Abstract
Food additives (e.g. artificial sweeteners, emulsifiers, dyes, etc.) are ingested by billions of individuals daily. Some concerning results, mainly derived from animal and/or cell-based experimental studies, have recently emerged suggesting potential detrimental effects of several widely consumed additives. Profiles of additive exposure as well as the potential long-term impact of multiple exposure on human health are poorly documented. This work aimed to estimate the usual intake of food additives among participants of the French NutriNet-Santé cohort and to identify and describe profiles of exposure (single substances and mixtures). Overall, 106,489 adults from the French NutriNet-Santé cohort study (2009-ongoing) were included. Consumption of 90 main food additives was evaluated using repeated 24 h dietary records including information on brands of commercial products. Qualitative information (as presence/absence) of each additive in food products was determined using 3 large-scale composition databases (OQALI, Open Food Facts, GNPD), accounting for the date of consumption of the product. Quantitative ingested doses were estimated using a combination of laboratory assays on food matrixes (n = 2677) and data from EFSA and JECFA. Exposure was estimated in mg per kg of body weight per day. Profiles of exposure to food additive mixtures were extracted using Non-negative Matrix Factorization (NMF) followed by k-means clustering as well as Graphical Lasso. Sociodemographic and dietary comparison of clusters of participants was performed by Chi-square tests or linear regressions. Data were weighted according to the national census. Forty-eight additives were consumed by more than 10% of the participants, with modified starches and citric acid consumed by more than 90%. The top 50 also included several food additives for which potential adverse health effects have been suggested by recent experimental studies: lecithins (86.6% consumers), mono- and diglycerides of fatty acids (78.1%), carrageenan (77.5%), sodium nitrite (73.9%), di-, tri- and polyphosphates (70.1%), potassium sorbate (65.8%), potassium metabisulphite (44.8%), acesulfame K (34.0%), cochineal (33.9%), potassium nitrate (31.6%), sulfite ammonia caramel (28.8%), bixin (19.5%), monosodium glutamate (15.1%) and sucralose (13.5%). We identified and described five clusters of participants more specifically exposed to five distinct additive mixtures and one additional cluster gathering participants with overall low additive exposure. Food additives, including several for which health concerns are currently debated, were widely consumed in this population-based study. Furthermore, main mixtures of additives were identified. Their health impact and potential cocktail effects should be explored in future epidemiological and experimental studies.
Similar content being viewed by others
Introduction
Food additives are substances intentionally added to foods during processing, treatment, packaging, transport, or storage. They are used for various technological, sensory and nutritional purposes, such as prolonging shelf life, sweetening, modifying or stabilizing consistency, enhancing taste, and enhancing or preserving color1. With a global market exceeding $64 billion, dozens of food additives are ingested daily by billions of individuals worldwide. At the international level, the Codex General Standard for Food Additives (GSFA, Codex STAN 192–19952) sets out the conditions for the use of food additives in food products. In Europe, they represent about 330 authorized compounds under the Regulation (EC) No 1333/2008, and their toxicity is evaluated by the European Food Safety Authority (EFSA). Despite the considerable amount of work for literature review and collective expertise performed by national and international institutions, these evaluations can only be based on currently available scientific data, i.e. mainly in-vitro or in-vivo experimental research and simulations of exposure in humans. Information regarding the health impact of regular and cumulative intake of food additives in humans and the potential ‘cocktail’ effects/interactions of mixtures is still missing.
Recently, evidence has accumulated suggesting an association between the consumption of “ultra-processed foods”3 (which generally contain a wide range of food additives) and increased risk of several chronic diseases, with more than 25 epidemiological studies published worldwide4,5,6, including several from the NutriNet-Santé cohort7,8,9,10,11,12,13. Beyond a poorer nutritional quality on average7,14,15,16,17, potential presence of neo-formed compounds and substances migrating from packaging, one of the main hypotheses postulated to explain these results is the presence of food additives18,19,20,21,22,23,24,25,26,27. New research is needed to better understand health impacts of food processing, formulation and packaging, to be able to provide consumers with safer and more sustainable food products in the coming years28,29.
