Abstract
The aim of this study was to cross-sectionally and longitudinally examine whether higher hemoglobin (Hb) levels within the normal variation associate with key components of metabolic syndrome and total and cardiovascular mortality. The study included 967 Finnish subjects (age 40–59 years) followed for ≥ 20 years. The focus was on Hb levels, cardiovascular diseases (CVDs) and mortality rates. Higher Hb levels associated positively with key anthropometric and metabolic parameters at baseline. At the follow-up similar associations were seen in men. The highest Hb quartile showed higher leptin levels and lower adiponectin levels at baseline and follow-up (p < 0.05) and lower plasma ghrelin levels at baseline (p < 0.05). Higher baseline Hb levels associated independently with prevalence of type 2 diabetes at follow-up (p < 0.01). The highest Hb quartile associated with higher serum alanine aminotransferase levels (p < 0.001) and independently with increased risk for liver fat accumulation (OR 1.63 [1.03; 2.57]) at baseline. The highest Hb quartile showed increased risk for total (HR = 1.48 [1.01; 2.16]) and CVD-related mortality (HR = 2.08 [1.01; 4.29]). Higher Hb levels associated with an adverse metabolic profile, increased prevalence of key components of metabolic syndrome and higher risk for CVD-related and total mortality.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a global epidemic which coincides with the increasing prevalence of obesity. MetS is defined as a group of metabolic disorders which increases the risk for type 2 diabetes (T2DM), cardiovascular diseases (CVD) and total mortality. The major components of MetS are visceral obesity, dyslipidemia and hypertension1,2. Non-alcoholic fatty liver disease (NAFLD) is also considered a co-morbidity of MetS3. The average prevalence of MetS was 31% in 20174. Individuals with MetS have a 46% increased risk of mortality compared to individuals without the syndrome5.
Hb is the main carrier of oxygen in the cardiovascular system. Hb levels are regulated genetically and environmentally and they vary by sex, race, age and living altitude6,7. However, individual Hb levels during adult life are relatively stable although Hb levels successively decrease with ageing in men and postmenopausal women6. In general, high-end Hb levels within the normal range are considered beneficial for health6. However, Hb levels can be elevated by factors such as smoking, a well-known risk factor for metabolic diseases and higher Hb levels are also observed in obesity, which likewise is a well-known risk factor for cardiometabolic diseases1,2.
Previous studies with selected cohorts, cross-sectional setting and often including only one sex have shown associations of higher Hb levels with individual components of MetS including insulin resistance, NAFLD, dyslipidemia and hypertension8,9,10,11,12,13,14,15. However, higher Hb levels have also been associated with lower glycated hemoglobin (HbA1c) levels16. Regarding obesity-related peptide hormones, Hb levels have previously been both positively and negatively associated with serum leptin levels and negatively associated with serum adiponectin levels17,18,19. Studies have also reported lower Hb levels (especially anemic) and very high Hb levels as predictors of total and CVD-related mortality20,21,22,23. The underlying mechanisms of the reported associations between higher Hb levels and metabolic markers have been poorly understood. Hyperviscosity or changes in plasma volume10,11,12,22,24, endothelial cell dysfunction 10 or higher iron/ferritin levels 13,15 have been suggested as mediators of these associations. However, we have recently shown that lower Hb levels associate with an overall healthier metabolic profile in males and females in two middle-aged Finnish sea level birth cohorts studied in a longitudinal setting until age of 46 years, and that these alterations are mediated by hypoxia25. All in all, the role of Hb levels as a risk factor of MetS and its comorbidities requires further studies.
The aims of this study were (1) to assess cross-sectionally the associations between Hb levels and some 20 key metabolic parameters, obesity-related peptides and ambulatory blood pressure (ABP) measurements in middle-age (average age 51 years) and senescence (average age 72 years), (2) evaluate in a cross-sectional design Hb levels as a risk factor for fatty liver disease, and in a longitudinal design evaluate the role of Hb levels (3) in prediction of the development of impaired glucose metabolism and (4) as a risk factor for CVD events and -related mortality and total mortality. Long-term studies considering the risk profile of the highly prevalent metabolic disorders are needed and this study offers a follow-up period of ~ 20 years, to our knowledge the longest in the literature. Increased information about the risk profile of MetS would eventually lead to decreased morbidity and mortality through improved primary prevention.
Materials and methods
Study population
OPERA (Oulu Project Elucidating Risk of Atherosclerosis) is an epidemiological cohort study designed to address the risk factors and clinical endpoints of CVDs. Study subjects were randomly selected between the years 1990 and 1993 as middle-aged, drug-treated hypertensives and their age- and sex-matched control subjects aged 40–59 years (Table S1, Figure S1). Participants were interviewed, examined and tested in our research laboratory. Mortality and hospital events of a total of 1045 subjects were followed up until year 2014. Of the 813 survivors, 600 (62–83 years of age) attended a follow-up examination between 2013 and 2014 (Figure S1).
Inclusion criteria for the current study were Hb levels within Finnish reference values (117–155 g/L for women and 134–167 g/L for men). The Finnish reference values for Hb represent the age-correlated 2.5–97.5% Hb reference range determined by the Finnish National Working Group for basic blood count reference intervals26. Of the 1045 subjects, 967 met the inclusion criteria. Of the 967 subjects 558 attended the follow-up. Of the 409 subjects missing the follow-up mortality accounted for 213. Of the 213 deaths 70 were classed as CVD-related. 231 fatal or non-fatal CVD events were recorded during the follow-up (Figure S1).
