Abstract
In this study, aged biochar (CCB350 and CCB650) were obtained from pyrolysis of corn stalk biochar (CB350 and CB650) at the degree of 350 °C and 650 °C by artificial oxidation with hydrogen peroxide (H2O2). Also, the mechanism of Pb2+ and Cd2+ on fresh and aged biochars was analyzed qualitatively and quantitatively by batch adsorption experiments combined with characterization. The adsorption isotherm results showed that aging treatment decreased the adsorption capacity of Pb2+ and Cd2+ and inhibited the competitive adsorption behavior of heavy metals. In the single-metal system, precipitation and cation exchange were considered as the main adsorption mechanisms for CB350 and CB650, with a ratio of 40.07–48.23% and 38.04–57.19%, respectively. Competition between Pb2+ and Cd2+ increased the relative contribution of mineral precipitation, but decreased the contribution of cation exchange mechanism. Aging resulted in the rise of the contribution of surface complexation to the adsorption of Pb2+ and Cd2+ on biochars, especially in low-temperature biochars, but weakened the contribution of mineral precipitation to the adsorption. Further, the contribution of other adsorption mechanisms was significantly enhanced for high-temperature aged biochars. These results are important to evaluate its long-term application prospects in the natural environment.
Similar content being viewed by others
Introduction
The problem of compound pollution of heavy metals in soil is a matter of widespread concern, which threatens both the environment and human health1,2. Cadmium and lead are elements that occur widely in contaminated soils and they are also classified as priority pollutants in many countries3,4. The combined contamination of the two heavy metals produces greater ecotoxicity than either one of them5. Therefore, it is important to explore suitable technologies to remediate the metals polluted soil. However, the interaction of multiple heavy metals limits the effective application of many remediation techniques6. Biochar is considered an eco-friendly, reliable, and low-cost adsorbent, which is widely used in the remediation of contaminated soil7,8,9,10,11,12. There are many resources for corn straw in northern China, but the harmless treatment of corn straw has always been a difficult problem to solve. Therefore, biochars produced from corn straw can contribute to environmental cleanup practices and waste resource recovery efforts.
Heavy metal adsorption depends not only on biochar characteristics but also on the competitive behaviors of the metals. Most previous studies have mainly focused on changes in competing sorption capacity and selective sequences of heavy metals using different biochars. For instance, Ni et al.13 evaluated the competing sorption by biochar from anaerobically digested sludge in an aqueous solution. Abdin et al.14 concentrated on the removal of the single and competing metals (Cd2+, Pb2+, Zn2+ and Cu2+) from aqueous solutions by mesocosms and fish bone biochar. Han et al.15 studied Pb2+ adsorption by three typical types of biochars (cattle manures, rice husks, and bamboo biochars) under the elevated competition of Cd2+ and Al3+. These studies illustrate that Pb2+ has a higher affinity than Cd2+, thus showing a better competitive advantage in the coexistence system. However, the relationship between competition and the adsorption mechanism of Cd2+ and Pb2+ has not attracted much attention.
The adsorption mechanism determines the removal rate of these metals. Therefore, studying on competitive adsorption mechanism of heavy metals plays a significant role16. The mechanisms of heavy metals adsorbed on biochars under single metal adsorption conditions involve precipitation or co-precipitation17, ion exchange18, complexation19,20, cation-π interaction21,22, and physical adsorption23. Although previous studies have investigated the dominant mechanisms of the metals adsorbed on biochars in a single-metal solution system, relevant information about the impact of competition on the change in the main adsorption mechanism is limited, especially those quantifying the effects.
Biochar properties are stable in the short term, but they may undergo a long-term changing process when added into soil, that is, aging10,11. Various environmental factors, such as freeze–thaw cycles24, wetting–drying cycles25, photochemical degradation26, and mild oxidation27 will lead to significant impact on the biochar, including its pH value, specific surface area (SSA), cation exchange capacity, oxygen-containing functional groups, elemental composition, ash content, and surface morphology28,29,30,31,32. However, it is very difficult to really learn about the features of biochar during the long natural aging process. Therefore, various artificial aging methods are often used to predict changes in the physiochemical properties and adsorption capacity of biochar33. The application of hydrogen peroxide (H2O2) can simulate the aging process of natural oxidation of biochar34. The competitive adsorption mechanism of Cd2+ and Pb2+ largely depend on biochars’ properties and the adsorbed solution, such as biochar surface characteristics, solution pH, and concentration of ions in solution, which are affected by aging process. However, qualitative and quantitative studies on the competitive adsorption mechanisms of Cd2+ and Pb2+ on aged biochar are not yet extensive and in-depth. Therefore, we assumed that the proportion of mechanisms for Cd2+ and Pb2+ adsorbed on biochars varied in co-existed adsorption conditions and biochar aging affected the contribution of adsorption mechanisms to heavy metals.
In this study, biochars were obtained by pyrolysis of corn straw at 350 °C and 650 °C and simulated aging process with H2O2, and the surface properties of fresh and aged biochar were characterized. Moreover, the Cd2+ and Pb2+ for kinetics and isotherms on fresh biochar (CB350 and CB650) and aged biochar (CCB350 and CCB650) were examined. In short, this paper has the following objectives. First, the effect of biochar aging on adsorption characteristics of Cd2+ and Pb2+ in single and binary adsorption conditions was clarified. Secondly, the effects of aging on the quantitative distributions of Cd2+ and Pb2+ adsorption mechanisms in single and binary conditions were evaluated.
Materials and methods
Preparation of biochars
Samples of corn straw used to produce biochar were collected from Baotou, Inner Mongolia, China. The preparation method for fresh biochar (CB350 and CB650) is provided in the supplementary materials.
Aged boichars were prepared as described by Nie et al.35. Briefly, the fresh biochars (10 g) and 20% H2O2 (200 mL) were mixed with the solid–liquid ratio of 1:20 (w/v) in a plastic bottle of 250 mL, and the samples were presented in a reciprocating shaker at 24 h room temperature. In the process of oxidation, the bottles were opened repeatedly to release air. After aging, the samples were filtered by centrifugation and rinsed several times. And the residual H2O2 was removed with distilled water. The aged biochars were dried at 80 °C and labeled as CCB350 and CCB650, respectively. The surface characteristics of the biochars were also analyzed using SEM–EDS, FTIR, XPS, XRD, and BET. The ash content, pH, surface zeta potential and total C, H, O, N and S contents of biochars were measured. The details were described in the supplementary materials.
Batch adsorption experiments
A certain amount of Cd(NO3)2·4H2O and Pb(NO3)2 were dissolved in the background solution (0.01 mol/L NaNO3) to prepare Cd2+ (1000 mg/L) and Pb2+ (1000 mg/L).
