Abstract
Metabolic rate has long been used in animal adaptation and performance studies, and individual oxygen consumption is used as proxy of metabolic rate. Stygofauna are organisms adapted to groundwater with presumably lower metabolic rates than their surface relatives. How stygofauna will cope with global temperature increase remains unpredictable. We studied the thermal acclimation and metabolic scaling with body mass of a stygobitic crustacean, Proasellus lusitanicus, in the climate change scenario. We measured oxygen consumption rates in a thermal ramp-up experiment over four assay temperatures and tested two hypotheses: (i) P. lusitanicus exhibits narrow thermal plasticity, inadequate for coping with a fast-increasing thermal regime; and (ii) oxygen consumption rates scale with the body mass by a factor close to 0.75, as commonly observed in other animals. Our results show that P. lusitanicus has low thermal plasticity in a fast-increasing thermal regime. Our data also suggest that oxygen consumption rates of this species do not follow mass-dependent scaling, potentially representing a new trait of metabolic optimization in groundwater habitats, which are often limited in food and oxygen. Species with limited dispersal capacities and rigid metabolic guilds face extinction risk due to climate change and omitting groundwater ecosystems from climate change agendas emphasizes the unprotected status of stygofauna.
Similar content being viewed by others
Introduction
Mean surface temperature increments due to climate change are incontrovertible1. Current carbon emissions suggest that the societal aspiration to restrict global warming to about 2.0 °C above the pre-industrial period will hardly be achieved2,3. Groundwater is generally characterized by thermal stability, with temperatures highly correlating with mean annual temperatures on the surface4. Therefore, a significant large-scale increase in groundwater temperatures is expected in the next years5. Since the 1980s, groundwater temperature increasing in the range of 0.7 to 3.0 °C has been observed in some unconsolidated alluvial aquifers6 and groundwaters of densely urbanized areas7. Temperatures of shallow groundwaters worldwide are projected to rise by 3–5 °C within the next century8,9. Although consequences of global climate change on aquatic biodiversity are widely predictable10, groundwater biodiversity is often disregarded11.
Groundwater is a highly diverse ecosystem, hosting over 7000 known animal species currently extinct on the surface (mostly arthropods12) and 2–6 × 1026 cells of microorganisms (prokaryotes13; fungi and a viral repository14), compressed into just ~ 19% of the world’s area15. Groundwater-adapted biodiversity is mainly composed of short-range species with restricted distributions [e.g.16,17]. Many are representatives of old lineages currently extinct at the surface, with high phylogenetic and conservationist value18. Obligate-groundwater species (hereafter referred as to “stygobites”) are adapted to environments with no light, limited food resources, low oxygen and low thermal amplitude19. Thermal acclimation is a phenotypic response to environmental temperature variation20,21 that has important implications for coping with global climate change22. Ectotherms from areas with high variability in temperature have greater acclimation abilities than those residing in habitats with low variability20. Therefore, stygobitic ectotherms are expected to exhibit a low thermal acclimation ability23,24,25,26,27,28,29. Recent studies suggest that some stygobitic species might have lost thermal acclimation mechanisms to adapt to groundwater21. However, our current knowledge on thermal acclimation of stygobitic species remains limited24,30,31.
Standard oxygen consumption rate (i.e., the oxygen required, in a time unit, for minimal resting lifestyle) is the most used variable in studies on animal short-term (hours or day) thermal acclimation32,33,34. When the environmental temperature rises, standard oxygen consumption rates of ectotherms also vary as a response to a sudden shift in temperature35. Standard oxygen consumption rates also change with the size of the organisms and this size-related effect is called scaling35,36. This relationship is described by a linear model where the scaling factor (also known as “mass exponent”) is in the range of 0.66 to 0.75 for most animal taxa35,36,37. Studies concerning thermal acclimation of stygobitic species are scarce27,38, only two studies investigated the scaling of standard oxygen consumption rates with body mass in stygobitic crustaceans28,39.
In this study, we measured the standard oxygen consumption rates of Proasellus lusitanicus (Frade, 1938), a stygobitic asellid (Crustacea Isopoda: Asellidae) endemic to a single karst aquifer in Portugal. Standard oxygen consumption rates were measured in a thermal ramp-up experiment from the groundwater temperature of the collection site to the one expected in the next 90 years in the aquifers of the 45° parallel North8. We also measured the scaling of oxygen consumption rates with body mass for this species. First, we hypothesized that P. lusitanicus would exhibit narrow thermal plasticity, inadequate for coping with a rapid-increasing thermal regime. Secondly, we assumed that the oxygen consumption rates of P. lusitanicus would scale with body mass showing a scaling exponent in the range of 0.66 to 0.75, as commonly measured for other animals. Findings are contextualized with previous studies.
