Abstract
Obesity is associated with the severity of asthma, which is characterized by airway obstruction. Pulmonary function testing is one of the important examinations for evaluating airway obstruction. However, the impact of obesity on pulmonary function in patients with asthma is not fully understood. A total of 193 patients with asthma and 2159 patients without asthma who visited Saga University Hospital were investigated retrospectively. Obesity was defined as a body mass index (BMI) greater than 25 kg/m2. Pulmonary functions including forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were compared between patients with and without asthma, focusing especially on obesity. FVC percent predicted and FEV1 percent predicted were significantly lower in patients with asthma than in those without asthma (p = 0.03, < 0.01 respectively). In patients with asthma, FVC percent predicted and FEV1 percent predicted were significantly lower in patients with obesity than in those without obesity (all p < 0.01). In addition, BMI was negatively correlated with FEV1 (r =− 0.21, p = 0.003) and FVC (r = − 0.15, p = 0.04), along with the percent predicted. On multivariate analysis in patients with asthma, FVC (β [95% confidence interval] 0.12 [0.02–0.22], p = 0.02) and FEV1 (0.13 [0.05–0.22], p < 0.01) were still significantly different between patients with and without obesity. However, these obesity-associated differences were not observed in patients without asthma. Obesity reduces pulmonary function, including FVC and FEV1, in patients with asthma, but not in those without asthma.
Similar content being viewed by others
Introduction
Asthma is a common respiratory disease the pathophysiology of which involves airway inflammation and airway hyperresponsivity1,2,3, which induce respiratory symptoms such as shortness of breath, coughing, and wheezing due to narrowing of the airway4,5. To evaluate the disease control level precisely and objectively, pulmonary function testing has been widely recognized as a useful tool6.
Pulmonary function testing is one of the important examinations for patients with asthma, and forced expiratory volume in 1 s (FEV1) in particular is a parameter that reflects the status of disease control related to airway obstruction7,8. For example, corticosteroid therapy, which is a pivotal treatment for asthma, increases FEV1 dramatically9, and, in contrast, severe asthma patients showed decreased FEV1 even with intense treatment10,11. In addition, a previous report showed that a decreased FEV1 is associated with poor outcomes in patients with asthma12. These data indicate that exploration of factors related to reduced FEV1 is important for the management of asthma in daily clinical care.
Obesity and asthma are closely related, and obesity contributes to clinical outcomes of patients with asthma13. For example, obesity increases the incidence of asthma beyond age and race differences14. Obesity also affects the severity of asthma, and for severe asthma patients in the United States, the prevalence of obesity was 57.3% in adults, which is substantially higher than that in the general United States population15. In addition, the annual rate of exacerbation, which is one of the characteristics of asthma severity, is higher in asthma patients with obesity than in those without obesity in Japan16,17. As for the mechanisms, it is considered that obesity increases systemic and airway inflammation and induces resistance to corticosteroid therapy, which augments asthma pathophysiology18,19,20,21. In terms of pulmonary function, it can be reduced by the excess adipose tissue on the chest wall in patients with obesity compared to those without obesity22,23. However, the impact of obesity on pulmonary function in patients with asthma is not fully understood. The aim of the present study is to assess the impact of obesity on pulmonary function in patients with asthma.
Results
Characteristics of patients with and without asthma
First, 193 patients with asthma and 2159 patients without asthma were analyzed (Figs. 1, 2). The body mass index (BMI) and height were not different between the groups. Patients with asthma were significantly younger than those without asthma (p < 0.01). There were more females and fewer with a smoking history in patients with asthma than in those without asthma (both p < 0.01). There were more never smokers in patients without asthma than in those with asthma (p = 0.01), but the rates of ex-smokers and current smokers were not different between the two groups. For comorbidities, the rates of hypertension, diabetes mellitus, and cardiovascular diseases were significantly lower in patients with asthma than in those without asthma (all p < 0.01), and the rate of hyperlipidemia was not different between the groups. On pulmonary function testing, FEV1 percent predicted and FVC percent predicted were significantly lower in patients with asthma than in those without asthma (p = 0.03, < 0.01 respectively) (Table 1). On univariate analysis, FEV1 (β [95% confidence interval] 0.11 [0.06–0.17], p < 0.01), but not FVC (0.04 [− 0.02–0.1], p = 0.24), was significantly different between the patients with and without asthma. On multivariate analysis adjusted by height, age, sex, and smoking history with respect to known cofounding factors for asthma incidence, pathophysiology, and pulmonary function, FVC (0.06 [0.03–0.1], p < 0.01) and FEV1 (0.17 [0.14–0.2], p < 0.01) were significantly different between patients with and without asthma. In particular, there was a corresponding reduction in FVC of 0.06 L and FEV1 of 0.17 L for patients with asthma compared to those without asthma (Table 2). Next, 189 patients from each group were extracted by the propensity score-matching (PSM) method to mitigate the risk of confounding due to differences between patients with and without asthma. Comorbidities such as hypertension, diabetes mellitus, hyperlipidemia, and cardiovascular diseases were not different, but FVC percent predicted, FEV1, and FEV1 percent predicted were still significantly different (p = 0.03, < 0.01, < 0.01 respectively) (Supplemental Table 1).
