Abstract
Late-onset noninfectious pulmonary complications (LONIPC) are a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). However, the clinical impact of lung function deterioration itself in long-term adult survivors of HSCT remains to be fully investigated. This retrospective, longitudinal study aimed to investigate pulmonary function following HSCT in terms of its change and the clinical significance of its decline. We examined 167 patients who survived for at least 2 years without relapse. The median follow-up period was 10.3 years. A linear mixed-effects model showed that the slope of pulmonary function tests values, including percent vital capacity (%VC), percent forced expiratory volume in one second (%FEV1), and FEV1/forced VC ratio (FEV1%), decreased over time. The cumulative incidence of newly obstructive and restrictive lung function impairment (LFI) at 10 years was 15.7% and 19.5%, respectively. Restrictive LFI was a significant, independent risk factor for overall survival (hazard ratio 7.11, P = 0.007) and non-relapse mortality (hazard ratio 12.19, P = 0.003). Our data demonstrated that lung function declined over time after HSCT and that the decline itself had a significant impact on survival regardless of LONIPC.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative treatment for hematological disorders. Advances in HSCT procedures, such as a less toxic conditioning regimen, anti-infection prophylaxis, and graft-versus-host-diseases (GVHD) management, have increased the number of long-term HSCT survivors1,2. If the original disease does not relapse within 2 years after HSCT, 85% of recipients survive for more than 10 years3. However, pulmonary complications are frequently encountered in HSCT and remain a major cause of morbidity and mortality4,5. Late-onset noninfectious pulmonary complications (LONIPC) are especially associated with a significantly poorer prognosis, larger healthcare resource utilization, and increased financial burden6.
Pre-transplant pulmonary dysfunction is linked to development of LONIPC as well as poorer outcomes7,8. Thus, pulmonary function tests (PFT) are performed before HSCT to predict pulmonary complications. Post-transplant PFT has also been shown to be predictive of LONIPC, especially bronchiolitis obliterans (BO)/bronchiolitis obliterans syndrome (BOS)9,10,11. However, because LONIPC rarely occurs beyond 2 years after HSCT12,13, the observational periods of previous studies were relatively short. Moreover, data on long-term pulmonary function transition in HSCT recipients are scarce. Although pulmonary function declines in survivors of childhood, adolescent, and early adulthood HSCT14,15,16, it is undetermined in older patients. Furthermore, the clinical significance of post-transplant pulmonary dysfunction itself in long-term survivors remains unknown. Therefore, this study aimed to investigate pulmonary function following HSCT in terms of its transition and the clinical significance of its decline.
Patients and methods
Patients
Patients who underwent their first HSCT at Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital between July 2004 and July 2012 and survived relapse-free for at least 2 years after HSCT were included3,17. The exclusion criteria were death from any cause, relapse of the underlying disease or requirement of a second HSCT within 2 years of the first HSCT, followed up < 2 years after HSCT, or unavailability of pre- or post-HSCT PFT data. Transplantation procedures have been described in detail elsewhere18,19.
This study was approved by the Institutional Ethics Committee of Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital (approval number 2651) and performed in accordance with the principles of the Declaration of Helsinki.
Pulmonary function tests and pulmonary complications
Pulmonary function was assessed using a multi-functional spirometer (CHESTAC-8800 and CHESTAC-8900; CHEST MI, Inc., Tokyo, Japan) following the guidelines of the Japanese Respiratory Society, which agree with those of the American Thoracic Society and the European Respiratory Society20,21. A PFT was routinely performed 1 month before HSCT and again after HSCT as part of long-term follow-up (LTFU), in accordance with the guideline recommendations22. To account for the effects of aging, vital capacity (VC) and forced expiratory volume in one second (FEV1) were evaluated and expressed as a percentage of the predicted, normal values after matching age, height, and weight to those of healthy individuals. In addition to percent VC (%VC) and percent FEV1% (%FEV1), FEV1/forced VC ratio (FEV1%) was also assessed. A slight decline in pulmonary function was considered to have no clinical impact, especially if the pulmonary function remained within the normal range. Therefore, the clinical impact of lung function impairment (LFI) was analyzed using the standard values23,24; restrictive LFI was defined as %VC < 80% and obstructive LFI was defined as FEV1% < 70%.