Materials and methods
Collection and acclimation to laboratory conditions
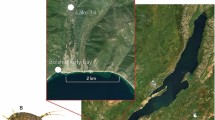
The stygobitic asellid Proasellus lusitanicus is an endemism living in the caves of Estremenho karst massif in Central Portugal (Fig. 1a), the largest karst aquifer of the Iberian Peninsula. The aquifer was the primary freshwater source for Lisbon in the last century40, and previous analyses indicated that groundwater from Estremenho karst massif was uncontaminated41, precisely, PAHs, PCBs, pesticides and heavy metals concentrations were below European detection limits41. Proasellus lusitanicus shows some of the most typical adaptive traits to groundwater lacks eyes and pigmentation, has appendage hypertrophy and breathes through branchiae (Fig. 1b)41,42,43. On September 2nd 2021, we collected only adults in a small stream running towards the ‘Christmas siphon’ of Almonda Cave (39° 30′ 20.05′′ N, 8° 36′ 56.63′′ W; Fig. 1a) using a plastic pipette. We put the individuals in a plastic container filled with groundwater and sediments from the collection site. We placed the container in a cooler and transported the individuals to the laboratory within a few hours from collection, as indicated in Castaño-Sánchez et al.43. We measured electrical conductivity (508 μS/cm), oxygen (6.9 mg/L), pH (7.1) and temperature (17 °C) in the field at the collection site using a portable multiparametric probe (WTW MULTI 3430).
In the laboratory, we selected up 20 adult individuals of different sizes (Fig. 2a). We kept them in a 1-L aquarium, in constant darkness, at the temperature of the collection site (17 °C ± 0.2 °C) in a thermo-regulated cabinet for 2 weeks to let them acclimate to laboratory conditions. Since guidelines suggest providing at least 1 mL of groundwater per individual44, we filled the aquarium with 800 mL of groundwater from the collection site. The dissolved oxygen concentration was measured three times during acclimation and was in the range of 6.5 to 7.0 mg/L. We did not change the groundwater in the aquarium during the two weeks to avoid providing additional stress to the animals44. According to previous studies44 and our personal observations, P. lusitanicus is a deposit feeder. For this reason, we provided the collected individuals with homogenized cave sediments by using a micro-spoon spatula (one scoop per individual). Five pebbles (5 cm in diameter) from the cave stream were provided as shelters. We offered no additional artificial food.
Thermal ramp-up experiment
We evaluated the thermal acclimation of P. lusitanicus by measuring standard oxygen consumption rates in a ramp-up experiment using four assay temperatures (17, 19.5, 21, 22.5 °C). Experimental phases are shown in Fig. 3. Phase I (acclimation to laboratory conditions; Fig. 3): this phase was described in the previous paragraph. Phase II (acclimation to new medium and vials; Fig. 3): after 14 days of acclimation to laboratory conditions, we gently picked up one individual at a time by using a soft brush and transferred them into 2-mL glass vials (one individual per vial). We filled each vial with 1 mL of commercial water (electrical conductivity: 110 µS/cm; pH: 6.40; SiO2−: 58 mg/L; Ca2+: 14 mg/L; Na+: 27 mg/L; Cl−: 20 mg/L; HCO3−: 469 mg/L) and 1 mL of groundwater from the collection site. We also added one scoop of homogenized cave sediments (dosed by using a micro-spoon spatula). Individuals were kept at 17 °C for acclimation to the new medium and vials for 24 h, according to Di Lorenzo et al.44. After 24 h, we replaced the medium with 2 mL of commercial water and removed the sediment to ensure that the digestive tracts of each individual would be empty during subsequent oxygen consumption measurements. We removed feces to avoid oxygen overshoot due to animal wastes. The specimens were kept like this further 24 h. Phase III (measurements; Fig. 3): before starting the measurements, we transferred the individuals into the measurement chambers. The chambers were 2-mL vials equipped with planar, pre-calibrated oxygen sensor spots (4 mm diameter) with optical isolation glued onto the bottom (Loligo Systems, Viborg, Denmark). We filled each chamber with 2 mL of commercial water. The chambers were integrated with a reader consisting of a 24-channel fluorescence-based respirometry system (SDR Sensor Dish Reader; PreSens Precision Sensing GmbH, Regensburg, Germany). We connected the reader to a computer to measure the oxygen consumption rates of 20 individuals of P. lusitanicus and four blank controls (i.e., vials without animals) simultaneously (Fig. 3). We placed the reader inside the thermostatic cabinet at the appropriate testing temperatures 18 h before the beginning of the measurements to equilibrate the equipment temperature. Water temperature in each chamber was recorded with a temperature logger already integrated into the device. After animal loading, the chambers were sealed by screw caps and inspected for air bubbles before being placed on the reader and into the thermostatic cabinet at 17 °C. We started the measurements after 2 h from animal loading to let temperature equilibrate29. Oxygen concentration in each chamber was recorded every 5 min for 4 h after temperature re-equilibration. Linear decrease of oxygen concentration was used to calculate