0.1 g biochar (CB350, CB650, CCB350 and CCB650) was mixed with 50 mL Cd2+ or Pb2+ solution for adsorption isotherm experiment. The mass ratio of Cd2+ to Pb2+ was chosen as 1:1 in the binary system. The initial pH of the solution was adjusted to 5.0 ± 0.1 by 0.1 mol/L NaOH or 0.1 mol/L HNO3. This pH ensured the maximum adsorption capacity of biochar while avoiding the effect of Pb2+ or Cd2+ adsorption due to precipitation. We added 0.01 mol/L of NaNO3 to all the solutions as background electrolyte. The concentration of Cd2+ and Pb2+ in both the single and binary metal systems range from 0 to 200 mg/L. The biochar and different concentrations of Cd2+ or Pb2+ solutions were then shaken in an HY-4 speed-controlled multipurpose shaker (Jintan Ronghua Instrument Manufacturing Company, Jinhua, China) at 200 rpm and 25 ± 1 °C for 2880 min.
As for adsorption kinetics, 0.1 g of biochars (CB350, CB650, CCB350, and CCB650) were mixed with 50 mL of 100 mg/L for single Cd2+, single Pb2+ and binary Cd2+ and Pb2+ at room temperature, and shaken at 200 rpm for 2880 min.
Finally, the suspension was centrifuged at 4000 rpm for 20 min and filtered by a 0.45 μm microporous filter. The concentrations of Cd2+ and Pb2+ in the filtrate were determined by flame atomic absorption spectrophotometry (Ice 3500, Thermo Fisher Scientific, USA). The adsorption amount of heavy metals on biochar (Qe) was calculated as follows:
where Qe (mg/g) is the adsorption capacity, C0 (mg/L) and Ce (mg/L) are the initial and equilibrium concentration, respectively. V (L) is the volume of mixed solution, and m (g) is the dry mass of biochar.
The adsorption isotherms were simulated using Langmuir, Freundlich and Langmuir–Freundlich models, and the adsorption kinetics were fitted using Lagergren’s pseudo-first-order (PFO), pseudo-second-order (PSO) and intra-particle diffusion (IPD) kinetic equations (see Supplementary Material).
Inherent minerals were removed by rinsing with 1.0 mol/L HCl to obtain demineralized biochars. And the untreated and demineralized biochars were mixed with solutions of Pb2+ and Cd2+ 200 mg/L. After adsorption, the solution concentration was measured. The fresh biochar was then mixed with 0.01 mol/L NaNO3 (blank) and metal salt solution, respectively. The contribution of cation exchange can be estimated by the difference of K+, Ca2+, Na+ and Mg2+ cations released into the solution after biochar adsorption of heavy metals and the number of cations released in the blank solution. To determine the role of complexation mechanisms, the difference of pH were calculated before and after adsorption. The reduced adsorption capacities of Cd2+ and Pb2+ on the biochars before and after demineralization attributed to the mineral precipitation. Flame atomic absorption spectroscopy was used to analyze the Na+, Mg2+, K+, Ca2+, Cd2+ and Pb2+ concentrations in the filtrates. Biochars carried with and without Cd2+, Pb2+ and the Cd2+/Pb2+ were accumulated for the measurements of SEM–EDS, XRD, and FTIR.
Quantitative contribution of adsorption mechanisms
The adsorption capacities of the fresh and aged biochars for Cd2+, Pb2+ and the Cd2+/Pb2+ were attributed to the following four fractions: (1) complexation between surface oxygen-containing functional groups (Qcom), (2) mineral co-precipitation (Qpre), (3) cation exchange (Qce), and (4) other potential mechanisms (Qoth)36. The adsorption capacity of each adsorption mechanism was calculated as follows:
-
1.
Complexation of oxygen functional groups (Qcom): Due to the adsorbing Cd2+ and Pb2+ by demineralized biochar, the carboxyl and hydroxyl groups of the biochar can release H+ through ion exchange, which in turn resulting in the changing of the pH. Therefore, the drop in pH was used as an indicator of H+ release and the amount of Cd2+ or Pb2+ adsorbed due to Qcom37.
-
2.
Metal adsorption resulting from cation exchange (Qce): The release amount of Na+, Mg2+, K+, Ca2+ was calculated by the net release amount of them by the comparison after biochar adsorption38 (Eq. (2)):
$$ Q_{{{\text{ce}}}} = Q_{{\text{K}}} + Q_{{{\text{Na}}}} + Q_{{{\text{Mg}}}} + Q_{{{\text{Ca}}}} $$(2)They are the net release amounts of K+, Ca2+, Na+ and Mg2+ during adsorption process, respectively.
-
3.
Mineral co-precipitation (Qpre): The demineralized biochar obtained after acid washing does not cause vital changing of the content of surface oxygen functional groups on its surface39. The adsorption capacity generated by mineral precipitation can therefore be measured as the difference of Cd2+ or Pb2+ by virgin biochar (fresh and aged biochar) and demineralized biochar. In turn, the adsorption due to mineral components was divided into ion exchange (Qce) and precipitation (Qpre). Therefore, it could be calculated as follows40 (Eqs. (3) and (4)):
$$ Q_{{{\text{pre}}}} = \, Q_{{\text{m}}} {-} \, Q_{{{\text{ce}}}} $$(3)$$ Q_{{\text{m}}} = \, Q_{{\text{t}}} {-} \, Q_{{\text{d}}} $$(4)Qm (mg/g) is the amount of Cd2+ or Pb2+ absorbed by biochar in the interaction with minerals, Qt (mg/g) is the total amount of metal absorbed by biochar, and Qd (mg/g) is the amount of Cd2+ as well as Pb2+ absorbed by demineralized biochar.
-
4.
Other mechanisms (Qoth): Except for the above mechanisms of adsorption, other mechanisms (e.g., cation-π interaction, electrostatic gravitational force, and physical adsorption) cannot be neglected as well. Therefore, the Qoth can be obtained as follows41 (Eq. (5)):
$$ Q_{{{\text{oth}}}} = \, Q_{{\text{d}}} {-}Q_{{{\text{com}}}} $$(5)
The contribution ratios of other mechanisms to the adsorption of metal are described below. The ratios of Qcom/Qt, Qce/Qt, Qpre/Qt, and Qoth/Qt can define the contribution percentages of different mechanisms to metal adsorption.
Statistical analysis
The experimental results were an average obtained from three adsorption experiments. According to Origin 9.0, we can obtain adsorption kinetics and isotherm data for Cd2+ and Pb2+ (Origin Lab, USA). In addition, mineral components of aged and fresh biochars were analyzed using Jade 6.5.
Ethical approval
This study did not involve human or animal research and did not require ethical approval.
Results and discussion
Changes in biochar properties with aging
Table S1 shows the main physicochemical properties of fresh and old biochar. As the pyrolysis temperature increased, for all the biochars, the pH and ash content also increased. The biomass material were mainly the evaporation of water and the volatilization of low boiling point substances at a low temperature. The high boiling point materials in biochars volatilized and inorganic mineral components increased with the temperature rising. As a result, the pH and ash of biochars were higher at higher pyrolysis temperatures42. After the chemical aging treatment, the pH and ash content showed a downward trend, especially for CCB350, which even became acidic. Oxidation reaction of hydrogen peroxide on the biochar surface may cause a rise in the functional groups of acidic oxygen, leading to a fall in the pH of the biochars23.