Study population was first divided to four sex-specific quartiles according to baseline Hb levels (Figure S1). The corresponding sex-specific Hb quartiles were then pooled to form four Hb quartiles (Hb quartile 1–4 where 1 is the lowest and 4 the highest) each of which consisted of the corresponding sex-specific Hb quartiles from both sexes (Figure S1).
The study was conducted according to the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medicine, University of Oulu. Written informed consent was obtained from each participant.
Clinical measurements
Lifetime smoking burden was determined as pack-years (1 pack-year = 20 cigarettes smoked/day in 1 year) and obtained from a questionnaire. Alcohol consumption was obtained from a questionnaire in number of standard drinks/wk which was converted to g/wk. Physical activity was assessed by a standardized health questionnaire covering physical activity using the method described by Grimby27. Physical activity was determined as none, mild, moderate or heavy physical activity. Lipid- and blood pressure medication users were determined from patient records. Lung diseases were classified as asthma, chronic obstructive pulmonary disease or any other lung disease based on anamnesis.
Blood pressure (bp) measurements were undertaken with an automatic oscillometric bp recorder (Dinamap, Critikon Ltd., Ascot, UK) and conducted according to the recommendations of the American Society of Hypertension28. Resting bp was measured three times at 1-min intervals from the right arm after the subject had been seated for at least 5 min. The mean value of the second and third bp measurements was used. Ambulatory blood pressure (ABP) measurements were obtained with a non-invasive fully automatic SpaceLabs90207 oscillometric unit (SpaceLabs Inc., Redmond, WA). The measurements were taken every 15 min from 04:00 am to 12:00 pm and every 20 min from 12:00 pm to 04:00 am. The accuracy and reproducibility of bp readings obtained with this device have previously been settled by the British Hypertension Society and the US Association for the Advancement of Medical Instrumentation29. The similarity (difference < 5 mmHg) between four SpaceLabs bp measurements and four auscultatory readings using a Y-connector was required to ensure the proper positioning of the cuff. Study subjects were instructed to relax their arm during the measurement. Systolic bp values ≤ 70 mmHg or ≥ 250 mmHg, diastolic bp values ≤ 40 mmHg or ≥ 150 mmHg, and heart rate ≤ 40 or ≥ 150 beats/min were automatically excluded from the analyses. Based on these criteria, less than 3% of the bp measurements were excluded as artefacts30.
Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Height was measured to the nearest centimetre (cm) without shoes using a stadiometer and a sliding horizontal headpiece which was adjusted to rest on the top of the head. Weight was measured to the nearest 0.1 kg with the subject wearing only light underwear without shoes using a SECA personal scale calibrated yearly and used for independent medical weighing and measuring. Waist and hip circumferences were measured using a tape measurer to the nearest 0.1 cm and waist-hip ratio was calculated from these values. All measurements were performed by the same specially trained nurses.
The determination of liver adiposity was based on liver-kidney contrast measured with ultrasonography by one trained radiologist (Markku Päivänsalo) with extensive experience in abdominal ultrasound examinations. The ultrasound examinations were carried out using a Toshiba SSA 270 ultrasound system (Toshiba Corp., Tokyo, Japan) with a scanning frequency of 5 MHz. The entire scanning procedure was captured on video with a Super-VHS video-cassette recorder (Panasonic Corp., Osaka, Japan) and the videotapes were analyzed later. The severity of hepatic steatosis was based on the brightness of the liver in ultrasound scanning and was classified into three groups ranging from 0 to 2 (0 = normal brightness, indicating a non-fatty liver, 1 = medium brightness, a moderate lipid content and 2 = clearly bright, a severe lipid content and fatty liver). Here, we compared subjects with normal brightness of liver (group 0) with those with fatty liver (= combined groups 1 and 2)31. Manuscript does not include information or images that could lead to identification of a study participant.
Laboratory analyses
Blood samples were analysed in NordLab Oulu (former name Oulu University Hospital, Laboratory), a testing laboratory (T113) accredited by Finnish Accreditation Service (FINAS) (EN ISO 15189). Blood Hb levels were determined using the sodium lauryl sulfate method (SLS) and blood leucocytes using the fluorescence flow cytometry method (FFC).
Very-low-density lipoprotein (VLDL) fraction was separated from plasma by ultracentrifugation at 10,500 × g for 18 h. Plasma high-density lipoprotein (HDL) cholesterol concentration was measured by mixing 0.5 mL of the VLDL-free fraction with 25 mL of 2.8% (wt/vol) heparin and 25 mL of 2 M manganese chloride and measuring the cholesterol concentration in the supernatant after centrifugation at 1000 × g and 4 °C for 30 min. Low-density lipoprotein (LDL) cholesterol concentration was calculated by subtracting the cholesterol concentration in HDL from that in the VLDL-free fraction.
Fasting glucose concentrations were measured with the glucose dehydrogenase method (Diagnostica, Merck, Darmstadt, Germany) and serum insulin levels with a two-site immunoenzymometric assay (AIA-PACK IRI, Tosoh Corp., Tokyo, Japan). HOMA-IR was calculated with the equation (fasting insulin (mU/mL) x fasting glucose (mmol/L) / 22.5). Oral glucose tolerance test (OGTT) was performed in the morning after a 12-h fast immediately after fasting blood had been drawn. Normal glucose tolerance, impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and T2DM were determined according to the World Health Organization (WHO) criteria32. Area under the curve (AUC) values for glucose and insulin were determined as integrates from 0 to 2 h time points in OGTT.