Previous study conducted by this group investigated the distribution and co-occurrence of food additives in a large-scale database of food products available on the French market30. Food additives were widespread with more than 50% of industrial food products containing at least 1 and 11.3% at least 5 additives. Most additives probably have no detrimental impact on health (some may even have beneficial effects: e.g. anti-microbial, antioxidants, polyphenols), however, some concerning results, mainly derived from animal and/or cell-based experimental studies, have recently emerged regarding several additives. For instance, nitrates/nitrites24,25,26, carrageenans31, glutamate32,33,34, bixin35,36, artificial sweeteners21,37,38,39,40, phosphates41,42, emulsifiers43,44,45,46, caramel47, tartrazine48,49 and butylated hydroxyanisole/ butylated hydroxytoluene (BHA/BHT)48 were previously linked to metabolic, gut microbiota or endocrine perturbations along with carcinogenic, inflammatory and/or oxidative stress effects. Also, in May 2021, following an updated evaluation, EFSA reviewed its position on titanium dioxide (TiO2) and stated that this additive could no longer be considered as safe22,50,51,52. Besides, some experimental results suggest potential interactions between some food additives and/or with the food matrix leading to synergistic or antagonist effects on health 4, yet profiles of exposure to additive mixtures in humans are poorly documented.
As a prerequisite for future etiological studies, the present work aimed (1) to estimate the average daily intake of a wide range of food additives in French adults, using detailed data collected in the NutriNet-Santé cohort and (2) to identify the main mixtures of food additives consumed and describe the corresponding profiles of consumers in terms of socio-demographic characteristics, food additive intake and food consumption. The novelty of this work lies in the fact that, to our knowledge, no large-scale population-based study previously estimated the exposure to such a wide variety of food additives with this degree of accuracy, identifying the main mixtures consumed.
Methods
Study population
NutriNet-Santé is a French ongoing web-based cohort launched in 2009, which aims to study the associations between nutrition and health as well as the determinants of eating behaviors and nutritional status. It was previously described in detail53. Briefly, the only eligibility criteria is to be aged 18 years or older and to have access to the internet. Various means are used for the recruitment of participants from the general population. First, large and repeated multimedia campaigns (television, radio, national and regional newspapers, posters, and Internet) disseminate information about the study and its website. The call for participation is also posted regularly on various websites (national health institutions, city councils, private firms) and regularly relayed by professional channels (e.g., general practitioners and medical specialists, pharmacists, dentists, municipalities)54. Recruitment is still open. Participants are tracked using an online platform connected to their email address and questionnaires are completed online on a dedicated website (https://etude-nutrinet-sante.fr). All participants provide an electronic informed consent. The NutriNet-Santé study is registered on ClinicalTrials.gov as NCT03335644 and is conducted according to the Declaration of Helsinki guidelines and is approved by the Institutional Review Board of the French Institute for Health and Medical Research (IRB Inserm) and the “Commission Nationale de l’Informatique et des Libertés” (CNIL n°908,450/n°909,216).
Data collection
At inclusion and at least each year thereafter, participants were asked to complete a set of five questionnaires related to sociodemographic and lifestyle characteristics (e.g., date of birth, sex, educational level, smoking status)55, anthropometry56,57, physical activity (validated seven day International Physical Activity Questionnaire, IPAQ)58, health status, and dietary intakes.
Dietary assessment
At inclusion, and every six months thereafter (to vary the season of completion) participants completed a series of three non-consecutive, web-based 24 h-dietary records (validated against an interview by a trained dietitian59 and against blood and urinary biomarkers60,61), randomly assigned over a two-week period (two week-days and one weekend day). Participants reported all foods and beverages consumed for the three main meals and on any additional eating occasions. Portion sizes were estimated by participants using validated photographs or usual serving containers62. To assess daily intakes of macronutrients, micronutrients, alcohol, and total calories, dietary consumption data were linked to the NutriNet-Santé food composition database which contains more than 3,500 generic items63. Besides, for each industrial product, the brand and commercial name were collected. Intakes from composite dishes were estimated by referring to French recipes as defined by nutrition professionals. Baseline habitual dietary intakes were averaged from all 24 h-dietary records provided during the first two years of follow-up (at least two 24 h records mandatory for inclusion in the study). Identification of dietary under-reporting was identified based on the method proposed by Black, by using the basal metabolic rate and Goldberg cut-off, and under-reporters of energy intake were excluded64.