The SSA and SEM images are illustrated in Table S1 and Fig. S1. The SSA of both fresh and aged biochar increased significantly with the pyrolysis temperature. The SSA of CB650 and CCB650 increased by 99.23% and 99.08%, respectively, compared with CB350 and CCB350, respectively. The increase of temperature removed more volatile matter, which led to the formation of many micropores and even nanostructures, and thus increased the biochar surface area43. SEM results showed that CB350 and CB650 exhibited a porous fibrous structure, and the pore structure of the biochar became smoother and clearer after ageing treatment. Furthermore, the SSA of CB350 and CB650 also increased by 43.59% and 32.12% after chemical oxidation, which could be illustrated by the finding that the oxidation process was usually accompanied by a more intense decomposition and consumption of organic matter, which led to the expansion of biochar pores and the creation of more pore structures44. Further, the reduction of impurities washed away by the water bath heating process led the pore structures to become smoother45, which was confirmed by the results of SEM images. The similar finding was evaluated by Mia et al.46, believing that the SSA of biochar rose after the oxidation of hydrogen peroxide.
With the exception of CB650, when the pyrolysis temperature was increased, the C content of the biochar rose, while the O, H and N content were on the decrease. The contents of O were higher for the aged biochars than the fresh biochars, but the contents of C were lower. The changing of C and O contents indicated that aging may decompose unstable aliphatic carbon in biochar and introduce more oxygen functional groups, resulting in a fall in C and an obvious rise in O47. For CB650, the C content increased by 7.32% after chemical oxidation treatment, which may be due to the mass loss caused by the activation of own oxygen and ash outweighing the oxidation loss of more resistant matrix carbon48. In general, O/C, H/C and (O + N)/C are used as evaluation indexes of hydrophilicity, aromatics, and polarity of biochar, respectively49. After chemical oxidation, the O/C values of CB350 and CB650 increased by 86.27% and 38.89%, respectively, indicating that chemical oxidation enhanced the hydrophilicity of biochar. At the same temperature, the low (O + N)/C meant that there were relatively fewer polar functional groups on the surface of fresh biochar.
Surface functional groups and crystalline minerals in the biochar were characterized by FTIR and XRD, as shown in Fig. S2. FTIR results showed that CCB350 had stronger intensity of bands at 3414 cm−1 and 1690 cm−1 compared to CB350, indicating that CCB350 exhibited a higher abundance of –OH and –COOH. CCB650 showed a more significant enhancement of the C–O–C stretching vibration peak at 1021 cm−1 than CB650. In summary, the aging treatment induced more O-containing functional groups. The XRD pattern showed that the main crystalline form on the surface of fresh biochar was KCl, and the KCl peak weakened or even disappeared after chemical aging, probably because of the high solubility of KCl in water.
In order to analyze the effects of aging treatments on the oxygen-containing functional components on the surfaces of biochar, XPS analysis was further performed. The fitted spectra of fresh and aged biochars are shown in Fig. S3. The main C1s peaks of biochars were divided into four peaks: C–C, C–O, C=O, and O=C–O19. In fresh biochars, the content of C–C increased from 59.04 to 70.24% with the increase of pyrolytic temperature. This indicated that the increase of pyrolytic temperature could reduce the content of oxygen-containing functional groups on the surface of biochar. After chemical oxidation aging, the C-O contents of CB350 and CB650 increased by 0.32% and 5.02%, respectively. The C=O contents of CB350 and CB650 increased from 6.24% and 14.62% to 14.91% and 17.47%, respectively. Overall, biochars exhibited an increasing trend for the oxygenated functional groups.
Effects of aging on the adsorption isotherms of Pb2+ and Cd2+ by biochars
The adsorption isotherms of Pb2+ and Cd2+ on fresh and aged biochars are shown in Fig. 1. The adsorption abilities of them on all biochars increased rapidly with increasing heavy metal concentrations at the initial stage. After that, it inclined to increase slowly and gradually saturate, except for the Pb2+ adsorption isotherms on CB650, indicating that the surface absorbing sites on CB650 were not totally covered by Pb2+. In the binary metal system, the presence of coexisting ions significantly altered the adsorption isotherms of them. The adsorption isotherms in binary metal systems had a shorter initial linear part and could reach equilibrium at lower concentrations. Therefore, this indicated that the presence of competitive ions achieved the effect of completely occupying the adsorption sites of biochar50. Aging treatment also had an effect on the sorption isotherms of Pb2+ and Cd2+. The adsorption capacity of aged biochar to heavy metals was lower than that of fresh biochar.
The adsorption isotherms were suitable for the Langmuire, Freundlich and Langmuir–Freundlich models in both metal systems. The fitted parameter values are shown in Table S2. All fresh biochar adsorption isotherms of Pb2+ and Cd2+ were better fitted by the Langmuire model (R2 = 0.9490–0.9822). Most of the adsorption isotherms for the aged biochars were better fitted by Langmuir–Freundlich model, which suggested that the adsorption of metal ions on biochar after aging transformed from uniform adsorption to heterogeneous surface monolayer adsorption23. That is, it was close to the Freundlich isotherm at low concentrations of metal ions, while it described monolayer adsorption in a similar way to the Langmuir isotherm at high concentrations [51]. The Qm of CB350 and CB650 for Pb2+ absorption using the Langmuir model were 84.91 mg/g and 149.98 mg/g, better than that of Cd2+ on CB350 and CB650 (35.66 mg/g and 65.43 mg/g, respectively). Competition affected the adsorption on biochars, the Qm values of Cd2+ on CB350, CCB350, CB650, and CCB650 decreased by 49.99%, 43.97%, 62.11%, and 20.66%, respectively. The Qm values of Pb2+ on CB350, CCB350, CB650, and CCB650 decreased by 42.45%, 20.73%, 25.91%, and 18.33%, respectively. The decrease in Qm values was greater for Cd2+ adsorption than for Pb2+ under competitive conditions. Therefore, compared to Cd2+, Pb2+ was more competitive.
