgNPs was developed as a paper-based sensor that could be easily handled10. The prepared paper-based sensor containing carbon dot functionalized paper coupled with AgNPs could stably detect H2O2 for 4 weeks with a detection limit of 1.38 µM11. For AgNP production, the green synthesis of AgNPs is an environmentally friendly process that has attracted considerable attention12. The properties of green-synthesized AgNPs, such as shape, size, and morphology, depended on several factors, i.e., pH, temperature, reaction time, and concentrations of the reducing agent and silver salt13,14. Various kinds of plant extracts, such as oregano essential oil15, gelatin16, and green tea17, have been applied as bioreducing agents for the preparation of AgNPs. Recently, the use of bioreducing agents derived from wastes, such as agricultural18, food19, and beverage20 wastes, is a new trend for the green synthesis of AgNPs. Srikhao et al. prepared AgNPs using sugarcane leaf extract18. The existing phenolic compounds acted as bioreducing agents. The obtained AgNPs were used as a sensor to detect ammonia and H2O2. Spent coffee ground (SCG) is a phenol-rich waste that has been produced approximately 6 million tons/year21. SCG waste is considered a pollution hazard when it is discarded in landfills22. Consequently, the use of SCG and its derivatives has certain benefits, including value added to SCG waste and the reduction of costs in waste management. Based on the literature, most green-synthesized AgNPs with SCG have been applied as antibacterial agents21,22 and catalysts23. Therefore, the search for new alternative uses of AgNPs would increase their usefulness.
At present, paper-based sensors are widely used because of their simplicity, low cost, and high optical contrast for the colorimetric detection of chemical substances24. Furthermore, the use of paper-based sensors can be combined with a smartphone as a detector to improve analysis capability and portability. Yoo et al. successfully used a smartphone camera as a readout device to analyze paper-based sensors for glucose detection in the range of 0–10 mM. After glucose detection, the color of the paper-based sensor changed. This color change was recorded using a smartphone camera. Then, the red, green, and blue (RGB) program in the smartphone was applied to analyze the glucose concentration25.
Currently, there is a dearth of information on the preparation and characterization of paper-based sensors containing green-synthesized AgNPs. Hence, this study aimed to prepare AgNPs using an extract of SCG (ex-SCG) for coating paper. The coated paper was applied as a new paper-based sensor for H2O2 detection. The selectivity and long-term stability of P-AgNPs were investigated. The use of P-AgNPs combined with a smartphone was applied to analyze the H2O2 concentration.
Materials and methods
Material
SCG used in this work was collected from a local coffee shop in Khon Kaen, Thailand. Polyvinyl alcohol (PVA) with an average molecular weight in the range of 1700–1800 was purchased from Loba Chemie Pvt. Ltd., Mumbai, India. Silver nitrate (AgNO3, with a purity of 99.8%) was purchased from RCI Labscan Limited, Bangkok, Thailand. Whatman filter paper no. 1 (GE Healthcare UK Limited) was used as a substrate for the preparation of the paper-based sensor. Gallic acid (with a purity of 98%) and Folin–Ciocalteu’s reagent (with a density of 1.27 g/cm3, equivalent acid of 2 N) were purchased from Sigma Aldrich, Singapore, and Loba Chemie Pvt. Ltd., Mumbai, India, respectively. H2O2 commercial test strips (Peroxide 1000) were obtained from Quantofix, Germany. H2O2 with a concentration of 30% was purchased from Merck Schuchardt OHG, Kenilworth, NJ, USA.
Preparation and characterization of ex-SCG
After obtaining SCG from the local coffee shop, it was first dried at a temperature of 80 °C for 24 h and then kept in a sealed plastic bag. ex-SCG was prepared using the SCG-to-deionized water ratio of 1:200 g/mL at a temperature of 95 °C for 5 min, according to the previous method of Trongchuen et al.26. The total phenolic content (TPC) of ex-SCG was determined following the Folin–Ciocalteu method27. The sample obtained using this method was analyzed by the Agilent Cary 60 UV–Vis spectrophotometer. The TPC of ex-SCG was reported as the gallic acid equivalent (GAE), which was determined to be 1.94 ± 0.16 mg GAE/g SCG.