Food additives
The determination of food additive exposure is described in detail in Appendix 1. Briefly, for each food (or beverage, candy, chewing-gum) consumed, the presence of each food additive (qualitative composition data) and if relevant, its dose (quantitative composition data) were searched by the research team (no specific knowledge on food additives or their classification was required from the participants). To determine the qualitative presence/absence of food additives, three databases were used: OQALI65, a national database hosted by the French food safety agency (ANSES) to characterize the quality of the food supply, Open Food Facts, an open collaborative database of food products marketed worldwide66, and Mintel GNPD67, an online database of innovative food products in the world. When several composition data existed for a same product at different dates (reformulations), the date of consumption in the cohort (year) was considered in the matching of composition data (dynamic matching).
The quantitative composition of additives has been derived from several sources. Firstly, 2677 ad-hoc laboratory assays were carried out for the main additive-vector food pairs (ad hoc assays commended by our laboratory or by the consumers’ association "UFC Que Choisir"), prioritizing the most consumed additives and those with suspected health effects. The second choice was the use of doses by food categories transmitted by EFSA. Last, when no dose was available neither from assays nor from EFSA, doses from the Codex General Standard for Food Additives (GSFA)2 were used. The decision tree in Appendix 1 describes this process in detail. Despite this multi-source assessment, no dose data was available for some additive-vector food pairs (however, it mostly concerned some additives that were consumed by less than 10% of the participants). Thus, only additives for which > 80% of the declarations could be matched to a dose were retained for analysis (i.e. 90 additives). For these, missing doses were imputed by taking the average dose in all food products containing the specific additive.
Statistical analyses
Descriptive analyses were weighted according to the 2016 French national census report data by using the CALMAR macro run by sex and based on categories for age, socio-professional status and housing area68. Intakes of each food additive were described in mg per day as well as in mg/kg bodyweight per day (% of consumers, mean, SD, median, 95th percentile). Toxicity of each food additive is assessed by EFSA to determine its Acceptable Daily Intake (ADI), which is then used to set maximum authorized levels in the different food groups. However, additives without a specified ADI can be used quantum satis, i.e., with ‘no limitations other than current good manufacturing practice'. Proportion of participants exceeding the ADI69 (when available) were calculated for each food additive. To evaluate the variation that may have been caused by reformulations across a 10y period, the top 50 most consumed food additives was compared between 3 different periods in a sensitivity analysis (2009–2013/2013–2017/2017–2020).
Nonnegative Matrix Factorization (NMF) was used to determine food additive profiles of exposure. This size reduction technique is specifically adapted to sparse matrixes containing positive values70. It is described in detail in Appendix 2. Choice of algorithm was carried out according to the measure of residuals and of sparseness71, and the number of ranks r was determined according to the method proposed by Brunet et al72, using the smallest value of r for which this coefficient starts decreasing. The NMF was performed using the R package NMF73. Then, the scores arising from the components were scaled and introduced to a k-means clustering process, and the number of clusters of participants was determined using the elbow method, which examines the percentage of variance explained depending on the number of clusters.
Clusters of participants were described in terms of socio-demographic characteristics, food additive intake and food consumption. Regarding food and food additive consumption, means adjusted for energy intake and number of dietary records were used for description. Comparisons between clusters were performed by Chi-square tests or linear regression, as appropriate.
Partial correlation corresponds to the degree of association between two variables, controlling for other variables. To visualize the partial correlations between food additives, a network was generated using the glasso package74 which computes a sparse gaussian graphical model with graphical lasso75. It can be interpreted as follows: when two food additives are connected by a blue line, it means that they are more consumed by the same participants, when they are connected by a red line, it means that they are rarely consumed by the same participants. Bolder is the line, higher is the correlation. The network was generated for the 90 analyzed food additives.