Impact of obesity in patients with and without asthma
Of the patients without asthma, 1,570 were non-obese (average BMI 21.1 kg/m2), and 589 were obese (average BMI 28.2 kg/m2). Age, sex, smoking history, and smoking status were not different. The rates of hypertension, diabetes mellitus, hyperlipidemia, and cardiovascular diseases were significantly higher in obese patients without asthma than in non-obese patients without asthma (p < 0.01. < 0.01, < 0.01, = 0.02, respectively). Parameters of pulmonary function testing including FVC percent predicted and FEV1 percent predicted were not different (Table 3). Additionally, FVC and FEV1 were not different even after adjustment for confounding factors including age, sex, and smoking history on multivariate analysis (Tables 4). Of the patients with asthma, 134 were non-obese (average BMI 20.9 kg/m2), and 59 were obese (average BMI 27.7 kg/m2). Obese patients with asthma were significantly older than non-obese patients with asthma (p < 0.01), but sex, smoking history, and smoking status were not different between the two groups. The rates of hypertension, diabetes mellitus, and hyperlipidemia, but not of cardiovascular diseases, were significantly higher in obese patients with asthma than in non-obese patients with asthma (p < 0.01, < 0.01. < 0.01, 0.09, respectively). Parameters of pulmonary function testing including FVC percent predicted and FEV1 percent predicted were significantly lower in obese patients with asthma than in non-obese patients with asthma (all p < 0.01) (Table 3). In addition, BMI was negatively correlated with FEV1 (r = − 0.21, p = 0.003), FEV1, percent predicted (r = − 0.19, p = 0.008), FVC (r = − 0.15, p = 0.04), and FVC percent predicted (r = − 0.16, p = 0.03), even though the correlation coefficients were low (Fig. 3a–d). On univariate analysis, FVC (0.23 [0.09–0.36], p < 0.01) and FEV1 (0.26 [0.14–0.38], p < 0.01) were significantly different between patients with and without obesity. On multivariate analysis with adjustment for age, sex, smoking history, and ICS dose, FVC (0.12 [0.02–0.22], p = 0.02) and FEV1 (0.13 [0.05–0.22], p < 0.01) were still significantly different between patients with and without obesity. In particular, there was a corresponding reduction in FVC of 0.12 L and FEV1 of 0.13 L for obese patients with asthma compared to non-obese patients with asthma (Table 4). Of the 189 patients extracted by the PSM method, 142 were non-obese, and 47 were obese in the group of patients without asthma. On the other hand, 130 patients were non-obese, and 59 patients were obese in the group of patients with asthma. The tendency of the results for pulmonary function testing were reproduced in that FVC, FVC percent predicted, FEV1, and FEV1 percent predicted were significantly lower in obese patients with asthma than in non-obese patient with asthma (all p < 0.01), but these differences were not observed between obese patients without asthma and non-obese patient without asthma (Supplemental Table 2).
Correlations between forced expiratory volume in 1 s, forced vital capacity, and the body mass index in patients with asthma. (a) The forced expiratory volume in 1 s (r = − 0.21, p = 0.003) and (b) the forced expiratory volume in 1 s, percent predicted (r = − 0.19, p = 0.008) are significantly negatively correlated with the body mass index. (c) The forced viral capacity (r = − 0.15, p = 0.04) and (d) the forced vital capacity, percent predicted (r = − 0.16, p = 0.03) are correlated with the body mass index, though the correlation coefficient is low.