Preparation of paper-based sensor for H2O2 detection
PVA (2.5 g) was dissolved in 100 mL ex-SCG at a temperature of 90 °C for 60 min. Then, 1.2 mL AgNO3 solution at concentrations of 0, 50, 100, and 150 mM was added to the PVA solution and stirred at a temperature of 90 °C for another 15 h under reflux. The obtained mixture was cast on a filter paper using a doctor blade with a gap of 200 µm. Finally, the coated paper was dried at a temperature of 50 °C for 10 min.
Characterization of the paper-based sensor for H2O2 detection
The UV–Vis spectra of green-synthesized AgNPs were analyzed by the Agilent Cary 60 UV–Vis spectrophotometer. X-ray diffraction (XRD) of paper coated with AgNPs was characterized by the SmartLab X-ray diffractometer (Rigaku, Japan) over diffraction angles of 10° to 80°. The diffractometer was equipped with a Cu Ka radiation source (wavelength l = 1.542 Å) operated at the scan rate of 0.01° at 40 kV and 30 mA. Transmission electron microscopy (TEM) images of the green-synthesized AgNPs at different AgNO3 concentrations were obtained using the TECNAI G2 20 transmission electron microscope (FEI, Hillsboro, OR, USA). The surface of P-AgNPs was analyzed and high-resolution energy-dispersive X-ray spectroscopy (EDX) mapping was conducted using a scanning electron microscope (Hitachi Miniscope Model TM-3000). All samples were coated with gold using an ion sputtering device. The sample solution was deposited on 400-mesh carbon-coated Cu grids and dried at room temperature for 24 h. The TEM images were taken at 200 kV accelerating voltage. The attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra (TENSOR 27, Bruker, Billerica, MA, USA) were used to examine the functional groups of the paper coated with AgNPs. All spectra were obtained at 4000–600 cm−1 with 64 scans at a resolution of 2 cm−1.
Colorimetric assay of H2O2
Paper-based sensors (with a circular area diameter of 6 mm) were immersed in 50 mL H2O2 standard solution with concentrations of 0–6000 mg/L and incubated at room temperature for 45 s. Then, the paper-based sensors were placed under a light-emitting diode (60 W) lamp. The color images of the paper-based sensors were taken by a smartphone (iPhone XR). The obtained images were analyzed by quantification using the ImageJ Version 1.8 software. The average color intensities of RGB were obtained to calculate the RGB distance (∆RGB) value according to Eq. (1)28,29:
where the R, G, and B values are values after immersing the paper sensors in known H2O2 solutions and the subscript 0 refers to the values before immersion. Then, the values of ∆RGB and the known H2O2 concentrations were curve-fitted to obtain the relationship needed to calculate the H2O2 concentration for a known ∆RGB.
Long-term stability test of the sensor
The sensor samples were kept in a plastic box and then stored at 25 °C under dark conditions for 100 days. The long-term stability was tested by examining the efficiencies of the sensors according to a colorimetric assay of H2O2.
Results and discussions
Characterization of green-synthesized AgNPs
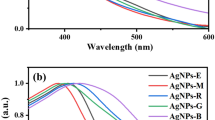
AgNPs are well known to show their unique optical properties for SPR in the range of 400–500 nm30. The SPR bands of green-synthesized AgNPs with ex-SCG at various synthesis times are depicted in Fig. 1a,b. After synthesis for 5–60 min, the SPR band at 431–435 nm was observed, as shown in Fig. 1a. This phenomenon indicated AgNP formation. The intensity of the SPR band increased with the increase in synthesis time from 5 min to 15 h. The increase in peak intensity was attributed to the continued reduction of silver ions to form AgNPs28,31. After the synthesis of AgNPs for 15 h, the intensity of the SPR band decreased possibly because of the agglomeration of AgNPs at longer synthesis times18. Furthermore, a blue shift in the wavelength of 435 nm for the synthesis time of 5 min to 431 nm for the synthesis time of 15 h was observed. The blue shift of SPR for NPs indicated a decrease in particle size20. The synthesis time of 15 h was determined to be the optimum condition. Therefore, the AgNPs green-synthesized for 15 h were used as paper coating. Figure 1b shows the UV–Vis spectra of AgNPs at AgNO3 concentrations of 0, 50, 100, and 150 mM. The peak of green-synthesized AgNPs with ex-SCG was detected in the range of 428–433 nm, whereas the absorption spectra of ex-SCG (0 mM AgNO3) did not show any characteristic peaks in this range. The peak intensity of AgNPs increased with the increase in AgNO3 concentration from 50 to 150 mM. Taesuwan et al.32 proposed that the increase in peak intensity can be attributed to the high yield of AgNP formation because of more available reactants. The red shift of SPR from 428 nm for synthesized AgNPs with 50 mM to 433 nm for synthesized AgNPs with 150 mM was observed with the increase in AgNO3 concentration. Amirjani et al.33 reported that the red shift of SPR indicated the increase in the size of AgNPs.