High-sensitivity C-reactive protein (hsCRP) was analysed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Diagnostic Systems Laboratories, Webster, TX) as described before33.
Fasting plasma leptin concentration was assessed with a commercial double antibody radioimmunoassay (RIA) (Human Leptin RIA Kit; Linco Research, Inc., St. Charles, MO). Plasma adiponectin concentrations were measured with an ELISA devised in our laboratory, described previously in detail34. At baseline the plasma total ghrelin concentration was determined using a commercial RIA kit (Phoenix Pharmaceuticals, Belmont, CA, USA) and at the follow-up using a commercial ELISA Kit (EZGRT-89 K Human Ghrelin ELISA, Merck/MilliporeSigma, Burlington, MO, USA). Correlation between these two methods for determination of total ghrelin concentration has been determined to be very strong35.
Alanine aminotransferase (ALT) levels were measured with the recommended method according to the European Committee for Clinical Laboratory Standards as previously described36.
Outcome classification
Information on causes of death and events leading to hospitalization were obtained from the Finnish Causes-of-Death Register and the Hospital Discharge Register. The diagnoses were classified according to the International Classification of Diseases (ICD), Eighth Revision (ICD-8) or Ninth Revision (ICD-9) before 1994 and the Tenth Revision (ICD-10) thereafter. Coronary heart disease (CHD) was defined as diagnoses I20, I21, I22 [ICD-10] and 410, 4110 [ICD-8/9], coronary artery bypass graft or coronary angioplasty as I20–I25, I46, R96, R98 [ICD-10] and 410–414, 798 (not 7980A) [ICD-8/9] as causes of death. CVD was defined as CHD or stroke that included I61, I63 (not I636), I64 [ICD-10] and 431, 4330A, 4331A, 4339A, 4340A, 4341A, 4349A, 436 [ICD-9] or 431 (excluding 43101, 43191), 433, 434, 436 [ICD-8] according to the FINRISK criteria37.
Statistical methods
Continuous variables were presented as mean and categorical variables as percentages. A Pearson correlation coefficient was determined between Hb and metabolic variables to linearly evaluate their associations at baseline and at the follow-up. Differences between Hb quartiles (main explanatory variable) and anthropometric and metabolic variables (outcome) at baseline and follow-up were calculated using general linear model (GLM). To adjust for potential confounding factors, smoking, alcohol consumption and BMI were included as explanatory variables for the Hb quartile comparisons. Statistical significances between the Hb quartiles at baseline and follow-up, and for the development of impaired glucose metabolism were tested using analysis of variance for continuous variables and chi-squared test for categorical variables. A Kruskal–Wallis test was used to determine statistical significance for alcohol consumption and smoking. For impaired glucose metabolism analysis age and sex were additionally included as covariates. A p value ≤ 0.05 was considered statistically significant. p values < 0.0001 were not given as exact values.
Risk of fatty liver disease according to Hb levels at baseline was estimated with a multivariable logistic regression model. The model included the following covariates: age, sex, BMI, physical activity, alcohol consumption, smoking, LDL cholesterol, HDL cholesterol and lipid medication.
Survival probabilities of total and CVD-related mortality and CVD events were assessed using the Kaplan–Meier survival curves for the Hb quartiles and sex-specific Hb quartiles. Statistical significances of the Kaplan–Meier survival curves were calculated using Log-Rank test. Cox proportional hazard model was used to estimate the association between Hb levels and CVD events, CVD-related mortality and total mortality, respectively. The regression models included Hb quartiles, age, sex, smoking, alcohol consumption, BMI, systolic and diastolic bp, bp medication, LDL and HDL cholesterol and lipid medication. Physical activity was not included in the mortality analysis due a large deviation and complete separation in the regression model. A person-year calculation for events per 100 person-years was used to further elaborate the mortality and CVD events data.
Statistical analyses were calculated using IBM SPSS statistics version 25.0 (IBM Corp, Armonk, NY).
Results
Association of Hb levels with key anthropometric, metabolic and other cardiovascular risk factors at baseline and follow-up
First, we examined the association of Hb levels with anthropometric and metabolic parameters in a longitudinal design at two timepoints; baseline at 40–59 years of age, average age 51 years and follow-up at 63–83 years of age, average age 72 years (Fig. 1, Tables S1-S3). At baseline Hb levels associated positively with anthropometric parameters including body weight, BMI and waist and hip circumference in both sexes (Fig. 1). In men these associations remained at the follow-up, but women no longer showed such associations (Fig. 1). For both sexes systolic bp associated positively with Hb levels at baseline but at the follow-up neither sex showed an association (Fig. 1). Diastolic bp associated with Hb levels at baseline in both sexes but at the follow-up the association weakened for women and strengthened for men (Fig. 1). In men heart rate associated with Hb levels at baseline but not at the follow-up (Fig. 1). To amend the resting bp data we used data from ABP monitoring which allows evaluating heart rate and bp continuously. For both sexes Hb levels associated with almost all ABP parameters at baseline (Fig. 1, Tables S2&S3). The positive associations of Hb levels and diastolic ABP were significantly stronger in men at follow-up compared to baseline, but were lost in women, while other associations with ABP were not observed at the follow-up (Fig. 1, Tables S2&S3). The average usage of bp medication increased from 49% at baseline to 78% at the follow-up (Table S1).