Allergic comorbidities, therapies, and laboratory data in patients with asthma focusing on obesity
Allergic rhinitis was less common in obese patients with asthma than in non-obese patients with asthma (p = 0.02), but atopic dermatitis and sinusitis were not different between the groups. Food allergy and drug allergy were more frequently seen in obese patients with asthma than in non-obese patients with asthma (both p = 0.01). In terms of therapies for the treatment of asthma, low-dose ICS was used significantly less often (p = 0.03), and high-dose ICS tended to be used more often (p = 0.05) in obese patients with asthma than in non-obese patients with asthma. The ICS doses calculated by beclomethasone equivalents were not different between the groups. Other therapies, long-acting β2 adrenergic agonists (LABAs), long-acting muscarinic antagonists (LAMAs), leukotriene receptor antagonists (LTRAs), and daily use of oral corticosteroid (OCS), were not different between the groups, but molecular targeting drugs were more often used in obese patients with asthma than in non-obese patients with asthma (p < 0.01), even though the absolute numbers were quite low. On laboratory testing, white blood cell counts and neutrophil counts were significantly higher in obese patients with asthma than in non-obese patients with asthma (p = 0.01, p = 0.03), and the percentages of eosinophils and eosinophil counts were not different between the two groups (Table 5).
Impact of sex differences on obesity for patients with and without asthma
Given the sex difference in obesity-induced asthma severity augmentation13,16,24, the impact of obesity on parameters of pulmonary function testing was examined by sex in patients with and without asthma. In patients of both sexes without asthma, FVC percent predicted and FEV1 percent predicted were not different between non-obese patients and obese patients. In patients with asthma, in males, FEV1 percent predicted, but not FVC percent predicted, was significantly lower in obese patients than in non-obese patients (p = 0.03). In females, FVC percent predicted and FEV1 percent predicted were significantly lower in obese patients than in non-obese patients (p = 0.01, < 0.01 respectively) (Table S3). On multivariate analysis, FEV1, but not FVC, was still significant after adjustment for confounding factors including age and smoking history in both sexes (male: 0.18 [0.01–0.35], p = 0.04, female: 0.09 [0.01–0.17], p = 0.03) (Table S4).
Discussion
In the present single-center, cross-sectional study, the impact of obesity on pulmonary function was examined in Japanese adult patients with asthma. Notably, all of the patients followed by pulmonary physicians with the disease name of asthma covered by medical insurance in our institute from 2005 to 2019 were included, and 193 patients definitely diagnosed as having asthma by a pulmonary physician were analyzed; this approach likely led to decreased rates of misdiagnosis and selection bias. In addition, the data of pulmonary function testing for patients without asthma were used for the comparison, which facilitated the evaluation of the effect of obesity in patients with asthma along with those without asthma. The present results showed that pulmonary functions were lower in patients with asthma than in those without asthma. Furthermore, FEV1 and FVC was negatively correlated with BMI in patients with asthma, though the correlation coefficients were relatively low, and obese patients with asthma showed significantly lower pulmonary functions than non-obese patients with asthma. Importantly, these differences were not seen between obese patients without asthma and non-obese patients without asthma.
Pulmonary function testing is an important examination for patients with asthma6 because decreased pulmonary function, including FEV1 and FVC, is associated with the severity of asthma. For example, decreased FEV1 is a major characteristic of severe asthma, along with increased symptoms and exacerbations25,26. Reduced FVC is also correlated with uncontrolled asthma defined by emergency department visits27 and is significantly lower in asthma patients with severe airflow obstruction than in those with moderate airflow obstruction based on their baseline FEV128. These data and the present results show that decreased pulmonary function is an important parameter reflecting asthma severity.
There is increasing evidence that obesity is related to the severity of asthma. In Japan, To et al. examined the impact of obesity defined by a BMI greater than 25 kg/m2 in 492 patients with severe asthma, and they found that obesity was associated with severe acute exacerbations in females16. We also reported that the annual exacerbation rate was significantly higher in overweight patients than in non-overweight patients, although there was no significant difference in pulmonary function given the small sample size17. In a cohort of 28,016 patients with asthma in the USA, seasonal exacerbations were significantly increased in overweight patients, defined as those whose BMI was 25–29.9 kg/m2, and obese patients, defined as those whose BMI was greater than 30 kg/m2, than in those with normal BMIs29. Importantly, in patients with asthma, exacerbations contribute to excess lung function decline30,31. Therefore, the present results indicating that obesity is related to lower pulmonary function in patients with asthma are consistent with those findings.