oxygen consumption rates in ng O2/h for each individual. We corrected oxygen consumption rates for microbial respiration measured in the control chambers. Phase IV) (acclimation to a new temperature; Fig. 3, time proportional to the thermal increment): at the end of the measurements at 17 °C, we returned the animals to the 2-mL glass vials filled with commercial water. We provided one scoop of homogenized cave sediments in each vial. We kept the vials in the thermostatic cabinet and increased the temperature by 0.041 °C/h. We deemed this ramping rate more appropriate for stygobitic species than the faster ramping rates of 1 °C/min or 1 °C/h used for other ectotherms [e.g.45]. When the next assay temperature was reached, we cleaned the sediment and feces from the vials and let the animals acclimate to the new temperature for 24 h before measurements. Phase V (measurements; Fig. 3): oxygen consumptions were measured as described in Phase III. The procedures described in phases IV and V (acclimation to a new temperature and measurements) were repeated to perform the measurements at 21 °C (Phases VI and VII) and 22.5 °C (Phases VIII and IX). We always used the same individuals, randomly switching the measurement chambers (and the relative oxygen sensors) to assure the independence of the measurements at each assay temperature. All measurements were run between 11 a.m. and 7 p.m. time period. Phase X) (dry mass estimates; Fig. 3): at the end of the experiment, the animals were individually photographed and then measured using ImageJ software46. Afterwards, wet mass was measured on a laboratory scale (A&D, model ER-120A) with 0.1 mg accuracy. Finally, dry mass was estimated by weighting the individuals after placing them between two sheets of blotting paper to remove water.
Phases of the thermal ramp-up experiment performed with twenty adult individuals of Proasellus lusitanicus. Circled numbers indicate phases. Phase I acclimation to laboratory conditions; Phase II acclimation to new medium and vials; Phase III measurements; Phase IV acclimation to a new temperature; Phase V measurements; Phases VI, VII, and VIII acclimation to a new temperature and measurements; and Phase X dry mass estimates.
The activity of adult individuals of P. lusitanicus in the measurement chambers was inspected in preliminary experiments with half-hourly interval observations during 4 h. In these preliminary trials, the individuals predominantly sat still on the top of the oxygen sensors (Fig. 2b), as observed for some cave amphipod species39. Therefore, the measured rates of oxygen decline were the best estimate of standard oxygen consumption rates, according to Clarke and Fraser33. Oxygen consumption rates of the individuals which died at temperatures > 17 °C (1 dead individual at 19.5 °C, 2 at 21 °C and 2 at 22.5 °C) and a female that became ovigerous during thermal acclimation at 21 °C, were discarded from the subsequent analyses.
We used a one-way permutational analysis of variance (PERMANOVA47) followed by a permutational pairwise t-test to highlight potential differences in oxygen consumption rates at the four temperatures. We used a resemblance matrix based on Euclidean distances of raw data. We applied a one-way design consisting of one fixed factor (temperature) with four levels. We examined the model under the Type I partitioning of sum of squares. We also used permutation of residuals under a reduced model as it yields the best power and the most accurate type I error for matrices with a few data47. We used PERMANOVA because it does not rely on ANOVA assumptions relating to the data distribution. Nevertheless, we performed a Levene’s test prior to PERMANOVAs to account for the potential heterogeneity of the variances among the four levels of the fixed factor47. The significance level (α) was set at 0.05 since permutational tests do not require alpha correction for multiple groups47. All analyses were performed with E-PRIMER and PERMANOVA + software v. 647.
Metabolic scaling
Oxygen consumption rates and body mass are related as in Eq. (1)48:
where OCR is oxygen consumption rate, M is dry mass, a is a proportionality constant and b is the scaling factor, which falls within the range of 0.66–0.75 for most animals [e.g.49]. Equation (1) can be conveniently log-transformed as in Eq. (2):
To assess the linear scaling of log oxygen consumption rates of P. lusitanicus with log body mass, we fitted a permutational Ordinary Least Square (OLS) regression model to the data collected at 17 °C (groundwater temperature at the collection site). We also fitted OLS models to data at the three assay temperatures > 17 °C to assess potential scaling variation due to thermal stress. We repeated the analyses after removing potential outliers from data at 19.5, 21 and 22.5 °C, where outliers were values ≤ 0.01 ng O2/mg h (Supplementary Table S1). Permutation tests are preferred when data distribution departs from normality50, as was the case for the present data even after the exclusion of the above mentioned extreme values. The significance (α) of the intercept and slope was set at 0.05, number of permutations of independent variable was 999, while the fit of the models was assessed by using R2. The analyses and plots were performed by using R vs. 4.1.1 and the library “ape”51.