Sex-specific associations of Hb levels with key anthropometric, metabolic and ambulatoric blood pressure (ABP) parameters. Forest plots representing the effect sizes and their 95% confidence intervals for one standard deviation (1-SD) change in key anthropometric measures and metabolic parameters at baseline (red) and at the the 20-year follow-up (blue). (A) males, (B) females. BMI body mass index, cx circumference, bp blood pressure, OGTT oral glucose tolerance test, LDL low-density lipoprotein, HDL high-density lipoprotein, ALT alanine aminotransferase.
Of the key parameters of glucose metabolism, fasting glucose levels associated positively with Hb levels in both sexes at both timepoints (Fig. 1). For both sexes fasting insulin levels associated positively with Hb levels at baseline but the association weakened for men and was lost for women at the follow-up (Fig. 1). Glucose levels 2 h after an OGTT associated positively with Hb levels in women at both time points, strengthening in the follow-up, but no association was seen in men at either timepoint (Fig. 1). Insulin levels 2 h after OGTT associated positively with Hb levels in both sexes at both time points (Fig. 1).
A positive association between Hb levels and total cholesterol levels was observed in men at both time points and but not in women (Fig. 1). For both sexes LDL cholesterol levels associated positively with Hb levels at the follow-up but not at baseline while HDL cholesterol levels associated negatively with Hb levels at baseline in both sexes but not at the follow-up (Fig. 1). For both sexes plasma triglyceride levels associated positively with Hb levels at baseline but not at the follow-up (Fig. 1). At the follow-up 49% of the subjects used lipid-lowering medication compared to 3% at the baseline (Table S1).
Blood leucocyte levels associated positively with Hb levels in both sexes at baseline and this association remained in women but not in men at the follow-up (Fig. 1). For both sexes leptin levels associated positively with Hb levels at baseline but not at the follow-up (Fig. 1). Ghrelin levels associated negatively with Hb levels in women at baseline and in men at the follow-up (Fig. 1). Adiponectin levels associated negatively with Hb levels in men and women at both time points (Fig. 1). For both sexes ALT levels associated positively with Hb levels at both time points (Fig. 1).
Distribution of cardiovascular risk factors in Hb quartiles at baseline and follow-up
When assessing the distribution of cardiovascular risk factors in Hb quartiles (see Figure S1 for Hb ranges), significant differences in several characteristics, including anthropometric parameters, heart rate, serum lipids, glucose levels, insulin resistance, inflammatory factors, obesity-associated peptide hormones and liver parameters, between the study quartiles were observed at both time points while significant differences in bp and ABP were only observed at baseline (Table 1). The subjects belonging to the highest Hb quartile smoked more, consumed more alcohol, had higher BMI and used bp medication more compared to the other quartiles at both time points (Table 1). The usage of lipid-lowering medication was much more common in all Hb quartiles at the follow-up than baseline, being significantly higher in Hb quartiles 2 and 4 at the follow-up (Table 1). Although the prevalence of lung diseases increased in all Hb quartiles during the follow-up, no difference between the Hb quartiles was observed at either time point (Table 1). The highest Hb quartile at baseline retained the highest mean Hb levels also at the follow-up.
When smoking and alcohol consumption were used as covariates, waist circumference, heart rate, HDL cholesterol, HOMA-IR, fasting glucose and -insulin levels, 2 h glucose levels in OGTT, leucocytes and leptin and adiponectin levels differed significantly between the Hb quartiles at both time points, the highest Hb quartile always having the most adverse values (Table 1). When a further adjustment by BMI was done only the difference in leucocytes between the Hb quartiles remained significant at both time points (Table 1). At baseline the differences in systolic and diastolic bp, diastolic ABP and heart rate, serum triglycerides and ALT levels between the Hb quartiles remained after adjustments for smoking, alcohol consumption and BMI, and at the follow-up those for fasting glucose levels and 2 h glucose levels in OGTT (Table 1).
Association of Hb levels with fatty liver disease in a multivariate risk model
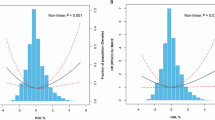
The highest Hb level quartile showed increased risk for liver fat accumulation in a multivariable logistic regression model after adjusting for covariates (Table 2). At baseline the Hb levels of the subjects with fat accumulation in the liver (n = 256) were significantly higher than those with no fat in the liver (n = 697) (Fig. 2A, Table S4) and the highest Hb level quartile showed higher serum ALT levels after adjusting for covariates (Table 1, Fig. 2B).
Association of Hb levels with fatty liver disease. (A) Hb levels of the subjects with no liver fat accumulation (n = 697) and subjects with liver fat accumulation (n = 256) at baseline. (B) Baseline mean ± SD of serum alanine aminotransferase (s-ALT) levels for the corresponding Hb quartiles. Hb quartile 1 has the lowest Hb and Hb quartile 4 the highest Hb levels. For S-ALT analysis was adjusted for smoking, alcohol consumption and BMI. Hb hemoglobin.
Association of baseline Hb levels with development of IFG, IGT and T2DM among normoglycemic subjects during the follow-up
We next assessed association of baseline Hb levels in normoglycemic subjects (n = 437) with the prevalence of IFG or IGT and T2DM at the 20-year follow-up (Fig. 3). The mean Hb levels had remained unchanged during the follow-up (Table S5). Mean baseline Hb levels were higher in subjects that developed IGF or IGT (n = 129) and subjects that developed T2DM (n = 98) during the follow-up compared to those who did not (Fig. 3). The highest baseline Hb levels were observed in those who developed T2DM during the follow-up after adjusting for covariates (Fig. 3, Table S5).