The present results showed that obese patients with asthma had significantly worse pulmonary functions than non-obese patients with asthma (Table 3). A population-based cohort study in The Netherlands of the epidemiology of obesity showed that, in 472 patients with asthma, obesity (BMI > 30 kg/m2) was associated with lower FEV1 and FVC than non-obesity32. Another longitudinal study also showed that obesity was significantly associated with decreased lung function (FVC), and the risk was higher in patients with asthma than in those without asthma33, which supported the present results. As for the mechanisms, decreased responses to corticosteroid therapy, which is one of the factors related to the severity of asthma with obesity21,34, might be involved. Indeed, the present study showed that obese patients with asthma were more often treated by high-dose ICS than non-obese asthma patients, even though their pulmonary functions were low (Table 5). In addition, the previous Japanese study mentioned above showed that obesity does not affect pulmonary functions when focusing on severe asthma patients treated by high-dose ICS compared to those without obesity, in contrast to the present study which included mild to severe patients with asthma. Notably, the present blood test results showed that obese patients with asthma had higher blood neutrophils, but not eosinophils, which might be related to the effectiveness of corticosteroid therapy35,36. These data remind us of the possibility that obese patients with asthma might generally include more patients with resistance to corticosteroid therapy and, consequently, have reduced pulmonary function compared to non-obese patients with asthma.
Pulmonary function, especially FEV1, was significantly lower in obese patients with asthma than in non-obese patients with asthma, and these differences were not seen between obese patients without asthma and non-obese patients without asthma (Tables 3, 4). Furthermore, the obesity-induced decline of FEV1 was greater than that of FVC on correlation analysis and multivariate analysis (Fig. 3a–d, Table 4). As for the mechanisms, augmented airway and systemic inflammation induced by obesity should be considered17,19,20,37, but airway dysanapsis, a physiological incongruence between the growth of lung parenchyma and the caliber of the airway, might be involved in obese patients with asthma38. Several studies reported that airway dysanapsis, explained by high FVC, normal FEV1, and low FEV1/FVC, was more frequently seen in overweight/obese asthmatic children than in those of normal weight39,40. Because obesity is associated with the incidence of asthma itself22, the result that obesity decreased FEV1 featured by airway dysanapsis, considered a diagnostic factor of asthma, might be consistent.
Interestingly, the present results showed that FVC was also lower in obese patients with asthma than in non-obese patients with asthma, and these differences were not seen between obese patients without asthma and non-obese patients without asthma (Table 3). These results cannot be explained by the obesity-induced physical effects caused by mobility regulation of the diaphragm and thorax by increased fat22,23. While we do not yet know the reason, obesity appears to have a specific impact on FVC along with FEV1 in patients with asthma, but not in those without asthma. Sex differences are normally involved in the pathophysiology of asthma with obesity, and studies in Japan of asthma incidence and exacerbation related to obesity showed more impact in female than male patients16,41. In the present study, there were no sex differences in pulmonary function associated with obesity (Tables S3, S4).
The present study has several limitations. First, the background characteristics were different between patients with and without asthma. This might have affected the impact of obesity on the results of pulmonary function testing in patients with and without asthma. However, the results of the PSM group comparison also confirmed that pulmonary functions were lower in patients with than in those without asthma. In addition, FEV1 and FVC were significantly lower in obese patients with asthma than in non-obese patients with asthma. These obesity-associated differences were not observed in patients without asthma, which we believe reduced the biases, and were confirmatory. Second, patients with chronic obstructive pulmonary disease may not have been completely excluded from the patients with and without asthma, which would have affected the results of pulmonary function testing. Third, it is unclear that the observed obesity-induced decreased pulmonary function in asthma contributes to poor outcomes, especially mortality. This was not assessed, and recently, overweight was found to be associated with improvement of long-term survival in a Japanese cohort42. Fourth, information on waist circumference was missing, which might have affected the present results, because the distribution of abdominal and thoracic fat mass is important for obesity-induced pathophysiology43,44 . Fifth, the range of time differences between the day of performing pulmonary function testing and obtaining clinical information was large up to 259 days, which might affect to the present results. Sixth, pulmonary function testing was not precisely performed according to the American Thoracic Society/European Respiratory Society guideline45, which might also affect to the present results. Finally, the present study involved a small number of patients at a single hospital with limited ethnic diversity. To confirm the validity of the present results, multicenter, prospective studies designed with appropriate controls and larger numbers of patients should be performed.