Respiration rates of aquatic isopod species
To contextualize the rates of oxygen consumption of P. lusitanicus with previous studies, we surveyed the literature for respiration rates of asellids by performing a search in Clarivate Analytics Web of Science. We used the keywords as follows: TS = "isopod*” AND TS = (“oxygen” OR “consumption” OR “SRR” OR “respiration”) AND TS = (“aquatic” OR “freshwater” OR “groundwater”), where TS denotes a search for ‘Topic’. The asterisk is used to match all words beginning with that string of characters. We used the PlotDigitizer 2.6.8 software (http://plotdigitizer.sourceforge.net/) to extract data available only in graphs. Data retrieved from the literature were converted to ng O2/mg h for comparative purposes whenever necessary. No temperature corrections were made, and temperatures at which each study was conducted were annotated.
Results
Dry mass of the adult individuals of Proasellus lusitanicus was in the range of 1.7–9.5 mg (mean ± SD: 4.7 ± 3.3 mg). Body length was 4.2–7.2 mm (mean ± SD: 5.2 ± 1.3 mm). Oxygen consumption rates of adult individuals were in the range of 21.3–259.5 ng O2/mg h at 17 °C, 0.04–191.4 ng O2/mg h at 19.5 °C, 0.03–142.4 ng O2/mg h at 21 °C and 0.01–91.9 ng O2/mg h at 22.5 °C (Supplementary Table S1). Mean values at the four assay temperatures are reported in Table 1. At temperatures > 17 °C, 8 individuals (with body mass in the range of 2.2–8.8 mg) had extremely low oxygen consumption rates. However, they remained alive until the end of the experiment (Supplementary Table S1). Five individuals died at temperatures > 17 °C. The main PERMANOVA test highlighted that oxygen consumption rates differed at the four assay temperatures (PERMANOVA: Pseudo-F3,55 = 4.45, p = 0.0048, perms = 9953). However, the differences were significant between 17.0 and 22.5 °C (pairwise t-test: t = 3.28, p = 0.0004, perms = 8417), 19.5 and 22.5 °C (pairwise t-test: t = 3.27, p = 0.0024, perms = 7990) and 21.0 and 22.5 °C (pairwise t-test: t = 2.52, p = 0.0179, perms = 7714).
The permutational OLS regression highlighted that log oxygen consumption rates of P. lusitanicus at 17 °C were not linearly correlated to log body mass (Table 2, Fig. 4). We obtained the same result across all assay temperatures. After outliers’ removal, the OLS model was significant at 19.5 °C, showing an intercept of 2.14 ng O2/h and a slope of 0.49 ng O2/h (Table 2, Fig. 4). However, the model explained 34% of data variability (Table 2).
Log–log scatter plots of oxygen consumption rates vs. body mass of adults of Proasellus lusitanicus at: (a) 17 °C; (b) 19.5 °C; (c) 21 °C; (d) 22.5 °C. Model parameters are reported in Table 2.
Oxygen consumption rates of other stygobitic isopod species (Table 3) varied from 20 ng O2/mg h (P. lusitanicus at 22.5 °C; this study) to 1750 ng O2/mg h (P. valdensis 127). Oxygen consumption rates of P. lusitanicus at 19 °C and 22.5 °C (mean values: 70 and 20 ng O2/mg h, respectively) were much lower than those of P. valdensis 1 (1250 ng O2/mg h at 19 °C and 1150 ng O2/mg h at 22 °C27), P. valdensis 2 (1200 ng O2/mg h at 19 °C;27) and P. valdensis n. sp. 2 (1700 ng O2/mg h at 19 °C27).
Discussion
The results of our study indicate that Proasellus lusitanicus has a low thermal acclimation ability. At 22.5 °C, oxygen consumption rates of adult individuals of this species were reduced by > 75% as compared to those at the temperature of the collection site (17 °C). In addition, some adults died at temperatures exceeding that of the collection site by ≥ 2.5 °C. Finally, our results indicate that eight adult individuals might have experienced heat rigor, i.e., rigor of living tissue caused by exposure to excessive but not immediately lethal temperatures53 at > 17 °C. We suspect that uropod movement was highly reduced in these individuals because their oxygen consumption rates were several orders of magnitude lower than those of other individuals engaged in the experiment. Our results suggest that P. lusitanicus is a stenothermic species, as observed for other stygobitic Proasellus species living in thermally buffered environments27. On the other hand, previous studies showed that P. valdensis (Chappuis, 1948), which lives in habitats with groundwater temperatures ranging from 4.1 to 11.8 °C (though each population resides in a thermally buffered habitat27), has a wider thermal tolerance than P. lusitanicus. These differences are consistent with theories suggesting that ectotherms living in areas with high variability in temperature acclimate better than those residing in habitats with low thermal variations20. Accordingly, P. lusitanicus exhibits a much-restricted distribution as compared to P. valdensis. The significant decrease in oxygen consumption rates at temperatures diverging from that at the collection site, also associated with some fatalities at temperatures > 17.0 °C in the case of P. lusitanicus, is suggestive of metabolic alteration during acclimation that should be further analyzed. Variability in metabolic end-products, such as lactate and succinate, and immune defenses are likely to be the causes27. Movement tracking and behavioral analyses could be useful for exploring how metabolic alterations at elevated temperatures reverberate, not only on single populations, but also on community and ecosystem services. For instance, some individuals of P. valdensis are known to reduce their locomotor activity by about 40% when temperature diverges from that of the collection site by ≥ 3 °C27. This behavioral alteration likely results in reduced efficiency in foraging and mating, and alterations in interactions associated with predation or competition. Our results suggest that if the worst global warming scenario is met in the next century, P. lusitanicus will likely go extinct. Population declines will be imputed to this species’ inability to acclimate fast enough to track increasing temperatures, as speculated for other species [e.g.31,54,55]. We speculate that there will not be possibility of recolonization from other geographic areas because of the restricted distribution of this endemic species56,57. This will inevitably have detrimental cascading effects involving essential groundwater ecosystem services23,24,58,59.