Prevalence of glucose intolerance/diabetes according to baseline Hb levels in previously normoglycemic subjects at the follow-up. Baseline mean ± SD Hb levels of 437 normoglycemic subjects shown in respect to glucose tolerance status at the follow-up. Normal glucose tolerance (normoglycemia), impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) and type 2 diabetes (T2DM) were determined according to the World Health Organization (WHO) criteria. Alcohol consumption, smoking, BMI, sex and age were included as covariates. Hb hemoglobin.
Survival rate analysis of CVD events and CVD-related and total mortality among the Hb quartiles
CVD events (n = 231, 23.8%), CVD-related mortality (n = 70, 7.2%) and total mortality (n = 213, 22%) were assessed with the Kaplan–Meier estimate survival analysis (Fig. 4). The highest Hb quartile had the highest risk for CVD events and CVD-related mortality (Fig. 4A,B). Interestingly, Hb quartile 2 had a similar CVD event-free survival probability to Hb quartile 4 while Hb quartiles 1 and 3 had significantly higher event-free probabilities (Fig. 4A). The highest Hb quartile also showed increased risk for total mortality while Hb quartiles 1–3 all had lower and similar survival probabilities (Fig. 4C). In sex-specific analyses, Hb quartile 4 in women had the lowest survival probability of all outcomes while in men it was the case for total and CVD-related mortalities but not for CVD events where Hb quartile 2 had the highest probability (Figure S2). When evaluated as events/100 person-years, total mortality, CVD mortality and CVD events were significantly highest in Hb quartile 4, the difference being more pronounced in women than men (Table S7, Figure S3).
Survival probability according to Hb quartiles. Kaplan–Meier graphs representing survival probabilities for CVD events (n = 231, 23.8%), CVD-related mortality (n = 70, 7.2%) and total mortality (n = 213, 22%) and in each Hb quartile. Hb quartile 1 has the lowest and Hb quartile 4 the highest Hb levels. (A) CVD events, (B) CVD-related mortality, (C) Total mortality. Hb hemoglobin.
Hazard ratios (HR) for CVD events, CVD-related mortality and total mortality among the Hb quartiles
HRs for CVD events, CVD-related mortality and total mortality were assessed with Cox regression models after adjusting for covariates. For CVD events there were no statistically significant differences between the Hb quartiles (Table 3, Table S6). For CVD-related and total mortality the highest Hb quartile (Hb quartile 4) showed a significantly higher HR than the other Hb quartiles (Table 3, Table S6).
Discussion
This long-term follow-up study presents higher Hb levels within Finnish reference values (117–155 g/L for women and 134–167 g/L for men) as an independent risk factor for deleterious cardiometabolic health and total and cardiovascular mortality.
We recently studied hypothesis-driven whether normal variation of Hb levels could be used as a surrogate measure for hypoxia25. We hypothesized that lower Hb levels were hypoxic and could result via hypoxia-inducible factor (HIF) prolyl 4-hydoxylase (HIF-P4H) inhibition to HIF-mediated reprogramming of energy metabolism and better metabolic health, as we had earlier shown for mice with genetic or pharmacologic inhibition of HIF-P4H38. We reported in a large (n = 7,175) systemic study in two Finnish birth cohorts studied until age of 46-years at cross-sectional and longitudinal design associations between Hb levels and many metabolic parameters25. Lower Hb levels associated with lower BMI, better glucose tolerance and other metabolic profiles, lesser inflammatory load and lower cholesterol and bp levels and similar associations were found for hematocrit levels and red blood cell counts with BMI25. The studied metabolic parameters associated with the expression on HIF target genes, such as glucose transporters, and suggest that Hb levels via regulation of tissue oxygenation can regulate energy metabolism via the HIF pathway, lower Hb levels being beneficial25. The findings reported here are in line and extend previous results by us25 and others.
The average Hb levels remained stable during the 20-year follow-up period in women and only declined 4 g/L (2.6%) in men. Associations with higher Hb levels and an adverse metabolic profile were observed in both sexes at baseline in average of 51-years suggesting that the individuals with the higher Hb levels possessed glucose intolerance, dyslipidaemia, hypertension, elevated heart rate and a higher inflammatory load. Most of these associations remained but weakened at the 20-year follow-up at average age of 72-years. The decline may have accounted from the increased usage of bp and lipid-lowering medication at the follow-up and by the increased mortality in the highest Hb quartile but likely other factors such as women becoming menopausal during the study contributed, too. In quartile comparisons the adverse metabolic profile was the most prominent in the highest Hb quartile mostly independent of smoking, alcohol consumption and in some cases of BMI. To extend the results we have previously reported in two cohorts studied until middle-age25, we show here that the association of Hb levels with an adverse metabolic profile is also observed in senescence. As an example, of the four Hb quartiles studied here, the highest Hb quartile was the only one that classified39 as obese by having an average BMI of 30.5 kg/m2 at the follow-up. High BMI is a major risk factor CVDs, musculoskeletal disorders (such as osteoarthritis) and some cancers (including breast, prostate and colon cancer)39.
Several associations between Hb levels and obesity-related peptide hormones were seen here.