BO/BOS was diagnosed based on clinical presentation, while the PFT, and CT findings were interpreted as per the National Institutes of Health consensus25,26. Although histopathological confirmation was not always performed, biopsy-proven BO was included as BO/BOS regardless of the PFT value. Interstitial lung disease (ILD) and organizing pneumonia (OP) were diagnosed using standard criteria27. All LONIPCs, other than BO/BOS were either ILD or OP. Because of the few cases of each event, we treated these events as ILD/OP in the analysis.
Statistical analysis
Linear mixed-effects (LME) model-fitting using the maximum likelihood t test and Satterthwaite’s method, was employed to assess the correlation between repeated measures (change in %VC, %FEV1, and FEV1% from transplantation to the last follow-up) and years after HSCT. The LME model accounts for fixed and random effects and is used especially in regression analysis involving dependent data from multiple observations in each subject28. Thus, this method is effective for investigating PFT values after HSCT because the baseline characteristics and interval between HSCT and PFT differed from patient to patient.
The cumulative incidence of a post-HSCT event (BO/BOS, ILD/OP, obstructive LFI, restrictive LFI, and relapse) was estimated using Gray’s method, considering death and a second HSCT without an event as a competing event29. Patients who had obstructive or restrictive LFI before HSCT were excluded from each analysis. Since there is a pathophysiological correlation between BO/BOS and obstructive LFI, patients with BO/BOS were excluded from causative factor analysis for obstructive LFI. For the same reason, patients with ILD/OP were also excluded from analysis for restrictive LFI. The cumulative incidence of BO/BOS or ILD/OP was estimated in all patients regardless of pre-transplant lung function. Non-relapse mortality (NRM) was defined as death without disease relapse or progression. The Fine–Gray model was used to estimate the hazard risk of these events29. Overall survival (OS) was defined either as the time from the date of the first HSCT until death from any cause and censored on the date the patient was last known to be alive. Data from patients who received a second HSCT were censored on the date of the second HSCT. All events were initiated by the date of HSCT. The final date of observation was April 30, 2021. For survival analysis (OS and NRM), patients who had any LFI before HSCT were excluded. Cox proportional hazards regression analysis was used to examine the relationship between the factors and OS. The results are as a hazard ratio (HR) with a 95% confidence interval (95% CI). The covariates in the univariate and multivariate models included: age (≥ 50 years), sex (female vs. male), refined disease risk index (low/intermediate vs. high/very high)30, hematopoietic cell transplantation comorbidity index (0–2 vs. ≥ 3)31, human leukocyte antigen (HLA) compatibility, donor type, stem cell source, conditioning regimen (myeloablative vs. non-myeloablative)32, total body irradiation (TBI) in conditioning, smoking history (pack-years), acute GVHD, chronic GVHD, BO/BOS, ILD/OP, restrictive LFI, and obstructive LFI. Post-HSCT events (acute GVHD, chronic GVHD, BO/BOS, ILD/OP, restrictive LFI, and obstructive LFI) were treated as time-dependent covariates.
All outcome analyses were performed on the date of HSCT. Statistical significance was set at P < 0.05. Statistical analyses were performed on EZR, a graphical user interface for R, version 4.1.2 (http://www.r-project.org)33.
Results
In total, 201 patients received their first HSCT and survived for at least 2 years without relapse, need for a second HSCT or loss to follow-up. Five patients were excluded owing to unavailability of pre-transplant PFT data and 29 due to unavailability of post-transplant PFT data, leaving 167 eligible patients. Table 1 summarizes the patient and transplant characteristics. The median age at transplant was 40 years (range: 15–71 years), and the median follow-up post-HSCT was 10.3 years (range: 2.2–16.2 years). Eleven patients reported relapse of the underlying disease, and nine of them received a second HSCT. A myeloablative conditioning regimen was employed for 84% of the patients. The most frequent indication for HSCT was acute myeloid leukemia (n = 43; 34%), and the most frequent stem cell source was bone marrow (n = 121; 72%).