Conclusion
The present cross-sectional study showed that parameters of pulmonary function testing including FEV1 and FVC were significantly lower in obese patients with asthma than in non-obese patients with asthma. These differences were not observed between obese patients without asthma and non-obese patients without asthma. High-dose inhaled corticosteroid therapy was more common in obese patients with asthma than in non-obese patients, which might indicate that obesity-related corticosteroid resistance is the mechanism.
Methods
Patients and diagnosis
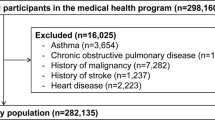
The purpose of the study was to clarify the effects of obesity, defined as a BMI greater than 25 kg/m2, on pulmonary function in patents with asthma and in those without asthma. To identify patients with asthma who underwent pulmonary function testing, 716 adult patients (age ≥ 18 years) who were followed by pulmonary physicians at Saga University Hospital with the International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] code of asthma (J459) from January 2005 to March 2019 were individually reviewed by a pulmonary physician. A total of 523 patients were excluded because of lack of medical information, other pulmonary diseases including COPD, chronic cough, and bronchitis, and 193 patients with asthma diagnosed by pulmonary physicians with reference to the Global Initiative for Asthma (GINA) guidelines46 were identified (Fig. 1). On the other hand, to identify patients without asthma who underwent pulmonary function testing, 2710 adult patients (age ≥ 18 years) who underwent pulmonary function testing at Saga University Hospital for preoperative examination in 2019 were individually reviewed by a pulmonary physician. A total of 551 patients were excluded because of lack of medical information or complications of asthma and other diseases that contribute to decreased pulmonary function, such as chronic obstructive pulmonary disease (COPD), interstitial pneumonia, pleural effusion, and neuromuscular diseases in the medical record. Patients with a history of lung resection were also excluded, and 2,159 patients were identified as patients without asthma (Fig. 2). The surgical procedures that the 2,159 patients underwent were as follows: abdominal surgery, 447 (20.7%) patients; cervical surgery, 394 (18.2%) patients; orthopedic surgery, 294 (13.6%) patients; urological surgery, 280 (13.0%) patients; gynecological surgery, 197 (9.1%) patients; cardiac surgery, 178 (8.2%) patients; brain surgery, 127 (5.9%) patients; skin surgery, 111 (5.1%) patients; lung surgery, 76 (3.5%) patients; ophthalmic surgery, 43 (2.0%) patients; surgical construction of an arteriovenous fistula for hemodialysis, 10 (0.5%) patients; and bone marrow transplantation, 2 (0.1%) patients. An obese patient was defined as one whose BMI was > 25 kg/m2, referring to criteria in Japan47.
Ethics approval and consent to participate
The present study was approved by the ethics committees of Saga University Hospital (approval number: 2021-11-R-01) and was performed in accordance with the 1964 Declaration of Helsinki. Informed consent of the participants was obtained in the form of opt-out on the website of Saga University Hospital, and no patients declined participation in the present study.
Data collection
The information for smoking history, comorbidities, asthma-related therapy, and laboratory data were collected from patients’ medical records at the time point nearest to when pulmonary function testing was performed. The average time between the day of performing pulmonary function testing and obtaining clinical information was 14.0 days (range 0–259 days). The data for BMI and age were obtained when pulmonary function testing was performed. Comorbidities and allergic comorbidities were diagnosed by the physicians. Cardiovascular disease included coronary artery disease, valvular disease, cardiac arrhythmias such as atrial fibrillation, and chronic heart failure diagnosed by echocardiography. Treatments for asthma were also selected at the physicians’ discretion, and doses of inhaled corticosteroid (ICS) were divided into 3 levels, low, moderate, and high, referring to the GINA guidelines46. In patients with asthma, spirometry parameters without using short-acting bronchodilators and withholding of controllers such as ICS was measured in a stable condition without exacerbation and airway infection. Pulmonary function testing was performed 2 or 3 times, and the highest parameters were obtained at a single point. The FVC, percent predicted, and FEV1, percent predicted were calculated with the LMS method, referring to the recommendations of the Japanese Respiratory Society48.