Oxygen consumption rates of P. lusitanicus fall within the range reported for other stygobitic isopod species and this supports the reliability of our measurements. However, P. lusitanicus has oxygen consumption rates lower than other Proasellus species. Such metabolic differences among closely phylogenetically-related species are not unlikely and are related to multiple factors. Previous studies have revealed that most of the variance in oxygen consumption rates of crustacean species can be attributed to temperature variability at the collection site and differences in body mass60. Thermally-stable environments require low energy for thermoregulation. Ammonia and phosphate excretion rates also play a role60. The epigean asellid species Asellus aquaticus has oxygen consumption rates twice the values of P. lusitanicus. Previous studies supported the theory that stygobitic species evolved a lower metabolism, compared to that of close-related surface species, as adaptations towards some environmental features of groundwater environments [e.g.28,38,39,52]. Reduced metabolism is advantageous for organisms living in habitats with chronically low and/or discontinuous food and oxygen supplies39,61. A metabolism independent on body mass may be seen a further advantage in groundwater habitats, as largest individuals may conserve energy more efficiently. Our results suggest that log oxygen consumption rates of P. lusitanicus do not depend linearly on log body mass (at least in the mass range of 1.7–9.5 mg) at the temperature of the collection site. We consistently observed the lack of linear scaling across the assay temperatures, except at 19.5 °C after outliers’ removal. Although the OLS model was significant at that temperature, its reliability is questionable because of the low percentage of explained variance. The apparent lack of linear scaling for P. lusitanicus is a result that diverges from most other animals36,37. Both endothermic and ectothermic animals have log oxygen consumption rates that scale linearly with log mass with a scaling factor b close to 0.7549, at least within the limited range of "biologically relevant" temperatures (0° and 40 °C36). However, the apparent lack of linear scaling is not an overall novelty for groundwater species. It has been already observed in two other stygobitic crustacean species. Log oxygen consumption rates of the stygobitic copepod Diacyclops belgicus Kiefer, 1936 seem not to scale linearly with body mass, at least in the mass range of 3.0–5.0 ng28. Similarly, log oxygen consumption rates of the stygobitic amphipod Gammarus acherondytes Hubricht & Mackins, 1940 do not scale with log body mass in the mass range of 1.5–9.3 mg39. Wilhelm et al.39 assumed that the lack of scaling could represent an adaptation to groundwater habitats where temporal unavailability of food and oxygen can be pronounced62,63. In particular, the independence of oxygen consumption rates from mass is an evident advantage for large individuals in food-deprived habitats. Other explanations had been called into question by Wilhelm et al.39 and then discarded. For instance, lack of scaling in G. acherondytes could have been due to differences in individual behavioral activity, with larger amphipods less active than the smaller ones. However, Wilhelm et al.39 observed that the individuals engaged in their experiment spent most of their time sitting on the oxygen sensors, showing no evident differences in movement or behavior. We can state the same for P. lusitanicus. A further explanation is relative to the mass range, which might have been too small to detect a statistically-significant relationship. Wilhelm et al.39 stated that they covered the range of mass (1.5–9.3 mg) for the collection site of G. acherondytes. We can say the same (1.7–9.5 mg) for the individuals of P. lusitanicus used in our experiment. However, data limitations in terms of both investigated species and measurements prevent claiming a novel metabolic guild limited to stygofauna, and further studies are necessary to explore this possibility. Furthermore, the apparent lack of linear scaling of log oxygen consumption rates with log mass only applies to adult individuals. Oxygen consumption rates of juvenile stages of P. lusitanicus and G. acherondytes have not been measured yet. Log oxygen consumption rates of juveniles of D. belgicus scale isometrically with log mass, i.e. with a scaling factor b close to 128. However, isometric scaling during ontogeny is commonly observed in many species and interpreted as a response to rapid growth rates necessary to face high juvenile mortality and predation64.