The morphology and size of AgNPs were analyzed using TEM, as depicted in Figs. 2a–d and 3a–c, respectively. A spherical shape was observed for all AgNPs at different AgNO3 concentrations. Furthermore, the amount of AgNPs increased with the increase in AgNO3 concentration from 50 to 150 mM. As illustrated in Fig. 3a–c, the particle size of AgNPs ranged from 8.64 to 38.99 nm. For 50 mM AgNO3, the size of AgNPs ranged from 15.37 to 27.98 nm. For 100 mM AgNO3, the size of AgNPs ranged from 8.64 to 35.68 nm. For 150 mM AgNO3, the size of AgNPs ranged from 15.40 to 38.99 nm. These results could be explained by the fact that, at high AgNO3 concentrations, the rate of spontaneous nucleation increased the growth rate of AgNPs. Then, large numbers of nuclei were formed during burst nucleation. The formation of a large amount of AgNPs with free and high surface tension leads to larger particles34,35. The size distributions and shapes of AgNPs in ex-SCG solution should be investigated using the dynamic light scattering technique to consolidate the material characterization in further study.
The crystalline nature of P-AgNPs was investigated using XRD analysis. Figure 4 shows the XRD patterns of pristine paper at 22.52°, which corresponds to the (220) crystallographic planes of the monolithic cellulose type I36,37. The paper coated with PVA/ex-SCG exhibited the characteristic peaks at 2θ = 29.50° to 67°. These peaks were attributed to the structures of PVA and the organic components of ex-SCG. Malik et al.30 detected a semicrystalline and a small amorphous peak of PVA at 19.5° and 39° to 40°, respectively. For ex-SCG, the peaks in the range of 20° to 80° can be assigned to chlorogenic acid38, caffeic acid39, and the bioorganic phase of ex-SCG40. In the case of AgNPs, the XRD pattern of Ag crystals consisted of four peaks in the range 2θ = 20° to 80°, which correspond to reflections of the (111), (200), (220), and (311) planes from the face-centered cubic unit cell. P-AgNPs-50, P-AgNPs-100, and P-AgNPs-150 showed weak reflections of the (111) plane at 38.80° and (200) plane at 47.05°. Furthermore, the peak intensity at 64.72° (220) increased with the increase in AgNP concentration. The XRD profile data of P-AgNPs corresponded to the standard JCPDS file no. 04-0783 and was consistent with the crystal planes of AgNPs reported by Satyanarayana41. The overlap of the AgNP characteristic peaks and those of other materials resulted in weak XRD signals42. The average crystallite size values of AgNPs can be calculated from the XRD pattern using the Scherrer equation, as expressed in Eq. (2). The average crystallite size was determined to be 19.09 nm for P-AgNPs-50, 24.58 nm for P-AgNPs-100, and 32.66 nm for P-AgNPs-150.
where D is the crystallite size (nm), k is the Debye–Scherrer constant (0.89), λ is the X-ray wavelength, β is the line broadening in radians obtained from the full width at half maximum, and θ is the Bragg angle or peak position (radians).
ATR-FTIR analysis was performed to detect the functional groups of bioreducing agents for the green synthesis of AgNPs as depicted in Fig. 5. The ATR-FTIR spectra of coated paper with ex-SCG were detected at 981 cm−1 (C=C bending), 1635 cm−1 (C=C stretching), 2945 cm−1 (C–H stretching), and 3293 cm−1 (O–H stretching). For P-AgNPs, the shift of peaks was only observed when compared with the spectra of coated paper with ex-SCG. The peaks at 1635 and 3293 cm−1 shifted to 1645 and 3277 cm−1, respectively. These shifted peaks indicated that the bioreducing agent could reduce silver ions to AgNPs and form a layer on the AgNP surface43. By integrating the hypotheses of previous reports44,45,46 with the results of the present study, a possible mechanism for the green synthesis of AgNPs using phenolic compounds in ex-SCG can be proposed, as illustrated in Supplementary Fig. S1. The main phenolic compounds, such as chlorogenic acid, ferulic acid, caffeic acid, and p-coumaric acid, could be obtained by extraction of SCG with water47,48. In green synthesis, phenolic compounds could release electrons to reduce silver ions and Ag cation reduction could be coupled with the oxidation of the hydroxyl group of phenolic compounds44,49.