Higher leptin levels and lower plasma ghrelin and adiponectin levels, a pattern well-established to be associated with obesity40,41, were observed in the highest Hb quartile at both time points. At baseline all differences were independent of smoking and alcohol consumption and in case of leptin levels, of BMI. At the follow-up the differences between Hb levels and leptin and adiponectin levels, respectively, were independent of smoking and alcohol consumption. Interestingly, the average leptin levels were significantly higher and those of ghrelin lower at the follow-up compared to baseline whereas adiponectin levels showed no obvious change. These results are most likely accounted by weight gain during the follow-up. In women this was probably also influenced by menopause as it increases leptin levels42 and lowers ghrelin levels43 by altering the levels of sex hormones but does not influence adiponectin levels44. To our knowledge this is the first study to report associations between Hb levels and serum ghrelin levels in adults while for leptin levels the previous reports have been conflicting our data agreeing with those in a Swedish senescent men but not middle-aged Japanese men18,19.
Several associations between Hb levels and ABP measurements were observed at baseline independent of covariates. Interestingly, many associations, most prominently those for systolic bp, disappeared at the follow-up. In sex-specific analysis a positive association between Hb levels and diastolic APB was observed at both time points in men while in women this was seen at both time points only when evaluated from the office bp measurements. This could reflect the treatment guidelines of the 1990’s where the focus was near-exclusively on treatment of systolic bp45,46. In addition, the usage of bp medication increased from 48 to 78% during the follow-up which may account for the loss of these associations. In addition, women are generally more aware of their condition and usually more committed to drug treatment than men47, which could explain the sex-specific differences observed. Incremental increases in 24 h ABP (seen here with increase in Hb levels at baseline) have been associated with greater risks of total and CVD-related mortality48. Although the increase in bp seen here was minor, it is evident that clustering of multiple cardiovascular risk factors increases morbidity of diseases such as coronary heart disease and consequent mortality49.
Higher Hb levels also associated with higher serum ALT levels and risk for liver fat accumulation after adjusting for covariates suggesting that higher Hb levels are an independent risk factor for fatty liver diseases. In addition, the highest Hb quartile was the only quartile with a mean s-ALT exceeding the previously reported thresholds for an increased risk for nonalcoholic steatohepatitis (> 35 U/I) and CVD-related mortality (> 40 U/I)50,51. Higher baseline Hb levels were also observed in previously normoglycemic subjects that had IFG, IGT or T2DM at the follow-up after adjusting for covariates, indicating that higher Hb levels could also be a risk factor for the development of T2DM. Furthermore, the highest Hb quartile was the only quartile at the follow-up having a mean 2 h glucose level in OGTT that exceeded the threshold of IGT or IFG set by the WHO (7.8 mmol/L).
The highest Hb quartile also had the lowest survival probability regarding total and CVD-related mortality. This could be the consequence of the positive associations of Hb levels with the key CVD predisposing parameters shown here. Furthermore, since only Hb values within the normal range were included in the analyses the HRs are most likely not affected by abnormally low or high Hb levels-related conditions, such as anaemia or thrombosis, respectively.
The outcomes of these data are in line with our previous data from mice. Mice that have chronically active HIF response due to deficiency in the key HIF-P4H isoenzyme, HIF-P4H-2, or wild-type mice treated with a small molecule HIF-P4H inhibitor, are leaner, have less adiposity and adipose tissue inflammation, they are protected against fatty liver disease of all aetiology and have better glucose tolerance38,52,53. In addition, these mice present several differences in the primary causes of death and comorbidities compared to wild-type mice, having e.g. less inflammation, liver diseases including hepatocellular cancer, and myocardial infarctions54. Furthermore, clinical trials for the treatment of renal anemia with novel HIF-P4H inhibitors roxadustat and daprodustat reported lowered serum cholesterol levels and an improved HDL/LDL lipoprotein profile in the subjects treated with the respected HIF-P4H inhibitors55,56.The similarities of these outcome with the Hb levels suggest that the protection against metabolic disorders, such as liver fat accumulation, dyslipidaemia and glucose intolerance, could be mediated by activation of the HIF response by lower tissue oxygen levels in the individuals with lower Hb levels and vice versa individuals with higher Hb levels being at risk.
One study limitation is the use of Hb levels as a surrogate measure for oxygen levels without saturation data. Lung diseases, such as chronic obstructive pulmonary disease or asthma could diminish tissue oxygenation via impaired pulmonary ventilation. This could lower Hb saturation which was not measured here. The prevalence of lung diseases however did not significantly differ between the Hb quartiles at either time point suggesting that the observed results were independent of them. Unfortunately, some of the well-known cardiovascular risk factors such as socioeconomic status, family history or nutritional information were not available for the regression analyses. However, the study population is Finnish representing one of the highest developed countries with free education and health care and highly developed welfare system. Although most data were adjusted for multiple covariates, residual confounding effects need to be considered as an explanatory factor. Also, we have not carried out correction for multiple testing. We believe, however that in the studies that are explanatory in nature, like the current one, it is more important to report all significant findings, even if suggestive, to be considered for replication in another study than taking the risk of rejecting the positive results.
In summary, higher Hb levels at middle-age associated extensively with co-morbidities of MetS at senescence proposing that high Hb levels are an additional risk factor of MetS. Although no clear evidence on causality is provided here, the association of Hb levels was seen extensively across the metabolic phenotype independent of key covariates. Altogether, these data suggest that slight tissue hypoxia, for example by lower endogenous Hb levels, mediate many beneficial effects to cardiometabolic health and increases survival.
Data availability
All data is provided in the paper.