R version 3.6.2 (R Foundation, Vienna, Austria) was used for the analyses.
Results
Most consumed food additives
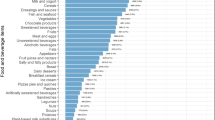
Up to 132,222 participants had at least two 24-h dietary records during the first two years of follow-up. After exclusion of under-reporters, 106,489 participants (69% women) were included for analyses (flowchart is in Appendix 3). Mean age at baseline was 42.9 years (SD = 16.1), and mean number of dietary records was 5.6 (SD = 3.1). Table 1 displays mean and median intakes, by percentage of consumers for each additive. Figure 1 illustrates the 50 most frequently consumed food additives, by percent of consumers. Forty-eight additives were consumed by more than 10% of the participants, with modified starches and citric acid consumed by more than 91.1%. The top 50 also included several food additives for which adverse health effects have been suggested by experimental studies: lecithins (86.6% consumers), mono- and diglycerides of fatty acids (78.1%), carrageenan (77.5%), sodium nitrite (73.9%), di-, tri- and polyphosphates (70.1%), potassium sorbate (65.8%), potassium metabisulphite (44.8%), acesulfame K (34.0%), cochineal (33.9%), potassium nitrate (31.6%), sulfite ammonia caramel (28.8%), bixin (19.5%), monosodium glutamate (15.1%) and sucralose (13.5%). Very little changes were observed in the top 50 generated across three different periods (2009–2013, 2013–2017, 2017–2020) (Appendix 4). Appendix 5 describes the main contributors to each food additive intake in term of number of declarations. In general, the proportion of participants exceeding the ADI was limited, but higher when considering consumers only, with highest proportions being observed for monosodium glutamate (7.07% among all participants, 46.74% among consumers of this specific additive), extracts of rosemary (0.32% overall, 1.36% among consumers), iron oxides and hydroxides (0.23% overall, 4.40% among consumers), sodium metabisulphite (0.18% overall, 4.35% among consumers), potassium sorbate (0.15% overall, 0.23% among consumers), beta-apo-8'-carotenal (0.13% overall, 6.95% among consumers), potassium metabisulphite (0.11% overall, 0.24% among consumers) and calcium phosphates (0.10% overall, 0.71% among consumers).
Food additive mixtures derived by NMF and clusters of participants according to additive intake
The NMF procedure resulted in 5 components that discriminated food additive exposure profiles (Appendix 6a). Figure 2 displays the network of partial correlation of food additives generated with graphical lasso. This method, complementary to NMF, yielded overall consistent results in terms of mixtures of additives identified.
After scaling NMF components of food additive mixtures, k-means clustering was performed and 6 clusters of participants were extracted using the elbow method. Appendix 6b shows the mean of each scaled NMF component, by cluster of participants. Each of the first 5 clusters mostly corresponded to one of the 5 specific food additive mixture NMF component, while cluster 6 corresponded to the participants with an overall low additive exposure. Table 2 displays sociodemographic and lifestyle characteristics according to clusters of participants. Table 3 and 4 display the mean (SD) consumption of food additives and food groups (respectively) by cluster, adjusted for energy intake and number of dietary records. Figure 3 displays a synthesis of cluster’s consumptions of food additives and food groups. The clusters were described as follows:
Cluster 1: Consumers of additives found in cookies and sweet cakes
This cluster constituted 9.8% of the study sample. Participants from this cluster presented notably the highest intakes of e322 lecithins, e471 mono-and diglycerides of fatty acids, e500 sodium carbonates, e450 diphosphates, e503 ammonium carbonates, e422 glycerol and e420 sorbitol. Participants from this cluster displayed the highest proportion of postgraduate and non-smoker individuals and had the lowest mean BMI. They had the highest caloric and lipid intakes, but the lowest protein, alcohol and sodium intakes. They were the highest consumers of fatty and sweet cakes and cookies (consistent with their higher intakes of lecithins, mono-and diglycerides of fatty acids, sodium carbonates and glycerol) and fatty and salty products.