Optimization of the detection conditions
The necessary detection conditions, such as the AgNP concentration and pH value of the H2O2 solution, were optimized using the ∆RGB values as a criterion. Figure 6a,b show that P-AgNPs-100 has the highest response to the color change and ∆RGB values, respectively, possibly because of the large amount of synthesized AgNPs with 100 mM AgNO3. Although the obtained amount of AgNPs was lower than the synthesized AgNPs with 150 mM AgNO3, the obtained AgNPs from 100 mM AgNO3 still had a more uniform and narrower size distribution, as observed in the UV–Vis spectral analysis in Fig. 1b and TEM analysis in Fig. 3. Furthermore, the EDX images in Supplementary Fig. S2 illustrated that P-AgNPs-100 had a homogeneous distribution of AgNPs on the surface, whereas P-AgNPs-150 had agglomerated AgNPs. The Ag content on the surface increased slightly with the increase in AgNO3 content. Figure 6c shows the effect of pH on H2O2 detection using P-AgNPs-100. The RGB values for pH values of 1–3, 4–9, and 10–14 were in the ranges of 15.15–20.93, 38.15–39.03, and 25.93–36.46, respectively. The maximum intensity of RGB values was observed in the pH range of 4–9. Farrokhnia et al.50 reported that H2O2 preferred to decompose in alkaline media. The intensity of RGB decreased at pH values of 1–3. For acidic media, the silver ions produced from AgNPs in the presence of H2O2 might not further oxidize and the reaction between hydronium ion and H2O2 at acidic pH enabled H2O2 detection by AgNPs51. A higher ∆RGB value indicated a higher detection response of the paper-based sensor24,52. Therefore, the optimum pH range for H2O2 detection was 4–9. A similar pH range of 5–7 for H2O2 detection using AgNPs was reported by Prapaporn et al.53.
H2O2 responsiveness of P-AgNPs-100
The colorimetric sensing capability of P-AgNPs-100 for H2O2 was determined, as illustrated in Fig. 7. After the H2O2 solution was deposited on P-AgNPs-100, color change with different shades depending on the H2O2 concentration was detected, which was attributed to the destruction of P-AgNPs-100 by the oxidation capability of H2O2. The AgNPs were oxidized to silver ions54. The possible reaction mechanism of AgNPs and H2O2 is expressed as Eq. (3):
The ∆RGB values increased linearly with the increase in H2O2 concentrations in the range of 50–1500 and 1500–6000 mg/L. Hence, the plot of ∆RGB values versus H2O2 concentrations was considered in the two ranges. The calibration curve plotting the signal intensity of color detected by P-AgNPs-100 integrated with the ImageJ software versus the H2O2 concentration.
The regression equation was Y = 13.249 + 0.021X, R2 = 0.99, for the H2O2 concentration range of approximately 50–1500 mg/L and Y = 35.942 + 0.0048X, R2 = 0.97, for the H2O2 concentration range of approximately 1500–6000 mg/L, where x is the H2O2 concentration (mg/L). The limit of detection (LOD) of response was calculated using Eq. (4)50:
The LOD of P-AgNPs-100 was 1.26 mmol/L. The obtained LOD value for H2O2 detection was relatively higher than those for paper-based sensors, which ranged from 1.75 to 100 µmol/L11,55,56. Notably, P-AgNPs-100 can be used as a sensor to detect H2O2 in the preparation of sanitizer agents, which normally contain H2O2 in the range of 97.06–11,426.47 mmol/L for household sanitizers57 and 8.16–970 mmol/L for fruit sanitizers2.
Effect of interferents on the selective determination of H2O2 using the P-AgNPs-100 sensor
The selectivity test of the paper-based sensor was conducted by depositing metal ions and anions in the sensor sample. The color change of the sensor referred to the tolerance limit24. Various types of metal ions and anions, viz., Fe2+, Cu2+, Cd2+, Mn2+, Mg2+, Ca2+, Cl−, ammonia, glucose, tannic acid, and ascorbic acid, were used to determine the selectivity of P-AgNPs-100, as shown in Fig. 8. Notably, the ∆RGB values obtained with H2O2 were higher than those with other agents, indicating that the proposed P-AgNPs100 exhibited high selectivity for H2O2 detection.