A total of 34 LONIPCs were diagnosed in 29 patients. Diagnoses included 17 cases of BO/BOS, 9 of ILD, 3 of OP, 3 of pleuroparenchymal fibroelastosis (PPFE), and 2 cases of air leak syndromes (ALS). PPFE and ALS were observed in patients with ILD. Histological confirmation was performed for seven patients (one with BO, four with ILD, and two with OP).
Changes in pulmonary function tests
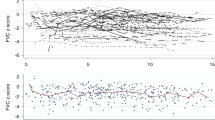
In total, 791 PFTs were performed over the study period. The median, minimum, and maximum number of PFTs per patient after HSCT was 4, 2, and 20, respectively. A total of 29 (17.4%) patients received 2 PFTs. Among these cases, PFT was performed at a median of 3.2 years (range: 0.2–15.7 years). The last PFT was performed at a median of 6.8 years (range: 0.2–15.9 years) post-HSCT. The transition of PFT values is shown in Fig. 1 [(a) %VC, (b) %FEV1, and (c) FEV1%]. During the study period, %VC decreased by > 10% in 58 patients and > 20% in 33 patients. Moreover, %FEV1, decreased by > 10% in 28 patients and > 20% in 12 patients. FEV1% decreased by > 10% in 63 patients and > 20% in 38 patients. Table 2 shows the results of the LME model for %VC, %FEV1, and FEV1%. %VC gradually declined after HSCT (mean slope difference: − 1.16; 95% CI: − 1.63 to − 0.70; P < 0.001). %FEV1 (mean slope difference: − 1.18; 95% CI: − 1.64 to − 0.74; P < 0.001) and FEV1% (mean slope difference: − 0.54; 95% CI: − 0.76 to − 0.32; P < 0.001) also declined.
Lung function impairment
A total of 41 patients developed LFI. Among them, 21 (51.2%) patients did not develop LONIPC during the long follow-up periods. Among the 20 patients who developed both LFI and LONIPC, the interval between these two events was within 1 month in 10 (50.0%) patients. There was more than a 1-year interval in 6 (30.0%) patients.
Obstructive LFI after HSCT developed in 24 patients. The cumulative incidence of obstructive LFI was 7.1% (95% CI: 3.8–11.9%) at 2 years, 15.4% (95% CI: 7.8–18.3%) at 5 years, 15.7% (95% CI: 10.3–22.1%) at 10 years, and 19.6% (95% CI: 11.1–29.8%) at 15 years (Fig. 2a). The median time to onset of obstructive LFI was 2.5 years (range: 0.2–14.6 years). During the study period, LFI resolved in only two patients. After excluding patients with BO/BOS, 13 patients developed obstructive LFI after HSCT. Of these, two symptomatic patients received inhaled drugs for chronic obstructive pulmonary disease (COPD). The cumulative incidence of obstructive LFI without BO/BOS was 0% (95% CI: 0–0%) at 2 years, 3.0% (95% CI: 1.0–7.0%) at 5 years, 10.6% (95% CI: 5.7–17.2%) at 10 years, and 35.6% (95% CI: 19.2–52.5%) at 15 years (Fig. 2b). The median time to onset of obstructive LFI without BO/BOS was 2.9 years (range: 0.2–14.6 years). Multivariate analysis identified HLA compatibility (HR: 4.74; 95% CI: 1.41–15.93; P = 0.01) and TBI in conditioning (HR: 0.23; 95% CI: 0.07–0.71; P = 0.01) as significantly associated with obstructive LFI (Supplementary Table 1).