Statistical analysis
Quantitative data are expressed as means ± standard deviation (SD). The clinical data were analyzed by the Mann–Whitney U test for continuous variables and the chi-squared test for categorical variables. For correlation analysis, Pearson’s correlation coefficient was calculated to determine whether it was zero. Multivariate analysis with linear regression analysis for continuous variables and logistic regression analysis for categorical variables were performed, and the regression coefficients (β) were calculated. Comparing patients with and without asthma, FVC and FEV1 were individually adjusted by confounding factors including height, age, sex, and smoking history. Comparing non-obese patients with asthma and obese patients with asthma, FVC and FEV1 were individually adjusted by confounding factors including age, sex, smoking history, and ICS dose. Comparing non-obese patients without asthma and obese patients without asthma, FVC and FEV1 were individually adjusted by confounding factors including age, sex, and smoking history. To mitigate the risk of confounding due to differences between patients without asthma and those with asthma, the PSM method was used to balance the groups with respect to known confounders including BMI, age, sex, and smoking history. Subjects were matched 1:1 using the fitted value on the logit scale and matching using the nearest neighbor approach without replacement with a caliper of 0.05. Therefore, 189 patients from each group were extracted. Significance was considered at a p value less than 0.05. Statistical analysis was performed with JMP Pro version 14.2.0 software (SAS Institute Inc., Cary, NC, USA).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bousquet, J. et al. Eosinophilic inflammation in asthma. N. Engl. J. Med. 15, 1033–1039 (1990).
Tashiro, H. et al. Interleukin-33 from Monocytes recruited to the lung contributes to house dust mite-induced airway inflammation in a mouse model. PLoS One 6, e0157571 (2016).
Tashiro, H. & Shore, S. A. The gut microbiome and ozone-induced airway hyperresponsiveness. Mechanisms and therapeutic prospects. Am. J. Respir. Cell Mol. Biol. 3, 283–291 (2021).
Aaron, S. D., Boulet, L. P., Reddel, H. K. & Gershon, A. S. Underdiagnosis and overdiagnosis of asthma. Am. J. Respir. Crit. Care Med. 8, 1012–1020 (2018).
Grainge, C. L. et al. Effect of bronchoconstriction on airway remodeling in asthma. N. Engl. J. Med. 21, 2006–2015 (2011).
Pellegrino, R. et al. Interpretative strategies for lung function tests. Eur. Respir. J. 5, 948–968 (2005).
Kitch, B. T. et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest 6, 1875–1882 (2004).
Lange, P. et al. A 15-year follow-up study of ventilatory function in adults with asthma. N. Engl. J. Med. 17, 1194–1200 (1998).
Donohue, J. F. & Ohar, J. A. Effects of corticosteroids on lung function in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 3, 152–160 (2004).
Reddel, H. K. et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 1, 59–99 (2009).
Chung, K. F. et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2, 343–73 (2014).
Ortega, H. et al. Asthma exacerbations associated with lung function decline in patients with severe eosinophilic asthma. J. Allergy Clin. Immunol. Pract. 3(980–6), e1 (2018).
Tashiro, H. & Shore, S. A. Obesity and severe asthma. Allergol. Int. 2, 135–42 (2019).
Akinbami, L. J. & Fryar, C. D. Current asthma prevalence by weight status among adults: United States, 2001–2014. NCHS Data Brief. 239, 1–8 (2016).
Schatz, M. et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J. Allergy Clin. Immunol. 6, 1549–1556 (2014).
To, M. et al. Obesity-associated severe asthma in an adult Japanese population. Respir. Investig. 6, 440–447 (2018).
Tashiro, H. et al. Biomarkers for overweight in adult-onset asthma. J. Asthma Allergy 13, 409–414 (2020).
Tashiro, H. et al. Saturated Fatty acid increases lung macrophages and augments house dust mite-induced airway inflammation in mice fed with high-fat diet. Inflammation 3, 1072–1086 (2017).
Tashiro, H. et al. Microbiota contribute to obesity-related increases in the pulmonary response to ozone. Am. J Respir. Cell Mol. Biol. 6, 702–712 (2019).
Peters, M. C. et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: A cross-sectional analysis of two cohorts. Lancet Respir. Med. 7, 574–584 (2016).
Sutherland, E. R. et al. Body mass and glucocorticoid response in asthma. Am. J. Respir. Crit. Care Med. 7, 682–687 (2008).
Zerah, F. et al. Effects of obesity on respiratory resistance. Chest 5, 1470–6 (1993).
Jones, R. L. & Nzekwu, M. M. The effects of body mass index on lung volumes. Chest 3, 827–833 (2006).