Application of P-AgNPs-100 in sanitizer preparation for H2O2 detection
To validate the applicability of P-AgNPs-100 for H2O2 detection, the H2O2 detection capability of P-AgNPs-100 was tested and compared with that of a commercial strip sensor. The recovery value from the actual value of H2O2 was calculated according to Eq. (5)58:
The recovery values of H2O2 at different concentrations in tap water are summarized in Table 1. The recovery values of P-AgNPs-100 and a commercial strip sensor ranged from 97.55 to 101.21% and from 105.37 to 106.49%, respectively. Notably, P-AgNPs-100 had recovery values comparable with those of a commercial strip sensor.
Furthermore, the H2O2 concentration in various types of sanitizers was tested using P-AgNPs-100. Three sanitizers, viz., fruit sanitizer, water for household settings57, and H2O2-based spray (against coronavirus) for dental settings59, were prepared with different H2O2 concentrations. These samples were spiked with known amounts of H2O2, and the recovery values were determined, as shown in Table 2. The recoveries of H2O2 at different concentrations were determined to be more than 99% with good precision (RSD ≤ 8%). The recovery values and RSD of P-AgNPs-100 were in an acceptable range, as reported in previous studies60,61. The obtained results indicated that P-AgNPs-100 exhibited high efficiency for the determination of H2O2 in real samples.
Long-term stability of P-AgNPs-100
The long-term stability of the sensor during storage was a necessary factor in practical application62. After P-AgNPs-100 was stored at room temperature under dark conditions for 100 days, no apparent change was detected in the sample, as illustrated in Fig. 9a. Furthermore, more than 95% of the initial activity was retained within 100 days, as depicted in Fig. 9b, possibly because of the oxidation of AgNPs by atmospheric oxygen63. However, the efficiency of P-AgNPs-100 after storage was comparable with that of other sensors in previous reports, as summarized in Table 3.
Conclusions
We have developed an H2O2 paper-based sensor using agricultural waste (i.e., ex-SCG) for the green synthesis of AgNPs. The optimum conditions for the preparation of P-AgNPs were determined to be 100 mM AgNO3 and 15 h reaction time. The combination of the paper-based sensor with smartphone readout is simple, efficient, and inexpensive. The detection limit of H2O2 for P-AgNPs-100 was observed at 1.26 mM. P-AgNPs-100 also had recovery values of H2O2 comparable with those of a commercial strip sensor. P-AgNPs-100 showed acceptable long-term stability of H2O2 detection with an efficiency of 95.62% when stored for 100 days. Furthermore, P-AgNPs-100 exhibited excellent recovery of H2O2 in the sanitizers and tap water, which confirmed possible application for H2O2 detection in real samples.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Caruso, A. A., Del Prete, A. & Lazzarino, A. I. Hydrogen peroxide and viral infections: A literature review with research hypothesis definition in relation to the current covid-19 pandemic. Med. Hypotheses 144, 109910 (2020).
Kniel, K. E. et al. Effect of organic acids and hydrogen peroxide on Cryptosporidium parvum viability in fruit juices. J. Food Prot. Food Prot. 66, 1650–1657 (2003).
Weber, D. J. et al. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials. Am. J. Infect. Control 44, e77–e84 (2016).
Şen, F. B., Bener, M., Bekdeşer, B. & Apak, R. Redox-based colorimetric sensing of H2O2 after removal of antioxidants with ABTS radical oxidation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 248, 119266 (2021).
Mahmood, A. et al. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci. Total Environ. 742, 140561 (2020).
Tran, H. V., Nguyen, T. V., Nguyen, L. T. N., Hoang, H. S. & Huynh, C. D. Silver nanoparticles as a bifunctional probe for label-free and reagentless colorimetric hydrogen peroxide chemosensor and cholesterol biosensor. J. Sci. Adv. Mater. Devices 5, 385–391 (2020).
Vasileva, P., Donkova, B., Karadjova, I. & Dushkin, C. Synthesis of starch-stabilized silver nanoparticles and their application as a surface plasmon resonance-based sensor of hydrogen peroxide. Colloids Surf. A Physicochem. Eng. 382, 203–210 (2011).
Jebril, S., Fdhila, A. & Dridi, C. Nanoengineering of eco-friendly silver nanoparticles using five different plant extracts and development of cost-effective phenol nanosensor. Sci. Rep. 11, 1–11 (2021).