Post-HSCT, 31 patients developed restrictive LFIs, which had a median time to onset of 2.6 years (range: 0.2–13.9 years) and a cumulative incidence of 7.4% (95% CI: 4.0–12.1%) at 2 years, 14.3% (95% CI: 9.4–20.2%) at 5 years, 19.5% (95% CI: 13.6–26.2%) at 10 years, and 22.7% (95% CI: 14.6–31.8%) at 15 years (Fig. 3a). Causal factors at the time of PFT were two of postoperative changes, one of non-tuberculosis mycobacterial lung disease, pericardial and pleural effusion due to serositis-type GVHD, lung cancer, and vertebral compression fracture. Six patients with restrictive LFI recovered. Of these, four (three with ILD and one with chronic GVHD) recovered after treatment. The remaining two patients recovered without any treatment. Ten and nine patients with restrictive LFI also had BO/BOS and ILD/OP, respectively. The cumulative incidence of restrictive LFI without ILD/OP was 3.4% (95% CI: 1.3–7.2%) at 2 years, 9.6% (95% CI: 5.5–15.1%) at 5 years, 14.5% (95% CI: 9.2–21.0%) at 10 years, and 18.0% (95% CI: 10.1–27.7%) at 15 years (Fig. 3b). The median time to onset of restrictive LFI without ILD/OP was 3.5 years (range: 0.2–13.9 years). Supplementary Table 2 shows that extensive chronic GVHD (HR: 3.46; 95% CI: 1.49–8.01; P = 0.004) and BO/BOS (HR: 16.32; 95% CI: 4.51–59.05; P < 0.001) were risk factors significantly associated with restrictive LFI in univariate analysis. In multivariate analysis, however, BO/BOS (HR 15.38, 95% CI: 4.35–54.46, P < 0.001) was the sole, independent risk factor for restrictive LFI.
Overall survival, non-relapse mortality and cause of death
Survival analysis was performed for the remaining 152 patients after excluding patients with any LFI before HSCT. Significant risk factors against OS identified through univariate analysis were stem cell source; peripheral blood (vs. bone marrow) (HR: 2.78; 95% CI: 1.11–6.95; P = 0.03), extensive chronic GVHD (HR: 3.05; 95% CI: 1.29–7.24; P = 0.01), BO/BOS (HR: 5.65; 95% CI: 2.06–15.5; P < 0.001), ILD/OP (HR: 3.28; 95% CI: 1.10–9.78; P = 0.03), obstructive LFI (HR: 2.93; 95% CI: 1.13–7.58; P = 0.03), and restrictive LFI (HR: 8.68; 95% CI: 3.63–20.74; P < 0.001) (Table 3). On multivariate analysis, restrictive LFI was a significant risk factor for OS (HR: 7.11; 95% CI: 1.71–29.49; P = 0.007). Obstructive LFI (HR: 3.16; 95% CI: 1.13–8.79; P = 0.03) and restrictive LFI (HR: 11.81; 95% CI: 4.39–31.77; P < 0.001) were also significant risk factors for NRM in univariate analysis (Table 4). Multivariate analysis identified restrictive LFI (HR: 12.19; 95% CI: 2.34–63.46; P = 0.003) as a significant risk factor for NRM. We also performed survival analysis for patients excluding BO/BOS and ILD/OP (Supplementary Tables 3 and 4). Restrictive LFI was a significant risk factor for OS (HR: 5.61; 95% CI: 1.47–21.42; P = 0.01) and NRM (HR: 7.66; 95% CI: 2.08–28.20; P = 0.002).
Causes of death in this study were a secondary malignancy (n = 6), BO (n = 2), heart failure (n = 2), underlying disease (n = 1), chronic lung GVHD (n = 1), renal failure (n = 1), infectious pneumonia (n = 1), interstitial pneumonia (n = 1), acute pancreatitis (n = 1), multiple sclerosis (n = 1), infectious peritonitis (n = 1), car accidents (n = 1), sudden death (n = 1), and suicide (n = 1). Among these patients, 12 (57.1%) developed restrictive LFI post-HSCT. The median interval from the development of restrictive LFI to death was 3.5 years (range: 0.1–9.2 years). Patients with restrictive LFI were less likely to die from a second malignancy than patients without restrictive LFI (8.3% vs. 44.4%).
Discussion
This study confirmed that post-HSCT pulmonary function declined over time, consistent with findings from studies on younger HSCT recipients14,16. As pulmonary function declines post-HSCT, the proportion of patients who develop obstructive and restrictive LFI increases, resulting in a cumulative incidence of 19.6% and 22.7% at 15 years, respectively. Generally, the incidence of LONIPC is thought to be higher in peripheral blood stem cell transplantation12. Although the majority of this study included bone marrow transplantation, not a few patients developed LFI. Especially, the cumulative incidence of obstructive LFI without BO/BOS showed steep increase from 10 to 15 years after HSCT. Since most LONIPC occur within 2 years after HSCT12,13, this finding suggests that LFI, at least in part, is another entity and long-term continuous PFT is warranted.