Matsunaga, K. Obesity and severe asthma in Japan: Similarities and differences with Western countries. Respir Investig. 6, 430–431 (2018).
Moore, W. C. et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 4, 315–323 (2010).
Wenzel, S. E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 5, 716–725 (2012).
Castelluccio, J. F. et al. Study of correlation between forced vital capacity and demand for healthcare services in severe asthmatics. Multidiscip. Respir. Med. 1, 22 (2015).
Azevedo, K. S., Luiz, R. R., Rocco, P. R. & Conde, M. B. Vital capacity and inspiratory capacity as additional parameters to evaluate bronchodilator response in asthmatic patients: A cross sectional study. BMC Pulm. Med. 12, 1–6 (2012).
Schatz, M. et al. Overweight/obesity and risk of seasonal asthma exacerbations. J. Allergy Clin. Immunol. Pract. 6, 618–622 (2013).
Bai, T. R., Vonk, J. M., Postma, D. S. & Boezen, H. M. Severe exacerbations predict excess lung function decline in asthma. Eur. Respir. J. 3, 452–456 (2007).
Sprio, A. E. et al. Clinical characterization of the frequent exacerbator phenotype in asthma. J. Clin. Med. 7, 2226 (2020).
Kasteleyn, M. J. et al. Pulmonary function, exhaled nitric oxide and symptoms in asthma patients with obesity: A cross-sectional study. Respir. Res. 1, 205 (2017).
Huang, Y. J. et al. The effects of asthma on the association between pulmonary function and obesity: A 16-year longitudinal study. J. Asthma Allergy 14, 347–359 (2021).
Boulet, L. P. & Franssen, E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir. Med. 11, 2240–2247 (2007).
Jang, A. S. et al. Factors influencing the responsiveness to inhaled glucocorticoids of patients with moderate-to-severe asthma. Chest. 3, 1140–1145 (2005).
Crisford, H. et al. Neutrophils in asthma: The good, the bad and the bacteria. Thorax 76, 835–844 (2021).
Scott, H. A., Gibson, P. G., Garg, M. L. & Wood, L. G. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur. Respir. J. 3, 594–602 (2011).
Manuel, S. S. & Luis, G. M. Nutrition, obesity and asthma inception in children. The role of lung function. Nutrients 13, 3837 (2021).
Forno, E. et al. Obesity and airway dysanapsis in children with and without asthma. Am. J. Respir. Crit. Care Med. 3, 314–23 (2017).
Jung, Y., Jean, T., Morphew, T. & Galant, S. P. Peripheral airway impairment and dysanapsis define risk of uncontrolled asthma in obese asthmatic children. J. Allergy Clin. Immunol. Pract. 10, 759–767 (2021).
Tomita, Y. et al. Obesity, but not metabolic syndrome, as a risk factor for late-onset asthma in Japanese women. Allergol. Int. 2, 240–246 (2019).
Yano, C. et al. Overweight improves long-term survival in Japanese patients with asthma. Allergol. Int. 2, 201–207 (2021).
Despres, J. P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 7121, 881–887 (2006).
Palmer, B. F. & Clegg, D. J. The sexual dimorphism of obesity. Mol. Cell Endocrinol. 402, 113–119 (2015).
Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J. 2, 319–338 (2005).
Reddel, H. K. et al. Global initiative for asthma strategy 2021: Executive summary and rationale for key changes. Am. J. Respir. Crit. Care Med. 1, 17–35 (2022).
Examination Committee of Criteria for 'Obesity Disease' in J, Japan Society for the Study of O. New criteria for 'obesity disease' in Japan. Circ. J 11: 987–992 (2002).
Kubota, M. et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir. Investig. 4, 242–250 (2014).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H.T., K.T., and Y.K. conceived and designed the project. H.T., Y.K., and H.S. analyzed clinical data. R.T. advised on the statistical analysis. H.T., K.T., and N.A. prepared the manuscript with input from all other authors. H.S., S.K., and N.A. performed the final check of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tashiro, H., Takahashi, K., Kurihara, Y. et al. Obesity affects pulmonary function in Japanese adult patients with asthma, but not those without asthma. Sci Rep 12, 16457 (2022). https://doi.org/10.1038/s41598-022-20924-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20924-y
This article is cited by
-
Forced vital capacity and body mass index of Xinjiang children and adolescents: an analysis based on seven successive national surveys, 1985–2014
BMC Public Health (2024)
-
Pulmonary and chest wall function in obese adults
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.