Abbreviations
- ABP:
-
Ambulatory blood pressure
- ALT:
-
Alanine aminotransferase
- BP:
-
Blood pressure
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- Hb:
-
Hemoglobin
- HbA1c:
-
Glycated hemoglobin
- HDL:
-
High-density lipoprotein
- HIF:
-
Hypoxia-inducible factor
- HIF-P4H:
-
HIF prolyl 4-hydroxylase
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- IGT:
-
Impaired glucose tolerance
- IFG:
-
Impaired fasting glucose
- LDL:
-
Low-density lipoprotein
- MetS:
-
Metabolic syndrome
- NAFLD:
-
Non-alcoholic fatty liver disease
- OGTT:
-
Oral glucose tolerance test
- OPERA:
-
Oulu project elucidating risk of atherosclerosis
- T2DM:
-
Type 2 diabetes mellitus
References
Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet 365, 1415–1428 (2005).
Alberti, K. G., Zimmet, P. & Shaw, J. The metabolic syndrome—a new worldwide definition. Lancet 366, 1059–1062 (2005).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 960, 1–17 (2017).
Wu, S. H., Liu, Z. & Ho, S. C. Metabolic syndrome and all-cause mortality: A meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 25, 375–384 (2010).
Patel, K. V. Variability and heritability of hemoglobin concentration: an opportunity to improve understanding of anemia in older adults. Haematologica 93, 1281–1283 (2008).
van der Harst, P. et al. Seventy-five genetic loci influencing the human red blood cell. Nature 492, 369–375 (2012).
Serum proteomic analysis revealed diagnostic value of hemoglobin for nonalcoholic fatty liver disease. J. Hepatol. 56, 241–247 (2012).
Göbel, B. O. et al. Arterial blood pressure. Correlation with erythrocyte count, hematocrit, and hemoglobin concentration. Am. J. Hypertens. 4, 14–19 (1991).
Atsma, F. et al. Hemoglobin level is positively associated with blood pressure in a large cohort of healthy individuals. Hypertension 60, 936–941 (2012).
Hematocrit and hemoglobin are independently related to insulin resistance and compensatory hyperinsulinemia in healthy, non-obese men and women. Metabolism 47, 831–835 (1998).
Böttiger, L. E. & Carlson, L. A. Relation between serum cholesterol and triglyceride concentration and haemoglobin values in non-anaemic healthy persons. BMJ 3, 731 (1972).
Hämäläinen, P., Saltevo, J., Kautiainen, H., Mäntyselkä, P. & Vanhala, M. Erythropoietin, ferritin, haptoglobin, hemoglobin and transferrin receptor in metabolic syndrome: a case control study. Cardiovasc. Diabetol. 11, 116 (2012).
Hämäläinen, P., Saltevo, J., Kautiainen, H., Mäntyselkä, P. & Vanhala, M. Hemoglobin level and lipoprotein particle size. Lipids Health Dis. 17, 2 (2018).
Hashimoto, Y. et al. Hemoglobin concentration and incident metabolic syndrome: A population-based large-scale cohort study. Endocrine 50, 390–396 (2015).
Bae, J. C. et al. Hemoglobin A1c values are affected by hemoglobin level and gender in non-anemic Koreans. J. Diab. Investig. 5, 60–65 (2014).
Streeter, E. A. et al. Adiponectin, hemoglobin, and cardiovascular risk in an indigenous siberian population. Am. J. Hum. Biol. 28, 580–583 (2016).
Togo, M. et al. Relationship between levels of leptin and hemoglobin in Japanese men. Blood 93, 4444–4445 (1999).
Lewerin, C. et al. High serum adiponectin is associated with low blood haemoglobin in elderly men: The Swedish MrOS study. J. Intern. Med. 278, 68–76 (2015).
Hemoglobin and Change in Hemoglobin Status Predict Mortality. Cardiovascular events, and bleeding in stable coronary artery disease. Am. J. Med. 130, 720–730 (2017).
Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in communities (aric) study. J. Am. Coll. Cardiol. 40, 27–33 (2002).
Lee, G. et al. Association of hemoglobin concentration and its change with cardiovascular and all-cause mortality. J. Am. Heart Assoc. 7, 2 (2018).
Kabat, G. C. et al. Association of hemoglobin concentration with total and cause-specific mortality in a cohort of postmenopausal women. Am. J. Epidemiol. 183, 911–919 (2016).
Zakai, N. A. et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: The cardiovascular health study. Arch. Intern. Med. 165, 2214–2220 (2005).
Auvinen, J. et al. Systematic evaluation of the association between hemoglobin levels and metabolic profile implicates beneficial effects of hypoxia. Sci. Adv. 7, 4822. https://doi.org/10.1126/sciadv.abi4822.Print2021Jul (2021).
Kairisto, V. et al. New Finnish reference limits for the basic blood count. Lääkärilehti 51–52, 5147–5153 (2003).
Grimby, G. Physical activity and muscle training in the elderly. Acta Med. Scand. Suppl. 711, 233–237 (1986).
Recommendations for routine blood pressure measurement by indirect cuff sphygmomanometry. Am. J. Hypertens. 5, 207–209 (1992).
Verdecchia, P. Prognostic value of ambulatory blood pressure: Current evidence and clinical implications. Hypertension 35, 844–851 (2000).
Ylitalo, A. Cardiovascular Autonomic Regulation in Systemic Hypertension (Oulun yliopisto, 1999).
Ballestri, S., Romagnoli, D., Nascimbeni, F., Francica, G. & Lonardo, A. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Exp. Rev. Gastroenterol. Hepatol. 9, 603–627 (2015).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998).