Conclusions
Our findings indicate that stenothermic species, such as the stygobitic P. lusitanicus, might be at extinction risk due to climate change, because of the reduced number of suitable habitats that may act as thermal refuges in next future and the inability to disperse to other habitats due to belowground isolation. Even if some adaptation is expected, the temporal scale at which key physiological traits (such as thermal plasticity) evolve is uncertain and the extinction risk of this stygobitic species is high19. Stygobitic crustaceans are key species for the groundwater ecosystem, and their extinction threatens the entire ecosystem services, which are vital for all life on Earth19,23,59. This study is particularly relevant to understand the thermal acclimation ability of stygobitic crustaceans, and by extension its implications in the ecological integrity of groundwater habitats24. Furthermore, the omission of groundwater ecosystems from climate change agendas65,66 marginalizes their ecological importance, leaving stygofauna substantially unprotected. Our findings also aim at stimulating open questions on metabolic adaptations of stygobitic species to thermally-stable, nutrient- and oxygen-poor, groundwater habitats.
Data availability
All data generated or analyzed during this study are included in this paper and Supplementary File.
References
Li, J. & Thompson, D. W. Widespread changes in surface temperature persistence under climate change. Nature 599(7885), 425–430. https://doi.org/10.1038/s41586-021-03943-z (2021).
Raftery, A. E., Zimmer, A., Frierson, D. M., Startz, R. & Liu, P. Less than 2 °C warming by 2100 unlikely. Nat. Clim. Change 7, 637–641 (2017).
Olabi, A. G. et al. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 153, 111710. https://doi.org/10.1016/j.rser.2021.111710 (2022).
Badino, G. Cave temperatures and global climatic change. Int. J. Speleol. 33(1), 103–114 (2004).
Wang, J. et al. Recent global decline in endorheic basin water storages. Nat. Geosci. 11(12), 926–932 (2018).
Figura, S., Livingstone, D. M., Hoehn, E. & Kipfer, R. Regime shift in groundwater temperature triggered by the Arctic Oscillation. Geophys. Res. Lett. 38(23), 401–405 (2011).
Mueller, M. H., Huggenberger, P. & Epting, J. Combining monitoring and modelling tools as a basis for city-scale concepts for a sustainable thermal management of urban groundwater resources. Sci. Total Environ. 627, 1121–1136 (2018).
Taylor, C. A. & Stefan, H. G. Shallow groundwater temperature response to climate change and urbanization. J. Hydrol. 375, 601–612 (2009).
Dehghani, R., Poudeh, H. T. & Izadi, Z. The effect of climate change on groundwater level and its prediction using modern meta-heuristic model. Ground. Sustain. Dev. 16, 100702. https://doi.org/10.1016/j.gsd.2021.100702 (2022).
Lenton, T. M. et al. Climate tipping points—Too risky to bet against. Nature 57, 592–595 (2019).
Albert, J. S. et al. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 50(1), 85–94 (2021).
Stein, H. et al. Stygoregions—A promising approach to a bioregional classification of groundwater systems. Sci. Rep. 2, 673. https://doi.org/10.1038/srep00673 (2012).
Baković, N., Matoničkin Kepčija, R. & Siemensma, F. J. Transitional and small aquatic cave habitats diversification based on protist assemblages in the Veternica cave (Medvednica Mt., Croatia). Subterr. Biol. 42, 43–60 (2022).
Magnabosco, C. et al. The biomass and biodiversity of the continental subsurface. Nat. Geosci. 11(10), 707–717 (2018).
Chen, Z. et al. The World Karst Aquifer Mapping project: Concept, mapping procedure and map of Europe. Hydrogeol. J. 25, 771–785 (2017).
Eme, D. et al. Do cryptic species matter in macroecology? Sequencing European groundwater crustaceans yields smaller ranges but does not challenge biodiversity determinants. Ecography 41(2), 424–436 (2018).
Manenti, R. et al. The stenoendemic cave-dwelling planarians (Platyhelminthes, Tricladida) of the Italian Alps and Apennines: conservation issues. J. Nat. Conserv. 45, 90–97 (2018).
Zagmajster, M., Malard, F., Eme, D. & Culver, D. C. Subterranean biodiversity patterns from global to regional scales. In Cave Ecology, Ecological Studies—Analysis and Synthesis (eds Moldovan, O. et al.) 19–227 (Springer, 2018).
Hose, G. C. et al. Invertebrate traits, diversity and the vulnerability of groundwater ecosystems. Funct. Ecol. 36, 2200. https://doi.org/10.1111/1365-2435.14125 (2022).
Angilletta, M. J. Jr. & Angilletta, M. J. Thermal Adaptation: A Theoretical and Empirical Synthesis (Oxford University Press, 2009).
Pallarées, S. et al. Loss of heat acclimation capacity could leave subterranean specialists highly sensitive to climate change. Anim. Conserv. 24(3), 482–490 (2020).
Vasseur, D. A. et al. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B 281, 20132612. https://doi.org/10.1098/rspb.2013.2612 (2014).