Moreover, the ratio of a*(Qe of Pb2+ or Cd2+ in coexisting systems)/a (Qe of Pb2+ or Cd2+ in single metal system) could also be used to indicate the degree of reduction in the adsorption capacity of Pb2+ or Cd2+ because of the competition for sites of adsorption in coexisting systems52,53. We evaluated the a*(Pb2+)/a (Pb2+) ratios for CB350, CCB350, CB650, and CCB650 were 0.54, 0.75, 0.95, and 0.85, respectively, while the a*(Cd2+)/a(Cd2+) ratios were 0.57, 0.68, 0.41, and 0.66, respectively. The a*(Pb2+)/a(Pb2+) ratios were greater than the a*(Cd2+)/a(Cd2+) ratios for all biochars except CB350, showing that the adsorption of Cd2+ was more influenced by Pb2+. In particular, the ratio a*(Pb2+)/a(Pb2+) (0.95) was close to 1, indicating that the existence of Cd2+ had limited impact on the adsorption of Pb2+ on CB650. This may be due to the following reasons: (1) compared to Cd2+ (4.26 Å), Pb2+ has a tinier hydrated radius (4.01 Å), and (2) Pb2+ has a higher electronegativity (2.33) than Cd2+ (1.69)13. Therefore, Pb2+ has stronger affinity for most functional groups and occupies more internal complexing sites in the coexistence system54.
After aging, the ratio of a*(Pb2+)/a(Pb2+) for CCB650 decreased to 0.85, while the ratio of a*(Cd2+)/a(Cd2+) increased to 0.66, indicating that aging inhibited the competitive adsorption of Pb2+ and Cd2+ by CB650. The competitive adsorption performance of low-temperature biochar was completely different from high-temperature biochar. The values of a*(Cd2+)/a(Cd2+) and a*(Pb2+)/a(Pb2+) on the fresh biochar (CB350) were close, while the a*(Cd2+)/a(Cd2+) and a*(Pb2+)/a(Pb2+) ratios of CCB350 grew to similar levels after aging. The above results indicated that there was no significant competitive adsorption in fresh and aged low temperature biochars. The Qm of biochars decreased after aging treatment, except for Cd2+ adsorption capacity of CCB350 in the binary metal system. This showed that the adsorption capacity had a tendency to decrease for most biochars when aged with H2O2. A similar trend was previously reported35,55. This might be due to the fact that aging introduced more O-containing functional groups, which could connect neighboring water molecules via strong H-bonding to form three dimensional water clusters. Thus, these clusters impeded the combination of heavy metal irons and functional groups on the surface of the biochar56.
Effects of aging on the adsorption kinetics of Cd2+and Pb2+ by biochars
Adsorption kinetics of Pb2+ and Cd2+ on fresh and aged biochars in the systems is shown in Fig. 2. It was evident that Pb2+ and Cd2+ adsorption on biochars rose sharply in the first 0–120 min, rose at a slow level for the next 120–720 min, and reached equilibrium in 720–2280 min. The adsorption process has three stages: (1) the rapid adsorption process, (2) the steady adsorption process, and (3) the process in equilibrium. The multi-linearity of the intra-particle diffusion plot suggested that the whole adsorption process was composed of three stages (Fig. S4). In the first stage, instantaneous adsorption resulted in the sharper portion in the graph. The Pb2+ and Cd2+ transferred from the aqueous phase to the external biochars surface. In the second step, there was internal diffusion of Pb2+ and Cd2+ through the pores of biochars. It showed a decrease in the slope due to the gradual decrease in the adsorption rate. The third region was the final equilibrium stage. The whole adsorption process was controlled by a rapid first stage, a slower second stage, and a much slower third stage until reaching equilibrium. At the end of the fast phase of adsorption, the adsorption amounts of Cd2+ and Pb2+ by biochars accounted for about 84.60–97.57% of the total adsorption amounts. These results showed that the rapid stage in adsorption was more prominent in this process. Aging treatment was without vital effect on the equilibrium time of heavy metals in adsorption.
The data were fitted by the model of pseudo-first-order (PFO) and pseudo-second-order (PSO), and the relevant parameters are shown in Table S3. The coefficient of determination (R2) obtained from the pseudo-first-order models (0.5617–0.9424) was lower than that of the pseudo-second-order model (0.9050–0.9786) for all biochars. Pb2+ and Cd2+ adsorption on fresh and aged biochars was more consistent with the pseudo-second-order model, suggesting that chemisorption (ion exchange, complexation, and precipitation) was the main rate-limiting step in this adsorption process57. Moreover, the calculated values (Qe) of the pseudo-second-order model were closer to the values of experiment (Qexp).
To further investigate the rate limiting steps involved in the adsorption process, the intra-particle diffusion (IPD) model was adopted in a kinetics analysis. In this study, data for the Cd2+ and Pb2+ adsorption on the fresh and aged biochars in the single and binary systems within 720 min were well fitted by the IPD model (Table S4), suggesting that intra-particle diffusion played a significant role in Cd2+ and Pb2+ adsorption. The sorption rate in stage 1 (ki1) was much higher than that in other stages (ki2 and ki3), reflecting the external diffusion was the rate-determining step during sorption. Diffusion resistance was evaluated by the intercept C of each stage, which provided information about the thickness of the boundary layer58. The intercept C of each stage followed the order of C1 (first stage) < C2 (second stage) < C3 (third stage). It indicated that external diffusion resistance was the lowest in early stages.
Effects of aging on the adsorption mechanisms of Pb2+ and Cd2+ by biochars
Cation exchange
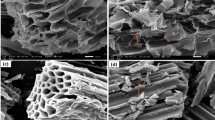
Many cations are remained on the surface of the biochar by direct electrostatic attraction, complexation or precipitation. In the process of the adsorption, these cations on the biochar are in exchange for Cd2+ and Pb2+ in solution through a cation exchanging reaction20,39. To illustrate this possible mechanism, the contents of cations in the solutions before and after adsorption of Pb2+ and Cd2+ by biochar (CB350, CB650, CCB350, and CCB650) were determined by SEM–EDS (Fig. 3). The percentage contents of Pb2+ and Cd2+ elements on the surface of biochars increased after adsorption, while the percentage contents of the metal elements (K+, Ca2+, Na+ and Mg2+) decreased or even lowered to the limit of detection. To further quantify the amount of the metals from the solution before and after the adsorption of Pb2+ and Cd2+ by biochar in single and binary metal systems, we calculated the net release amount of metal ions (Fig. 4). After adsorption of Pb2+ and Cd2+, the net release amounts of the elements from the single and binary metal systems were 0.0196–0.2385, 0.0806–0.4648, 0.0333–0.1550, and 0.0639–0.2454 meq/L, respectively. The net emissions of Ca2+ and Mg2+ were much greater than those of K+ and Na+, indicating a vital role in the cation exchange process. This was mainly because K+ and Na+ could only be retained on the surface of biochars by external spherical electrostatic attraction to the negatively charged position on the surface. However, Ca2+ and Mg2+ remained on biochar due to precipitation (CaCO3 or MgCO3) or complexation of inner sphere for the acid groups on the surface of biochar59.
In comparison with the fresh biochars, the total release amounts of K+, Ca2+, Na+ and Mg2+ of the metal systems obviously decreased after absorbing the Pb2+ and Cd2+ by aged biochars, which may be related to the leaching of soluble salt ions during the aging process60.