Song, H. et al. Triangular silver nanoparticle U-bent fiber sensor based on localized surface plasmon resonance. AIP Adv. 9, 085307 (2019).
Gokul Eswaran, S. et al. Preparation of a portable calorimetry kit and one-step spectrophotometric nanomolar level detection of l-Histidine in serum and urine samples using sebacic acid capped silver nanoparticles. J. Sci. Adv. Mater. Dev. 6, 100–107 (2021).
Zhang, X., Chen, S., Zhuo, S., Ji, Y. & Li, R. A carbon dots functionalized paper coupled with AgNPs composites platform: Application as a sensor for hydrogen peroxide detection based on surface plasmon-enhanced energy transfer. New J. Chem. 45, 6025–6032 (2021).
Giri, A. K. et al. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria full width at half maximum. Sci. Rep. 12, 1–9 (2022).
Jain, S. & Mehata, M. S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 7, 15867 (2017).
Długosz, O., Chwastowski, J. & Banach, M. Hawthorn berries extract for the green synthesis of copper and silver nanoparticles. Chem. Pap. 74, 239–252 (2020).
Srikhao, N. et al. Bioactive nanocomposite film based on cassava starch/polyvinyl alcohol containing green synthesized silver nanoparticles. J. Polym. Environ. 29, 672–684 (2021).
Jung, J., Raghavendra, G. M., Kim, D. & Seo, J. One-step synthesis of starch-silver nanoparticle solution and its application to antibacterial paper coating. Int. J. Biol. Macromol. 107, 2285–2290 (2018).
Vatandost, E., Ghorbani-Hasan Saraei, A., Chekin, F., NaghizadehRaeisi, S. & Shahidi, S.-A. Green tea extract assisted green synthesis of reduced graphene oxide: Application for highly sensitive electrochemical detection of sunset yellow in food products. Food Chem. X 6, 100085 (2020).
Srikhao, N., Kasemsiri, P., Lorwanishpaisarn, N. & Okhawilai, M. Green synthesis of silver nanoparticles using sugarcane leaves extract for colorimetric detection of ammonia and hydrogen peroxide. Res. Chem. Intermed. 47, 1269–1283 (2021).
Nasiriboroumand, M., Montazer, M. & Barani, H. Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J. Photochem. Photobiol. B Biol. 179, 98–104 (2018).
Ounkaew, A. et al. Green synthesis of nanosilver coating on paper for ripening delay of fruits under visible light. J. Environ. Chem. Eng. 9, 105094 (2021).
Panzella, L. et al. Silver nanoparticles on hydrolyzed spent coffee grounds (HSCG) for green antibacterial devices. J. Clean. Prod. 268, 122352 (2020).
Chien, H. W., Kuo, C. J., Kao, L. H., Lin, G. Y. & Chen, P. Y. Polysaccharidic spent coffee grounds for silver nanoparticle immobilization as a green and highly efficient biocide. Int. J. Biol. Macromol. 140, 168–176 (2019).
Mangindaan, D., Lin, G. Y., Kuo, C. J. & Chien, H. W. Biosynthesis of silver nanoparticles as catalyst by spent coffee ground/recycled poly(ethylene terephthalate) composites. Food Bioprod. Process. 121, 193–201 (2020).
Shrivas, K. et al. Colorimetric and paper-based detection of lead using PVA capped silver nanoparticles: Experimental and theoretical approach. Microchem. J. 150, 104156 (2019).
Yoo, S., Min, K., Tae, G. & Han, M. S. A long-term stable paper-based glucose sensor using a glucose oxidase-loaded, Mn2BPMP-conjugated nanocarrier with a smartphone readout. Nanoscale 13, 4467–4474 (2021).
Trongchuen, K. et al. Bioactive starch foam composite enriched with natural antioxidants from spent coffee ground and essential oil. Starch/Staerke 70, 1–9 (2018).
Al-Dhabi, N. A., Ponmurugan, K. & Maran Jeganathan, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 34, 206–213 (2017).
Cho, Y. B., Jeong, S. H., Chun, H. & Kim, Y. S. Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sensors Actuators B Chem. 256, 167–175 (2018).
Singh, G., Raj, P., Singh, H. & Singh, N. Colorimetric detection and ratiometric quantification of mercury(ii) using azophenol dye: ‘Dip & read’ based handheld prototype device development. J. Mater. Chem. C 6, 12728–12738 (2018).