Castaño-Sánchez, A., Hose, G. C. & Reboleira, A. S. P. Ecotoxicological effects of anthropogenic stressors in subterranean organisms: A review. Chemosphere 244, 125422. https://doi.org/10.1016/j.chemosphere.2019.125422 (2020).
Castaño-Sánchez, A., Hose, G. C. & Reboleira, A. S. P. Salinity and temperature increase impact groundwater crustaceans. Sci. Rep. 10(1), 1–9 (2020).
Issartel, J., Hervant, F., Voituron, Y., Renault, D. & Vernon, P. Behavioural, ventilatory and respiratory responses of epigean and hypogean crustaceans to different temperatures. Comp. Biochem. Physiol. Mol. Amp Integr. Physiol. 141, 1–7 (2005).
Issartel, J., Voituron, Y. & Hervant, F. Impact of temperature on the survival, the activity and the metabolism of the cave-dwelling Niphargus virei, the ubiquitous stygobiotic N. rhenorhodanensis and the surface-dwelling Gammarus fossarum (Crustacea, Amphipoda). Subterr. Biol. 5, 9–14 (2007).
Mermillod-Blondin, F. et al. Thermal tolerance breadths among groundwater crustaceans living in a thermally constant environment. J. Exp. Biol. 216, 1683–1694 (2013).
Di Lorenzo, T. et al. Metabolic rates of a hypogean and an epigean species of copepod in an alluvial aquifer. Freshw. Biol. 60, 426–435 (2015).
Di Lorenzo, T. & Galassi, D. M. P. Effect of temperature rising on the stygobitic crustacean species Diacyclops belgicus: Does global warming affect groundwater populations? Water 9, 951. https://doi.org/10.3390/w9120951 (2017).
Mammola, S. et al. Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthr. Rev. 6(1–2), 98–116 (2019).
Jones, K. et al. The critical thermal maximum of diving beetles (Coleoptera: Dytiscidae): A comparison of subterranean and surface-dwelling species. Curr. Opin. Insect. Sci. 1, 100019 (2021).
Pörtner, H. O. Physiological basis of temperature-dependent biogeography: Trade-offs in muscle design and performance in polar ectotherms. J. Exp. Biol. 205, 2217–2230 (2022).
Clarke, A. & Fraser, K. P. P. Why does metabolism scale with temperature? Funct. Ecol. 18, 243–251 (2004).
Dell, A. I., Pawar, S. & Savage, V. M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl. Acad. Sci. 108, 10591–10596 (2011).
Willmer, P., Stone, G. & Johnston, I. Environmental Physiology of Animals (Wiley, 2009).
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M. & Charnov, E. L. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001).
Gillooly, J. F., Charnov, E. L., West, G. B., Savage, V. M. & Brown, J. H. Effects of size and temperature on developmental time. Nature 417, 70–73 (2002).
Hervant, F., Mathieu, J., Barré, H., Simon, K. & Pinon, C. Comparative study on the behavioural, ventilatory, and respiratory responses of hypogean and epigean crustaceans to long-term starvation and subsequent feeding. Comp. Biochem. Physiol. B 118A, 1277–1283 (1997).
Wilhelm, F. M., Taylor, S. J. & Adams, G. L. Comparison of routine metabolic rates of the stygobite, Gammarus acherondytes (Amphipoda: Gammaridae) and the stygophile, Gammarus troglophilus. Freshwat. Biol. 51, 1162–1174 (2006).
Reboleira, A. S. P. S., Borges, P., Gonçalves, F., Serrano, A. R. M. & Oromí, P. The subterranean fauna of a biodiversity hotspot region—Portugal: An overview and its conservation. Int. J. Speleol. 40(1), 23–37 (2011).
Reboleira, A. S. P. S., Abrantes, N., Oromí, P. & Gonçalves, F. J. M. Acute toxicity of copper sulfate and potassium dichromate on stygobiont Proasellus: General aspects of groundwater ecotoxicology and future perspectives. Water Air Soil Pollut. 224, 1550. https://doi.org/10.1007/s11270-013-1550-0 (2013).
Morvan, C. et al. Timetree of Aselloidea reveals species diversification dynamics in groundwater. Syst. Biol. 62(4), 512–522 (2013).
Castaño-Sánchez, A., Malard, F., Kalčikova, G. & Reboleira, A. S. P. S. Novel protocol for acute in situ ecotoxicity test using native crustaceans applied to groundwater ecosystems. Water 13(8), 1132. https://doi.org/10.3390/w13081132 (2021).
Di Lorenzo, T. et al. Recommendations for ecotoxicity testing with stygobiotic species in the framework of groundwater environmental risk assessment. Sci. Total Environ. 681(1), 292–304 (2019).
Rezende, E. L., Tejedo, M. & Santos, M. Estimating the adaptative potential of critical thermal limits: Methodological problems and evolutionary implications. Funct. Ecol. 25, 111–121 (2011).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9(7), 671–675 (2012).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46 (2001).