Complexation with oxygen-containing functional groups
The oxygenated groups (e.g., –OH, –COOH, –R–OH) distributed on surfaces of the biochar formed metal complexes with heavy metal ions, which reduced the concentration and mobility of heavy metals61. A lot of studies have revealed that the complexation between heavy metals and oxygenated functional groups is a significant mechanism for biochar adsorption20,39,62. The changes of these groups in biochar adsorption were analyzed by FTIR (Fig. 5). The dominant bands at 3414 cm−1, 2932 cm−1, 1701–1680 cm−1, 1564–1597 cm−1 and 1021–1084 cm−1 represented –OH, C–H of aromatic groups, –COOH, C=O/C=C, and C–O–C, respectively23,61. These functional groups were transformed after the adsorption. For CB650, the intensity of the stretching vibration peak of –OH did not change evidently after the adsorption of Pb2+ and Cd2+, which was due to the low content of –OH in the high-temperature biochar. However, the intensity and peak position of –OH changed after the adsorption by CCB350 and CCB650, indicating that –OH was in the process of the adsorption of these metals28. The intensity of aromatic C–H weakened after the heavy metal adsorption by CB350 and CCB350, while CCB650 + Pb + Cd was enhanced, indicating that the aromatic group C–H was in the adsorption of Pb2+ and Cd2+63. The strength of the –COOH stretching vibration peaks of CB350 + Pb, CB350 + Cd and CCB350 + Pb + Cd weakened after the adsorption of the heavy metals Pb2+ and Cd2+. The band at 1441–1437 cm-1 was classified to CO32−, at 1104 cm−1 was because of the P–O stretching vibrations64, which may be due to the metallic carbonate and metallic phosphate precipitates formation, respectively. The C=O/C=C intensities of CB650 + Pb, CB650 + Cd, CB650 + Pb + Cd, CCB650 + Pb, and CCB650 + Cd were weakened. The stretching vibration peak of C–O–C showed a small shift and change. These results indicated that these groups were in the adsorption of Pb2+ and Cd2+ by biochar.
Precipitation with minerals
Some inorganic components of biochar (CO32−, SO42−, OH−, and PO43−) can also be precipitated with heavy metal irons65. The function of these anions in the adsorption of Pb2+ and Cd2+ on biochar has been reported by Fei et al.66. In the present study, after adsorbing heavy metals, SEM results showed that white granular crystals were formed on the surface of biochar. Furthermore, EDS analysis believed that the white granular crystals involved Cd and Pb. Hence, the ability of mineral precipitation to adsorb heavy metals cannot be ignored.
To further determine their precipitate crystals, all biochars before and after the adsorption of Pb2+ and Cd2+ were scanned by XRD (Fig. 6). The XRD patterns also showed the presence of CdCO3, PbCO3, Pb3(CO3)2(OH)2, and PbSe on the surface of the biochars after adsorption. Biochar is rich in inorganic minerals. The higher the ash contents of biochar, the stronger the precipitation of heavy metals67. The ash contents of biochar at high temperature (CB650 and CCB650) were higher than those of low-temperature biochars (CB350 and CCB350). Similarly, the ash content of the fresh biochars (CB350 and CB650) was greater than that of the aged biochars (CCB350, CCB650). This showed that a low pyrolysis temperature and aging could reduce the precipitation of heavy metals.
Other potential mechanisms
Except for cation exchange, complexation, and precipitation, there are other adsorption mechanisms68. Except for functional groups (–OH, –COOH, –R–OH), the influence of other functional groups (γ-CH, C=C) and basic groups (C=N, –NH2) on the adsorption of Pb2+ and Cd2+ by biochar cannot be neglected38. More aromatic structures were formed in the biochar with a rising pyrolysis temperature, which could allow the donation of π-electron to interact with Pb2+ and Cd2+, resulting in an excellent concordant affinity for heavy metals69. Figure 5 showed that in comparison with low-temperature biochar (CB350, CCB350), the high temperature biochar (CB650, CCB650) caused more changes in aromatic C–H functional groups at 400–700 cm-1 after adsorption, indicating that more aromatic functional groups, such as the γ-CH of furan and the β-ring of pyridine. This was similar to previous results70,71.
Furthermore, biochar is able to adsorb heavy metal cations (Cd2+, Pb2+, Zn2+, Cu2+, Ni2+, etc.) due to its negative surface charge72. To elucidate the role played by electrostatic attraction under experimental conditions, the surface zeta potential values of CB350, CB650, CCB350 and CCB650 were examined at pH 5. The zeta-potential values for CB350, CB650, CCB350 and CCB650 were − 53.5 mV, − 45.5 mV, − 57.5 mV and − 56.4 mV, respectively, indicating that all biochar surfaces were negatively charged under these experimental conditions. Therefore, electrostatic attraction was expected to generate between positively charged metal cations and negatively charged functional groups.
Microporous structures and the specific surface area is important for the biochar physical absorption72,73. In this experiment, the SSA of high temperature and aged biochar was greater than that of low temperature and fresh biochar, so physical adsorption might play a major role in high temperature and aged biochar.
Effects of aging on the quantitative contribution of adsorption mechanisms by biochars
The contribution of cation exchange (Qce), precipitation with minerals (Qpre), surface complexation (Qcom) and other mechanisms (Qoth) within the fresh and aged biochars to the adsorption of the metals in the systems were calculated (Fig. 7). Cation exchange was the major adsorption mechanism for the fresh low-temperature biochar (CB350) in the single system. The values of Qce for Cd2+ and Pb2+ reached 20.02 and 30.89 mg/g, accounting for 48.23% and 40.07% of the total adsorption capacity, respectively. The contribution of Qce was going down with the increase of pyrolysis temperature. This might be due to the fact that mineral crystallization inhibits the release of cations at high pyrolysis temperatures74. The main adsorption mechanism of CB650 in the single system changed from cation exchange to mineral precipitation. The Qpre of Cd2+ and Pb2+ were 32.84 and 36.76 mg/g, corresponding to 57.19% and 38.04% of the adsorption contribution, respectively. Under competitive conditions, the Qce/Qt values were at a lower level, compared to those in single metal systems. Precipitation with minerals formed the biggest fraction for CB350 and CB650 in the binary metal system, which contributed 44.36% and 49.20%, respectively.
After aging, the values of Qcom and Qcom/Qt for CCB350 and CCB650 increased to varying degrees under both single and binary metal systems, and this phenomenon was more obvious for low-temperature biochar. For CB350, the Qcom/Qt values of Cd2+ and Pb2+ were 2.12% and 0.28%, respectively. However, after aging, the Qcom/Qt values of Cd2+ and Pb2+ showed 10.20% and 0.53%, respectively, which increased by 8.08% and 0.25%, respectively. Further, the Qpre and Qpre/Qt values of CCB350 and CCB650 decreased significantly compared with fresh biochars in all systems, and the Qoth/Qt values of CCB650 increased significantly after aging.