Hietaniemi, M., Pöykkö, S. M., Ukkola, O., Päivänsalo, M. & Antero Kesäniemi, Y. IGF-I concentrations are positively associated with carotid artery atherosclerosis in women. Ann. Med. 37, 373–382 (2005).
Santaniemi, M., Kesäniemi, Y. A. & Ukkola, O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur. J. Endocrinol. 155, 745–750 (2006).
Falkén, Y., Hellström, P. M., Holst, J. J. & Näslund, E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J. Clin. Endocrinol. Metab. 96, 2227–2235 (2011).
Rantala, A. O. et al. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J. Intern. Med. 245, 163–174 (1999).
Pajunen, P. et al. Body fat measured by a near-infrared interactance device as a predictor of cardiovascular events: The FINRISK’92 cohort. Obesity (Silver Spring) 19, 848–852 (2011).
Rahtu-Korpela, L. et al. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 63, 3324–3333 (2014).
WHO obesity and overweight key fact sheet. https://www.who.int/health-topics/obesity#tab=tab_1 (2016).
Tschöp, M. et al. Circulating ghrelin levels are decreased in human obesity. Diabetes 50, 707–709 (2001).
Arita, Y. et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 (1999).
Di Carlo, C., Tommaselli, G. A. & Nappi, C. Effects of sex steroid hormones and menopause on serum leptin concentrations. Gynecol. Endocrinol. 16, 479–491 (2002).
Sowers, M. R. et al. Change in adipocytokines and ghrelin with menopause. Maturitas 59, 149–157 (2008).
Sieminska, L. et al. Effect of postmenopause and hormone replacement therapy on serum adiponectin levels. Metabolism 54, 1610–1614 (2005).
Izzo, J. L. Jr., Levy, D. & Black, H. R. Clinical advisory statement. Importance of systolic blood pressure in older Americans. Hypertension 35, 1021–1024 (2000).
Flint, A. C. et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N. Engl. J. Med. 381, 243–251 (2019).
Wolf-Maier, K. et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension 43, 10–17 (2004).
Yang, W. Y. et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA 322, 409–420 (2019).
Berenson, G. S. et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N. Engl. J. Med. 338, 1650–1656 (1998).
Verma, S., Jensen, D., Hart, J. & Mohanty, S. R. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 33, 1398–1405 (2013).
Yun, K. E., Shin, C. Y., Yoon, Y. S. & Park, H. S. Elevated alanine aminotransferase levels predict mortality from cardiovascular disease and diabetes in Koreans. Atherosclerosis 205, 533–537 (2009).
Laitakari, A. et al. HIF-P4H-2 inhibition enhances intestinal fructose metabolism and induces thermogenesis protecting against NAFLD. J. Mol. Med. (Berl) 98, 719–731 (2020).
Laitakari, A. et al. Systemic inactivation of hypoxia-inducible factor prolyl 4-hydroxylase 2 in mice protects from alcohol-induced fatty liver disease. Redox Biol. 22, 101145 (2019).
Laitakari, A. et al. Systemic long-term inactivation of hypoxia-inducible factor prolyl 4-hydroxylase 2 ameliorates aging-induced changes in mice without affecting their life span. FASEB J. 34, 5590–5609 (2020).
Olson, E. et al. Short-term treatment with a novel HIF-prolyl hydroxylase inhibitor (GSK1278863) failed to improve measures of performance in subjects with claudication-limited peripheral artery disease. Vasc. Med. 19, 473–482 (2014).
Provenzano, R. et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin. J. Am. Soc. Nephrol. 11, 982–991 (2016).
Acknowledgements
The authors thank Markku Päivänsalo for liver ultrasound examinations, and Saija Kortetjärvi, Heidi Häikiö and Leena Ukkola for the excellent technical assistance. Elina Malo and Meiju Saukko are thanked for their co-operation in organizing cardiovascular event and mortality data.
Funding
This study was supported by the Academy of Finland Grant 308009, the S. Jusélius Foundation and the Jane and Aatos Erkko Foundation to P.K. and by the Finnish Foundation for Cardiovascular Research to O.U.
Author information
Authors and Affiliations
Contributions
J.T.: conceptualization, investigation, writing original draft, visualization. H.V.: formal analysis, review and editing writing. Y.A.K.: resources. O.U.: conceptualization, investigation, resources, review and editing writing. P.K.: conceptualization, investigation, writing original draft, supervision, project administration, funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tapio, J., Vähänikkilä, H., Kesäniemi, Y.A. et al. Higher hemoglobin levels are an independent risk factor for adverse metabolism and higher mortality in a 20-year follow-up. Sci Rep 11, 19936 (2021). https://doi.org/10.1038/s41598-021-99217-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99217-9
This article is cited by
-
Hemoglobin levels are associated with retinal vascular caliber in a middle-aged birth cohort
Scientific Reports (2024)
-
The Importance of Hypoxia-Related to Hemoglobin Concentration in Breast Cancer
Cell Biochemistry and Biophysics (2024)
-
Retrospective cohort study of pulmonary arterial hypertension associated with connective tissue disease effect on patients’ prognosis
Clinical Rheumatology (2023)
-
An efficient edge/cloud medical system for rapid detection of level of consciousness in emergency medicine based on explainable machine learning models
Neural Computing and Applications (2023)
-
Higher hemoglobin levels are an independent risk factor for gestational diabetes
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.