Because advanced BO/BOS is usually irreversible and is associated with a high mortality rate, early detection of high-risk patients may allow enhanced monitoring and pre-emptive or prompt therapy before significant lung dysfunction occurs. As up to 20% of patients with BO/BOS remain asymptomatic at diagnosis34, PFT is a very useful screening tool for early diagnosis10,11. Accordingly, the efficacy of PFT has been studied and proven mainly in the early after HSCT. However, our long-term follow-up study did reveal that some patients developed irreversible airway obstruction after HSCT regardless of BO/BOS. Clinically, they are usually considered to develop COPD; however, only three patients had 20 or higher pack-years, and six patients were never smokers. In the present study, HLA mismatch rather than smoking history, were significant predictive factors of obstructive LFI after HSCT. COPD is a systemic disease, and lung injury may be a part of a global, vascular process that damages other organs35,36. Martinez et al. suggested that the hematopoietic and immune systems are crucial to COPD progression37. Thus, we speculate that HSCT, along with miscellaneous complications, induces lung injury and airway obstruction which may, at least in part, be exacerbated by immune reactions.
Only BO/BOS was found to be an independent risk factor for restrictive LFI. Although a restrictive spirometric pattern can be caused by increased residual volume, in this study it was observed in only three patients, including one patient with OP and, therefore, cannot have a causal role in all cases of restrictive LFI. Moreover, it is possible that a mix of restrictive and obstructive lung processes may occur in BO/BOS38. Restrictive lung impairment is caused by multiple factors, including intrinsic (caused by lung parenchymal disorders) and/or extrinsic (caused by extraparenchymal disorders) factors. Although the pathogenesis of restrictive LFIs in patients with HSCT have been thought to center on ILD/OP39, they are more heterogeneous. This may account for the fact that LFI more frequently resolved in patients with restrictive rather than obstructive LFI. Causal factors can be readily detected if there is a single, apparent factor such as postoperative change, pleural effusion, and fracture. However, statistical analysis cannot always include all factors. If multiple, complex factors are present, many patients are needed for accurate statistical analysis. Indeed, factors that are causally linked to a spirometric restriction pattern often remain indeterminate owing to their complexity8,12,24. Palmar et al. also studied restrictive ling disease; however, their data are from patients with cGVHD, and their factors and prognostic impact have not been fully analyzed40. Future studies with a larger patient cohort are necessary to more accurately characterize the relationship between restrictive LFI and its causal factors.
Patients with restrictive LFI are at an increased risk of mortality from various causes, most commonly those other than an underlying disease or secondary malignancy. Of note, LONIPC was not the main cause of death. In this study, patients who developed LONIPC and died within 2 years were excluded. This finding suggests that the unfavorable prognostic impact of restrictive LFI cannot be explained by LONIPC only. We were unable to determine the reason for this, however, numerous population-based studies have reported that a restrictive spirometric pattern is associated with morbidity such as poor physical performance and cognitive impairment41,42, as well as all-cause mortality23,43,44. This high mortality rate may be caused not only by respiratory, but also by a systemic dysfunction. Pleural or pericardial effusion, which is caused by organ failure or severe serositis-type GVHD, could display a restrictive PFT pattern. Anorexia or steroid use also cause restrictive PFT pattern through respiratory muscle weakness in the long run. One large retrospective study of HSCT recipients (n = 2545) revealed that the presence of pre-transplant pulmonary restriction was significantly associated with NRM8. The authors speculated that this was partially caused by respiratory muscle weakness. Generally, frailty, including systemic muscle weakness seems to worsen after HSCT45. Therefore, restrictive LFI may not be the direct cause of death, but may reflect organ dysfunction or frailty.