The above results showed that cation exchange (Qce) belonged to the main mechanism for CB350 and CCB350 (low temperature biochar) in a single-metal system, with the Qce/Qt values ranging from 35.63% to 48.23%. Precipitation with minerals (Qpre) and other mechanisms (Qoth) were foremost for CB650 and CCB650 in the single-metal system, respectively. The competitive interaction between Cd2+ and Pb2+ increased the relative contribution of mineral precipitation. Aging resulting in the contribution of surface complexation to Cd2+ and Pb2+ adsorption in all biochars, especially in low temperature biochars but reduced the contribution of mineral precipitation to adsorption. Notably, the contribution of other mechanisms to the adsorption increased significantly after the aging of the high-temperature biochar.
Conclusions
The aging of corn straw biochar significantly changed its physicochemical characteristics, affecting the competitive adsorption behavior and mechanism of Cd2+ and Pb2+. In the single-metal system, cation exchange was the key adsorption mechanism for biochar (35.63–48.23%) at a low temperature. However, mineral precipitation dominated Cd2+ and Pb2+ adsorption (38.04–57.19%) on fresh high-temperature biochars. Competitive interactions between Cd2+ and Pb2+ increased the relative contribution of mineral precipitation, but the contribution of cation exchange mechanism was reduced. Biochar aging increased the contribution of surface complexation to the adsorption of Cd2+ and Pb2+ on biochars, especially in low-temperature biochars, but weakened the contribution of mineral precipitation to the adsorption. Further, the contribution of other adsorption mechanisms was significantly enhanced for high-temperature aged biochars. Therefore, the results in this study are expected to provide a theoretical basis for the long term application of biochar in the remediation of heavy metal compound polluted soil.
Data availability
The data used to support the findings of this study are included within the article and the supplementary information files.
References
Bashir, S., Zhu, J., Fu, Q. L. & Hu, H. Q. Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 194, 579–587. https://doi.org/10.1016/j.chemosphere.2017.11.162 (2018).
Fajardo, C. et al. Heavy metals immobilization capability of two iron-based nanoparticles (nZVI and Fe3O4): Soil and freshwater bioassays to assess ecotoxicological impact. Sci. Total. Environ. 656, 421–432. https://doi.org/10.1016/j.scitotenv.2018.11.323 (2019).
Sellaoui, L. et al. Insights on the statistical physics modeling of the adsorption of Cd2+ and Pb2+ ions on bentonite-chitosan composite in single and binary systems. Chem. Eng. J. 354, 569–576. https://doi.org/10.1016/j.cej.2018.08.073 (2018).
Yan, Y. B. et al. Recycling flue gas desulphurization (FGD) gypsum for removal of Pb(II) and Cd(II) from waste water. J. Colloid Interface Sci. 457, 86–95. https://doi.org/10.1016/j.jcis.2015.06.035 (2015).
Lian, C., Zhou, S. L., Shi, Y. X. & Wang, C. H. Heavy metals in food crops, soil, and water in the Lihe River Watershed of the Taihu Region and their potential health risks when ingested. Sci. Total. Environ. 615, 141–149. https://doi.org/10.1016/j.scitotenv.2017.09.230 (2017).
Jeon, E. K., Jung, J. M., Kim, W. S., Ko, S. H. & Beak, K. In situ electrokinetic remediation of As-, Cu-, and Pb-contaminated paddy soil using hexagonal electrode configuration: A full scale study. Environ. Sci. Pollut. Res. 22(1), 711–720. https://doi.org/10.1007/s11356-014-3363-0 (2015).
Chen, D. et al. Effects of biochar on availability and plant uptake of heavy metals: A meta-analysis. J. Environ. Manag. 22, 76–85. https://doi.org/10.1016/j.jenvman.2018.05.004 (2018).
Cheng, S. et al. Application research of biochar for the remediation of soil heavy metals contamination: A review. Molecules 25(14), 3167. https://doi.org/10.3390/molecules25143167 (2020).
El-Naggar, A. et al. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 337, 536–554. https://doi.org/10.1016/j.geoderma.2018.09.034 (2019).
Wang, L. et al. New trends in biochar pyrolysis and modification strategies: Feedstock, pyrolysis conditions, sustainability concerns and implications for soil amendment. Soil Use Manag. 36, 1–29. https://doi.org/10.1111/sum.12592 (2020).
Wang, L. W. et al. Biochar aging: Mechanisms, physicochemical changes, assessment, and implications for field applications. Environ. Sci. Technol. 54(23), 14797–14814. https://doi.org/10.1021/acs.est.0c04033 (2020).
Gholizadeh, M. & Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 9, 105830. https://doi.org/10.1016/j.jece.2021.105830 (2021).
Ni, B. J. et al. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 219, 351–357. https://doi.org/10.1016/j.chemosphere.2018.12.053 (2019).
Abdin, Y., Usman, A., Ok, Y. S., Tsang, Y. F. & Al-Wabel, M. Competitive sorption and availability of coexisting heavy metals in mining-contaminated soil: Contrasting effects of mesquite and fishbone biochars. Environ. Res. 181, 108846. https://doi.org/10.1016/j.envres.2019.108846 (2019).
Han, L. et al. Lead adsorption by biochar under the elevated competition of cadmium and aluminum. Sci. Rep. 7(1), 1–11. https://doi.org/10.1038/s41598-017-02353-4 (2017).
Deng, Y. Y., Huang, S., Laird, D. A., Wang, X. G. & Meng, Z. W. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single- and binary-metal systems. Chemosphere 218, 308–318. https://doi.org/10.1016/j.chemosphere.2018.11.081 (2019).
Fan, S. S. et al. Cadmium removal from aqueous solution by biochar obtained by co-pyrolysis of sewage sludge with tea waste. Res. Chem. Intermed. 44, 135–154. https://doi.org/10.1007/s11164-017-3094-1 (2017).
Sun, J. K., Fei, L., Liu, Z. Q., Zhu, L. Y. & Song, Z. G. Biochars derived from various crop straws: Characterization and Cd(II) removal potential. Ecotoxicol. Environ. Saf. 106, 226–231. https://doi.org/10.1016/j.ecoenv.2014.04.042 (2014).
Chang, R. H., Sohi, S. P., Jing, F. Q., Liu, Y. Y. & Chen, J. W. A comparative study on biochar properties and Cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation. Environ. Pollut. 254, 113123. https://doi.org/10.1016/j.envpol.2019.113123 (2019).
Lu, H. L. et al. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 46, 854–862. https://doi.org/10.1016/j.watres.2011.11.058 (2012).
Liu, L. & Fan, S. S. Removal of cadmium in aqueous solution using wheat straw biochar: Effect of minerals and mechanism. Environ. Sci. Pollut. Res. 25, 8688–8700. https://doi.org/10.1007/s11356-017-1189-2 (2018).
Wang, Z. Y. et al. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 177, 308–317. https://doi.org/10.1016/j.biortech.2014.11.077 (2015).