Malik, R. S., Soni, U., Chauhan, S. S., Kumar, D. & Choudhary, V. Semi-interpenetrating polymer networks of poly (vinyl alcohol)-functionalized nanocrystals/sulfonated poly (ether ether ketone) (PVA-FNCs/SPEEK) as fuel cell membrane. Mater. Today Commun. 29, 102897 (2021).
Ibrahim, H. M. M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 8, 265–275 (2015).
Taesuwan, I. et al. Smart conductive nanocomposite hydrogel containing green synthesized nanosilver for use in an eco-friendly strain sensor. Cellulose 29, 273–286 (2021).
Amirjani, A., Firouzi, F. & Haghshenas, D. F. Predicting the size of silver nanoparticles from their optical properties. Plasmonics 15, 1077–1082 (2020).
Abdel-Mohsen, A. M. et al. Green synthesis of hyaluronan fibers with silver nanoparticles. Carbohydr. Polym. 89, 411–422 (2012).
Singh, M., Sinha, I. & Mandal, R. K. Role of pH in the green synthesis of silver nanoparticles. Mater. Lett. 63, 425–427 (2009).
Chien, H. W., Tsai, M. Y., Kuo, C. J. & Lin, C. L. Well-dispersed silver nanoparticles on cellulose filter paper for bacterial removal. Nanomaterials 11, 1–12 (2021).
Alula, M. T. & Feke, K. Peroxidase-like activity of silver nanoparticles loaded filter paper and its potential application for sensing. J. Clust. Sci. 5, 1–9 (2021).
Buskaran, K., Hussein, M. Z., Moklas, M. A. M., Masarudin, M. J. & Fakurazi, S. Graphene oxide loaded with protocatechuic acid and chlorogenic acid dual drug nanodelivery system for human hepatocellular carcinoma therapeutic application. Int. J. Mol. Sci. 22, 5786 (2021).
Ballesteros, L. F., Cerqueira, M. A., Teixeira, J. A. & Mussatto, S. I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 127, 347–354 (2015).
Desai, N. M., GilbertStanley, J. & Murthy, P. S. Green coffee nanoparticles: Optimisation, in vitro bioactivity and bio-release property. J. Microencapsul. 37, 52–64 (2020).
Satyanarayana, B. M., Reddy, N. V., Kommula, S., Kesava, R. & Rao, J. V. Biogenesis of silver nanoparticles using leaf extracts of Asparagus racemosus and Sophora interrupta: Structure characterization, antibacterial and anticancer studies. SN Appl. Sci. 2, 1–15 (2020).
Kamal, T. et al. Silver nanoparticles embedded in gelatin biopolymer hydrogel as catalyst for reductive degradation of pollutants. J. Polym. Environ. 28, 399–410 (2020).
Dutta, T. et al. Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: Experimental and theoretical studies. J. Environ. Chem. Eng. 8, 104019 (2020).
Aziz, S. et al. Fabrication of interconnected plasmonic spherical silver nanoparticles with enhanced localized surface plasmon resonance (LSPR) peaks using quince leaf extract solution. Nanomaterials 9, 1557 (2019).
Kanniah, P. et al. Green synthesis of multifaceted silver nanoparticles using the flower extract of Aerva lanata and evaluation of its biological and environmental applications. ChemistrySelect 5, 2322–2331 (2020).
Liu, Y.-S., Chang, Y.-C. & Chen, H.-H. Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. J. Food Drug Anal. 26, 649–656 (2018).
Vu, D. C. et al. Evaluation of fatty acids, phenolics and bioactivities of spent coffee grounds prepared from Vietnamese coffee. Int. J. Food Prop. 24, 1548–1558 (2021).
Okur, I., Soyler, B., Sezer, P., Oztop, M. H. & Alpas, H. Improving the recovery of phenolic compounds from spent coffee grounds (SCG) by environmentally friendly extraction techniques. Molecules 26, 613 (2021).
Barwant, M. et al. Eco-friendly synthesis and characterizations of Ag/AgO/Ag2O nanoparticles using leaf extracts of Solanum elaeagnifolium for antioxidant, anticancer, and DNA cleavage activities. Chem. Pap. 76, 4309–4321 (2022).
Farrokhnia, M., Karimi, S., Momeni, S. & Khalililaghab, S. Colorimetric sensor assay for detection of hydrogen peroxide using green synthesis of silver chloride nanoparticles: Experimental and theoretical evidence. Sensors Actuators B Chem. 246, 979–987 (2017).