Harvey, P. H. & Pagel, M. D. The Comparative Method in Evolutionary Biology (Oxford University Press, 1991).
Dodds, P. S., Rothman, D. H. & Weitz, J. S. Re-examination of the “3/4” law of metabolism. J. Theor. Biol. 209, 9–27 (2001).
Manly, B. F. J. Randomization, Bootstrap and Monte Carlo Methods in Biology (Chapman & Hall/CRC Press, 2006).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (R Foundation for Statistical Computing, Vienna, Austria, 2018).
Simčič, T. & Sket, B. Comparison of some epigean and troglobiotic animals regarding their metabolism intensity. Examination of a classical assertion. Int. J. Speleol. 48, 133–144 (2019).
Hazell, S. P., Pedersen, B. P., Worland, M. R., Blackburn, T. M. & Bale, J. S. A method for the rapid measurement of thermal tolerance traits in studies of small insects. Physiol. Entomol. 33(4), 389–394 (2008).
Cohen, J. M., Lajeunesse, M. J. & Rohr, J. R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Change 8, 224. https://doi.org/10.1038/s41558-018-0067-3 (2018).
Ficetola, G. F., Lunghi, E. & Manenti, R. Microhabitat analyses support relationships between niche breadth and range size when spatial autocorrelation is strong. Ecography 43(5), 724–734 (2020).
Sánchez-Fernández, D., Rizzo, V. & Bourdeau, C. The deep subterranean environment as a model system in ecological, biogeographical and evolutionary research. Subterr. Biol. 25, 1–7 (2018).
Pallarés, S. et al. Loss of heat acclimation capacity could leave subterranean specialists highly sensitive to climate change. Anim. Conserv. 24(3), 482–490 (2021).
Griebler, C. & Avramov, M. Groundwater ecosystem services: A review. Freshw. Sci. 34(1), 355–367 (2015).
Saccò, M. et al. Stygofaunal diversity and ecological sustainability of coastal groundwater ecosystems in a changing climate: The Australian paradigm. Freshw. Biol. https://doi.org/10.1111/fwb.13987 (2022).
Ikeda, T., Kanno, Y., Ozaki, K. & Shinada, A. Metabolic rates of epipelagic marine copepods as a function of body mass and temperature. Mar. Biol. 139, 587–596 (2001).
Mezek, T., Simčič, T., Arts, M. T. & Brancelj, A. Effect of fasting on hypogean (Niphargus stygius) and epigean (Gammarus fossarum) amphipods: A laboratory study. Aquat. Ecol. 44(2), 397–408 (2010).
Hüppop, K. The role of metabolism in the evolution of cave animals. NSS Bulletin 47, 136–146 (1985).
Humphreys, W. F. Hydrogeology and groundwater ecology: Does each inform the other? Hydrogeol. J. 17(1), 5–21 (2009).
Glazier, D. S. The 3/4-power law is not universal: Evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience 56(4), 325–332 (2006).
Sánchez-Fernández, D., Galassi, D. M. P., Wynne, J. J., Cardoso, P. & Mammola, S. Don’t forget subterranean ecosystems in climate change agendas. Nat. Clim. Change 11, 458–459 (2021).
Reboleira, A. S. P. S. et al. Nutrient-limited subarctic caves harbour more diverse and complex bacterial communities than their surface soil. Environ. Microbiome 17, 41 (2022).
Acknowledgements
The authors thank Marta Palma, Claudia Duarte and Maria João Medina for all kinds of help in lab work, and Pedro Souto for support in fieldwork. All specimens were collected under permits of the Instituto de Conservação da Natureza e das Florestas. This research was funded by CNR Short Term Mobility Program (2021), MUR-FOE-PROJECT Capitale Naturale—Task Biodiversità. This work was supported by the VILLUM FONDEN (research Grant 15471), by the project “Sustainability of subterranean ecosystems” financed by the Cooperation protocol with the Municipality of Alcanena, and by Portuguese National Funds through “Fundação para a Ciência e a Tecnologia” (FCT) within the cE3c Unit funding UIDB/00329/2020.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.D.L., A.S.R.; methodology, T.D.L.; validation, T.D.L., A.S.R.; formal analysis, T.D.L.; investigation, T.D.L., A.S.R.; resources, T.D.L., A.S.R.; data curation, T.D.L., A.S.R.; writing-original draft preparation, T.D.L., A.S.R.; writing-review and editing T.D.L., A.S.R.; project administration, T.D.L., A.S.R.; funding acquisition, T.D.L., A.S.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Lorenzo, T., Reboleira, A.S.P.S. Thermal acclimation and metabolic scaling of a groundwater asellid in the climate change scenario. Sci Rep 12, 17938 (2022). https://doi.org/10.1038/s41598-022-20891-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20891-4
This article is cited by
-
Environmental risk of diclofenac in European groundwaters and implications for environmental quality standards
Scientific Reports (2024)
-
Temperature variation in caves and its significance for subterranean ecosystems
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.