Tan, L. S. et al. Effect of three artificial aging techniques on physicochemical properties and Pb adsorption capacities of different biochars. Sci. Total. Environ. 699, 134223. https://doi.org/10.1016/j.scitotenv.2019.134223 (2020).
Fu, Q. et al. Effects of biochar addition on soil hydraulic properties before and after freezing-thawing. CATENA 176, 112–124. https://doi.org/10.1016/j.catena.2019.01.008 (2019).
Meng, Z. W. et al. Transport and transformation of Cd between biochar and soil under combined dry-wet and freeze-thaw aging. Environ. Pollut. 263, 114449. https://doi.org/10.1016/j.envpol.2020.114449 (2020).
Quan, G. X. et al. Simulated photocatalytic aging of biochar in soil ecosystem: Insight into organic carbon release, surface physicochemical properties and cadmium sorption. Environ. Res. 183, 109241. https://doi.org/10.1016/j.envres.2020.109241 (2020).
Hua, Y. et al. Microbial aging of hydrochar as a way to increase cadmium ion adsorption capacity: Process and mechanism. Bioresour. Technol. 300, 122708. https://doi.org/10.1016/j.biortech.2019.122708 (2020).
Huff, M. D. & Lee, J. W. Biochar-surface oxygenation with hydrogen peroxide. J. Environ. Manag. 165, 17–21. https://doi.org/10.1016/j.jenvman.2015.08.046 (2016).
Li, H. Y. et al. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 304, 40–48. https://doi.org/10.1016/j.jhazmat.2015.10.048 (2016).
Mia, S., Dijkstra, F. A. & Singh, B. Aging induced changes in biochar’s functionality and adsorption behavior for phosphate and ammonium. Environ. Sci. Technol. 51, 8359–8367. https://doi.org/10.1021/acs.est.7b00647 (2017).
Ren, X. H., Sun, H. W., Wang, F. & Cao, F. M. The changes in biochar properties and sorption capacities after being cultured with wheat for 3 months. Chemosphere 144, 2257–2263. https://doi.org/10.1016/j.chemosphere.2015.10.132 (2016).
Shi, K. S., Xie, Y. & Qiu, Y. P. Natural oxidation of a temperature series of biochars: Opposite effect on the sorption of aromatic cationic herbicides. Ecotoxicol. Environ. Saf. 114, 102–108. https://doi.org/10.1016/j.ecoenv.2015.01.015 (2015).
Hale, S. E., Hanley, K., Lehmann, J., Zimmerman, A. R. & Cornelissen, G. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ. Sci. Technol. 45(24), 10445–10453. https://doi.org/10.1021/es202970x (2011).
Li, H. X., Lu, X. Q., Xu, Y. & Liu, H. T. How close is artificial biochar aging to natural biochar aging in fields? A meta-analysis. Geoderma 352, 96–103. https://doi.org/10.1016/j.geoderma.2019.06.006 (2019).
Nie, T. T., Hao, P. L., Zhao, Z. D., Zhou, W. J. & Zhu, L. J. Effect of oxidation-induced aging on the adsorption and co-adsorption of tetracycline and Cu2+ onto biochar. Sci. Total Environ. 673, 522–532. https://doi.org/10.1016/j.scitotenv.2019.04.089 (2019).
Cao, X. D., Ma, L., Gao, B. & Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 43, 3285–3291. https://doi.org/10.1021/es803092k (2009).
Deng, Y. Y., Huang, S., Laird, D. A., Wang, X. G. & Dong, C. Q. Quantitative mechanisms of cadmium adsorption on rice straw-and swine manure-derived biochars. Environ. Sci. Pollut. Res. 25, 32418–32432. https://doi.org/10.1007/s11356-018-2991-1 (2018).
Huang, F., Gao, L. Y., Wu, R. R., Wang, H. & Xiao, R. B. Qualitative and quantitative characterization of adsorption mechanisms for Cd2+ by silicon-rich biochar. Sci. Total. Environ. 731, 139163. https://doi.org/10.1016/j.scitotenv.2020.139163 (2020).
Cui, X. Q. et al. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total. Environ. 562, 517–525. https://doi.org/10.1016/j.scitotenv.2016.03.248 (2016).
Ho, S. H. et al. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 246, 142–149. https://doi.org/10.1038/s41598-017-02353-4 (2017).
Deng, Y. Y., Huang, S., Dong, C. Q., Meng, Z. W. & Wang, X. G. Competitive adsorption behaviour and mechanisms of cadmium, nickel and ammonium from aqueous solution by fresh and ageing rice straw biochars. Bioresour. Technol. 303, 122853. https://doi.org/10.1016/j.biortech.2020.122853 (2020).
Cao, X. D. & Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 101, 5222–5228. https://doi.org/10.1016/j.biortech.2010.02.052 (2010).
Shinogi, Y. & Kanri, Y. Pyrolysis of plant, animal and human waste: Physical and chemical characterization of the pyrolytic products. Bioresour. Technol. 90(3), 241–247. https://doi.org/10.1016/s0960-8524(03)00147-0 (2003).
Zhao, Y. Q., Huang, L. & Chen, Y. C. Biochars derived from giant reed (Arundo donax L.) with different treatment: characterization and ammonium adsorption potential. Environ. Sci. Pollut. Res. 24, 25889–25898. https://doi.org/10.1007/s11356-017-0110-3 (2017).
Qian, L. B., Chen, M. F. & Chen, B. L. Competitive adsorption of cadmium and aluminum onto fresh and oxidized biochars during aging processes. J. Soil. Sediment. 15(5), 1130–1138. https://doi.org/10.1007/s11368-015-1073-y (2015).
Mia, S., Dijkstra, F. A. & Singh, B. Long-term aging of biochar: A molecular understanding with agricultural and environmental implications. Adv. Agron. 141, 1–51. https://doi.org/10.1016/bs.agron.2016.10.001 (2017).
Yang, K., Wang, X. L., Cheng, H. F. & Tao, S. Effect of aging on stabilization of Cd and Ni by biochars and enzyme activities in a historically contaminated alkaline agricultural soil simulated with wetedry and freezeethaw cycling. Environ. Pollut. 268, 115846. https://doi.org/10.1016/j.envpol.2020.115846 (2021).
Sanford, J. R., Larson, R. A. & Runge, T. Nitrate sorption to biochar following chemical oxidation. Sci. Total Environ. 669(15), 938–947. https://doi.org/10.1016/j.scitotenv.2019.03.061 (2019).
Jing, F. Q., Sohi, S. P., Liu, Y. Y. & Chen, J. W. Insight into mechanism of aged biochar for adsorption of PAEs: Reciprocal effects of ageing and coexisting Cd2+. Environ. Pollut. 242, 1098–1107. https://doi.org/10.1016/j.envpol.2018.07.124 (2018).
Park, J. H. et al. Comparative sorption of Pb and Cd by biochars and its implication for metal immobilization in soils. Water Air Soil Pollut. 224(12), 1711–1122. https://doi.org/10.1007/s11270-013-1711-1 (2013).