Owing to its retrospective nature, our study has some limitations. First, although most patients who visited LTFU routinely received a PFT, several patients did not especially if they appeared to be healthy or dropped out of the LTFU. In addition, the number of PFT was decreased in 2020–2021 because of the coronavirus disease 2019 pandemic. These could affect the LME model analysis. Second, there was paucity of data on lung function after bronchodilator use. Third, our study included patients who survived for 2 years without relapse. In addition, our patient population was up to 2012 to ensure a long observational period. Thus, haplo-identical transplantation was not included, and mainly cases with bone marrow transplantation were assessed. Although this was favorable for investigating the transition of pulmonary function, it may have affected the survival analysis. Finally, LONIPC and LFI sometimes overlap each other and could complicate interpreting the analysis. As the sample size of this study is small, we could not perform multivariate analysis for patients without LONIPC to confirm the results. However, LFI and LONIPC often do not occur at the same time. In addition, they need LTFU periods to determine whether these overlap or not. Therefore, the multivariate analysis considering these events as time-dependent covariates together could be in line with the clinical setting. Owing to these limitations, our conclusion needs validation in a larger and prospective cohort.
In conclusion, this study confirmed that pulmonary function declined over time after HSCT and highlighted the clinical impact of newly developed LFI, regardless of the presence of LONIPC. Further, large-scale studies are warranted for establishing an appropriate treatment strategy.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Gooley, T. A. et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 363, 2091–2101 (2010).
Hahn, T. et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J. Clin. Oncol. 31, 2437–2449 (2013).
Wingard, J. R. et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 29, 2230–2239 (2011).
Patel, S. S. et al. Noninfectious pulmonary toxicity after allogeneic hematopoietic cell transplantation. Transplant. Cell. Ther. 28, 310–320 (2022).
Wilhelmsson, M. et al. Adverse health events and late mortality after pediatric allogeneic hematopoietic SCT-two decades of longitudinal follow-up. Bone Marrow Transplant. 50, 850–857 (2015).
Sacks, N. C. et al. The economic burden of NIPC and BOS following allogeneic HSCT in patients with commercial insurance in the United States. Blood Adv. 6, 1566–1576 (2022).
Duque-Afonso, J. et al. Impact of lung function on bronchiolitis obliterans syndrome and outcome after allogeneic hematopoietic cell transplantation with reduced-intensity conditioning. Biol. Blood Marrow Transplant. 24, 2277–2284 (2018).
Ramirez-Sarmiento, A., Orozco-Levi, M., Walter, E. C., Au, M. A. & Chien, J. W. Influence of pretransplantation restrictive lung disease on allogeneic hematopoietic cell transplantation outcomes. Biol. Blood Marrow Transplant. 16, 199–206 (2010).
Thompson, P. A. et al. Screening with spirometry is a useful predictor of later development of noninfectious pulmonary syndromes in patients undergoing allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 20, 781–786 (2014).
Abedin, S. et al. Predictive value of bronchiolitis obliterans syndrome stage 0p in chronic graft-versus-host disease of the lung. Biol. Blood Marrow Transplant. 21, 1127–1131 (2015).
Jamani, K. et al. Early post-transplantation spirometry is associated with the development of bronchiolitis obliterans Syndrome after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 26, 943–948 (2020).
Bergeron, A. et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur. Respir. J. 51, 1702617 (2018).
Astashchanka, A. et al. Pulmonary complications in hematopoietic stem cell transplant recipients—A clinician primer. J. Clin. Med. 10, 3227 (2021).
Inaba, H. et al. Pulmonary dysfunction in survivors of childhood hematologic malignancies after allogeneic hematopoietic stem cell transplantation. Cancer 116, 2020–2030 (2010).
L’Excellent, S., Yakouben, K., Delclaux, C., Dalle, J. H. & Houdouin, V. Lung evaluation in 10 year survivors of pediatric allogeneic hematopoietic stem cell transplantation. Eur. J. Pediatr. 178, 1833–1839 (2019).
Myrdal, O. H. et al. Late-onset, noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation: A nationwide cohort study of long-term survivors. Respiration 101, 544–552 (2022).
Atsuta, Y. et al. Late mortality and causes of death among long-term survivors after allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 22, 1702–1709 (2016).
Shingai, N. et al. Urinary liver-type fatty acid-binding protein linked with increased risk of acute kidney injury after allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 20, 2010–2014 (2014).
Kurosawa, S. et al. Bone turnover markers as an aid to monitor osteoporosis following allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 99, 1873–1882 (2020).
Tojo, N., Suga, H. & Kambe, M. Lung function testing—The Official Guideline of the Japanese Respiratory Society. Rinsho Byori 53, 77–81 (2005).