Elgamouz, A. et al. Encapsulation capacity of β-cyclodextrin stabilized silver nanoparticles towards creatinine enhances the colorimetric sensing of hydrogen peroxide in urine. Nanomaterials 11, 1897 (2021).
Wang, K. et al. Smartphone-imaged multilayered paper-based analytical device for colorimetric analysis of carcinoembryonic antigen. Anal. Bioanal. Chem. 412, 2517–2528 (2020).
Prapaporn, S., Arisara, S., Wunpen, C. & Wijitar, D. Nanocellulose films to improve the performance of distance-based glucose detection in paper-based microfluidic devices. Anal. Sci. 36, 1447–1452 (2020).
Amirjani, A., Bagheri, M., Heydari, M. & Hesaraki, S. Label-free surface plasmon resonance detection of hydrogen peroxide; A bio-inspired approach. Sensors Actuators B Chem. 227, 373–382 (2016).
Cheng, D., Qin, J., Feng, Y. & Wei, J. Synthesis of mesoporous cuo hollow sphere nanozyme for paper-based hydrogen peroxide sensor. Biosensors 11, 258 (2021).
Sharma, L., Gouraj, S., Raut, P. & Tagad, C. Development of a surface-modified paper-based colorimetric sensor using synthesized Ag NPs-alginate composite. Environ. Technol. 42, 3441–3450 (2021).
Totaro, M. et al. Role of hydrogen peroxide vapor (HPV) for the disinfection of hospital surfaces contaminated by multiresistant bacteria. Pathogens 9, 408 (2020).
Monisha, et al. Inkjet-printed paper-based colorimetric sensor coupled with smartphone for determination of mercury (Hg2+). J. Hazard. Mater. 414, 125440 (2021).
da Mota Santana, L. A., Andrade Pinho, J. N., de Albuquerque, H. I. M. & de Almeida Souza, L. M. Virucidal potential of H2O2-based spray against SARS-CoV-2 and biosafety in a dental environment. Oral Dis. 1, 1–2 (2021).
Scroccarello, A. et al. Plasmonic active film integrating gold/silver nanostructures for H2O2 readout. Talanta 222, 121682 (2021).
Apyari, V. V. et al. Potentialities of differently-stabilized silver nanoparticles for spectrophotometric determination of peroxides. Talanta 202, 51–58 (2019).
Roushani, M. & Shahdost-fard, F. A glassy carbon electrode with electrodeposited silver nanoparticles for aptamer based voltammetric determination of trinitrotoluene using riboflavin as a redox probe. Microchim. Acta 185, 1–8 (2018).
Yu, S. J., Yin, Y. G. & Liu, J. F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 15, 78–92 (2013).
Hosu, O. et al. Colorimetric multienzymatic smart sensors for hydrogen peroxide, glucose and catechol screening analysis. Talanta 204, 525–532 (2019).
Nguyen, P. T., Kim, Y. I. & Kim, M. I. Reagent-free colorimetric cholesterol test strip based on self color-changing property of nanoceria. Front. Chem. 8, 1–7 (2020).
Fu, Y. et al. A novel non-enzymatic electrochemical hydrogen peroxide sensor based on a metal-organic framework/carbon nanofiber composite. Molecules 23, 2552 (2018).
Bai, Z. et al. Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 82, 185–194 (2016).
Sekar, N. C., Ge, L., Mousavi Shaegh, S. A., Ng, S. H. & Tan, S. N. A mediated turnip tissue paper-based amperometric hydrogen peroxide biosensor. Sensors Actuators B Chem. 210, 336–342 (2015).
Acknowledgements
This research project is supported by National Research Council of Thailand (NRCT): NRCT5-RGJ63003-049 and Research and Graduate Studies, Khon Kaen University, Thailand.
Author information
Authors and Affiliations
Contributions
N.S.: Investigation, Writing-original draft, Formal analysis. A.O.: Visualization, Investigation. P.K.: Conceptualization, Methodology, Supervision, Writing-review & editing. S.T.: Writing-review & editing. M.O.: Supervision. S.H.: Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Srikhao, N., Ounkaew, A., Kasemsiri, P. et al. Green synthesis of silver nanoparticles using the extract of spent coffee used for paper-based hydrogen peroxide sensing device. Sci Rep 12, 20099 (2022). https://doi.org/10.1038/s41598-022-22067-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22067-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.