Jeppu, G. P. & Clement, T. P. A modified Langmuir–Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 129–130, 46–53 (2012).
Liu, Y. Y. et al. Oxidative ageing of biochar and hydrochar alleviating competitive sorption of Cd(II) and Cu(II). Sci. Total. Environ. 725, 138419. https://doi.org/10.1016/j.scitotenv.2020.138419 (2020).
Park, J. H. et al. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142, 77–83. https://doi.org/10.1016/j.chemosphere.2015.05.093 (2016).
Ding, Z. H., Xin, H., Wan, Y. S., Wang, S. S. & Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 33, 239–245. https://doi.org/10.1016/j.jiec.2015.10.007 (2016).
Wu, W. D. et al. Unraveling sorption of lead in aqueous solutions by chemically modified biochar derived from coconut fiber: A microscopic and spectroscopic investigation. Sci. Total. Environ. 576, 766–774. https://doi.org/10.1016/j.scitotenv.2016.10.163 (2017).
Ghaffar, A. & Abbas, G. Adsorption of phthalic acid esters (PAEs) on chemically aged biochars. Green Process. Synth. 5(4), 407–417. https://doi.org/10.1515/gps-2016-0014 (2016).
Ahmad, Z. et al. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 180, 437–449. https://doi.org/10.1016/j.jclepro.2018.01.133 (2018).
Tan, X. et al. Biochar amendment to lead-contaminated soil: Effects on fluorescein diacetate hydrolytic activity and phytotoxicity to rice. Environ. Toxicol. Chem. 34, 1962–1968. https://doi.org/10.1002/etc.3023 (2015).
Zhang, F. et al. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J. Environ. Manag. 153, 68–73. https://doi.org/10.1016/j.jenvman.2015.01.043 (2015).
Bakshi, S., Aller, D. M., Laird, D. A. & Chintala, R. Comparison of the physical and chemical properties of laboratory and field-aged biochars. J. Environ. Qual. 45(5), 1627–1634. https://doi.org/10.2134/jeq2016.02.0062 (2016).
Fan, Q. Y. et al. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 207, 33–40. https://doi.org/10.1016/j.chemosphere.2018.05.044 (2018).
Huang, F., Wen, X. H., Cai, Y. X. & Cai, K. Z. Silicon-mediated enhancement of heavy metal tolerance in rice at different growth stages. Int. J. Environ. Res. Public Health 15(10), 2193. https://doi.org/10.3390/ijerph15102193 (2018).
Lawrinenko, M., Laird, D. A., Johnson, R. L. & Jing, D. Accelerated aging of biochars: Impact on anion exchange capacity. Carbon 103, 217–227. https://doi.org/10.1016/j.carbon.2016.02.096 (2016).
Qian, L. B. & Chen, B. L. Dual role of biochars as adsorbents for aluminum: The effects of oxygen-containing organic components and the scattering of silicate particles. Environ. Sci. Technol. 47(15), 8759–8768. https://doi.org/10.1021/es401756h (2013).
Gao, R. L. et al. High-efficiency removal capacities and quantitative sorption mechanisms of Pb by oxidized rape straw biochars. Sci. Total. Environ. 699, 134262. https://doi.org/10.1016/j.scitotenv.2019.134262 (2020).
Fei, H., Gao, L. Y., Deng, J. H., Chen, S. H. & Cai, K. Z. Quantitative contribution of Cd2+ adsorption mechanisms by chicken-manure-derived biochars. Environ. Sci. Pollut. Res. 25, 1–13. https://doi.org/10.1007/s11356-018-2889-y (2018).
Zhang, C., Shan, B. Q., Tang, W. Z. & Zhu, Y. Y. Comparison of cadmium and lead sorption by Phyllostachys Pubescens biochar produced under a low-oxygen pyrolysis atmosphere. Bioresour. Technol. 238, 352–360. https://doi.org/10.1016/j.biortech.2017.04.051 (2017).
Mahdi, Z., Yu, Q. J. & Hanandeh, A. E. Investigation of the kinetics and mechanisms of nickel and copper ions adsorption from aqueous solutions by date seed derived biochar. J. Environ. Chem. Eng. 6, 1171–1181. https://doi.org/10.1016/j.jece.2018.01.021 (2018).
Yu, W. C., Lian, F., Cui, G. N. & Liu, Z. Q. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 193, 8–16. https://doi.org/10.1016/j.chemosphere.2017.10.134 (2018).
Gao, L. Y. et al. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 272, 114–122. https://doi.org/10.1016/j.biortech.2018.09.138 (2018).
Uchimiya, M. et al. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 58(9), 5538–5544. https://doi.org/10.1021/jf9044217 (2010).
Tran, H. N., You, S. J., Hosseini-Bandegharaei, A. & Chao, H. P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 120, 88–116. https://doi.org/10.1016/j.watres.2017.04.014 (2017).
Shen, Z. T., Zhang, Y. Y., Jin, F., McMillan, O. & Al-Tabbaa, A. Qualitative and quantitative characterization of adsorption mechanisms of lead on four biochars. Sci. Total. Environ. 609, 1401–1410. https://doi.org/10.1016/j.scitotenv.2017.08.008 (2017).
Wang, R. Z. et al. Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour. Technol. 261, 265–271. https://doi.org/10.1016/j.biortech.2018.04.032 (2018).
Acknowledgements
The study was helped the following associations, including National key research and development program (2018YFC1802904), the National Science Foundation of China (41867061), Inner Mongolia Science & Technology Plan Program (2019 and 2020), the National Science Foundation of Inner Mongolia (2020MS02005), Inner Mongolia Engineering Research Center of Evaluation and Restoration in the Mining Ecological Environment and Inner Mongolia Special Fund for Transformation of Scientific and Technological Achievements, No. 2019CG062. The authors sincerely acknowledge the anonymous reviewers for their insights and comments to further improve the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data curation, writing-Original draft preparation were performed by Z.W. Formal analysis and investigation were performed by C.G. Investigation and visualization were performed by Y.B. Investigation were performed by G.Z. Conceptualization, writing- reviewing and editing and resources were performed by C.Z. Supervision and validation were performed by C.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Geng, C., Bian, Y. et al. Effect of oxidative aging of biochar on relative distribution of competitive adsorption mechanism of Cd2+ and Pb2+. Sci Rep 12, 11308 (2022). https://doi.org/10.1038/s41598-022-15494-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15494-y
This article is cited by
-
Effects of Biotic and Abiotic Aging Techniques on Physiochemical and Molecular Characteristics of Biochar and Their Impacts on Environment and Agriculture: A Review
Journal of Soil Science and Plant Nutrition (2023)
-
Removal of cadmium from aqueous solution by magnetic biochar: adsorption characteristics and mechanism
Environmental Science and Pollution Research (2023)
-
Biochar Modification Methods for Augmenting Sorption of Contaminants
Current Pollution Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.