Kawabata, R. et al. Relationships between body composition and pulmonary function in a community-dwelling population in Japan. PLoS One 15, e0242308 (2020).
Majhail, N. S. et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 18, 348–371 (2012).
Kubota, M. et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir. Investig. 52, 242–250 (2014).
Guerra, S. et al. Morbidity and mortality associated with the restrictive spirometric pattern: A longitudinal study. Thorax 65, 499–504 (2010).
Filipovich, A. H. et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 11, 945–956 (2005).
Jagasia, M. H. et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol. Blood Marrow Transplant. 21, 389-401.e1 (2015).
Schlemmer, F. et al. Late-onset noninfectious interstitial lung disease after allogeneic hematopoietic stem cell transplantation. Respir. Med. 108, 1525–1533 (2014).
Kinoshita, Y. et al. Clinical outcomes in donors and recipients of kidney transplantations involving medically complex living donors—A retrospective study. Transpl. Int. 33, 1417–1423 (2020).
Fine, J. P. & Gray, R. J. A Proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509 (1999).
Armand, P. et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood 123, 3664–3671 (2014).
Sorror, M. L. et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 106, 2912–2919 (2005).
Giralt, S. et al. Reduced-intensity conditioning regimen workshop: Defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol. Blood Marrow Transplant. 15, 367–369 (2009).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Soubani, A. O. & Uberti, J. P. Bronchiolitis obliterans following haematopoietic stem cell transplantation. Eur. Respir. J. 29, 1007–1019 (2007).
Roversi, S., Fabbri, L. M., Sin, D. D., Hawkins, N. M. & Agustí, A. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am. J. Respir. Crit. Care Med. 194, 1319–1336 (2016).
Polverino, F. et al. A pilot study linking endothelial injury in lungs and kidneys in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 195, 1464–1476 (2017).
Martinez, F. J. et al. At the root: Defining and halting progression of early chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 197, 1540–1551 (2018).
Kitko, C. L. et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: IIa. The 2020 clinical implementation and early diagnosis working group report. Transplant. Cell Ther. 27, 545–557 (2021).
Yanik, G. & Cooke, K. R. The lung as a target organ of graft-versus-host disease. Semin. Hematol. 43, 42–52 (2006).
Palmer, J. et al. Pulmonary symptoms measured by the national institutes of health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 20, 337–344 (2014).
Mannino, D. M., Ford, E. S. & Redd, S. C. Obstructive and restrictive lung disease and functional limitation: Data from the Third National Health and Nutrition Examination. J. Intern. Med. 254, 540–547 (2003).
Scarlata, S. et al. Restrictive pulmonary dysfunction at spirometry and mortality in the elderly. Respir. Med. 102, 1349–1354 (2008).
Mannino, D. M., Buist, A. S., Petty, T. L., Enright, P. L. & Redd, S. C. Lung function and mortality in the United States: Data from the First National Health and Nutrition Examination Survey follow up study. Thorax 58, 388–393 (2003).
Mannino, D. M., Doherty, D. E. & Buist, A. S. Global initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir. Med. 100, 115–122 (2006).
Arora, M. et al. Longitudinal trajectory of frailty in blood or marrow transplant survivors: Report from the Blood or Marrow Transplant Survivor Study. Cancer 127, 794–800 (2021).
Acknowledgements
We thank the nursing staff in the LTFU program, medical staff in Physiological Examination Laboratory, and Dr. Makoto Saito in the clinical research support center, for their valuable contributions to the study.
Author information
Authors and Affiliations
Contributions
Y.K. and N.S. designed the study, interpreted the data analysis and drafted the manuscript. N.S. and K.H. conducted statistical analysis. Y.K., N.S., M.Y., C.K., S.S., Y.K., Y.A., R.K., A.W., D.M., S.N., Y.U., D.O., A.H., A.N., T.T., H.S., Y.N., T.K., H.S., K.O., and N.D. were involved in treatment of the patients and commented on the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kishida, Y., Shingai, N., Hara, K. et al. Impact of lung function impairment after allogeneic hematopoietic stem cell transplantation. Sci Rep 12, 14155 (2022). https://doi.org/10.1038/s41598-022-18553-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18553-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.