Cluster 2: Consumers of additives found in broths, meal substitutes, butter and bread
This cluster constituted 14.7% of the sample. They were the highest consumers of e14xx modified starches, e621 monosodium glutamate, e304 fatty acid esters of ascorbic acid and e320 butylated hydroxyanisole (BHA). Participants from this cluster were notably the oldest and the most physically active, with the lowest proportion of current smokers. They had the highest intake of sodium, the lowest lipid intake and were among the highest consumers of butter and margarines, meal substitutes and broth (consistent with the high intakes of monosodium glutamate and BHA).
Cluster 3: Consumers of additives found in dairy desserts, breakfast cereals and pastries
This cluster constituted 8.4% of the sample. They were notably the highest consumers of e407 carrageenan, e270 lactic acid, e282 calcium propionate, e452 polyphosphates, e160b annatto, e1442 hydroxy propyl distarch phosphate. These participants had relatively high carbohydrate intakes. They were the highest consumers of dairy desserts, which could explain the high intakes of carrageenans, lactic acid and hydroxy propyl distarch phosphate. They were also high consumers of pastries (consistent with higher intakes of calcium propionate), sweetened breakfast cereals and cereal bars.
Cluster 4: Consumers of additives found in industrial sauces and processed meat
This cluster constituted 12.3% of the sample. They notably had the highest intakes of e250 sodium nitrite, e316 sodium erythorbate, e451 triphosphates and e120 cochineal which are particularly used in processed meat. They also had the highest intakes of e330 citric acid, e415 xanthan gum, e202 potassium sorbate, e412 guar gum, e224 potassium metabisulphite and e150a plain caramel. This cluster included the highest proportion of men and had an overall lower level of education. They had the lowest carbohydrate intake. They were the highest consumers of bread, fish, rice, semolina, dressings and sauces (the latest being consistent with higher intakes of e415 xanthan gum, e202 potassium sorbate, e412 guar gum, e224 potassium metabisulphite). They were also high consumers of processed meat and pork and poultry hams (consistent with higher intakes of sodium nitrite, sodium erythorbate, triphosphates and cochineal).
Cluster 5: Consumers of additives found in sugary and artificially sweetened beverages
This cluster constituted 2.6% of the sample. They were notably the highest consumers of the 4 main sweeteners (e950 acesulfame K, e951 aspartame, e955 sucralose, e960 steviol glycosides), and of e440 pectins, e160a carotenes, e331 sodium citrates, e301 sodium ascorbate, e160c paprika extract, e150d sulphite ammonia caramel, e100 curcumin, e252 potassium nitrate, e338 phosphoric acid, e161b lutein, e211 sodium benzoate, e472 esters of mono- and diglycerides and e212 potassium benzoate. These participants had higher BMI, were the youngest, had the lowest physical activity and were more likely to be smokers. They had an intermediate caloric intake, the highest protein and UPF intakes, and the lowest proportion of organic food in their diet. They were notably the highest consumers of non-alcoholic sweetened and unsweetened drinks (including sugary and artificially sweetened sodas, in line with higher intakes of sweeteners, sodium and potassium benzoates, sodium citrates, phosphoric acid and sulphite ammonia caramel), processed meat, pork and poultry hams (consistent with higher intakes of potassium nitrate), and table-top sweeteners in powder.
Cluster 6: Consumers of various staple foods with low additive content
This cluster constituted 52.1% of the study sample, with the highest proportion of women (74.3%). It presented the lowest mean intakes for all food additives. It was characterized by its lower caloric intake, higher proportion of organic food and lower proportion of UPF in the diet, and higher alcohol intake. Participants of this cluster were high consumers of "staple foods": whole-grain products, pulses, breakfast cereals with little or no added sugar, vegetable juice, oleaginous fruits, vegetable oils, and cheese.
Discussion
To our knowledge, this large population-based study was the first to estimate chronic exposure to food additive mixtures based on detailed consumption and composition data for a wide range of substances. Forty-eight additives were consumed by more than 10% of the participants, with modified starches and citric acid consumed by more than 90%. The top 50 also included several food additives for which potential adverse health effects have been suggested by recent experimental studies. We identified and described five clusters of participants more specifically exposed to five additive mixtures and one additional cluster gathering participants with overall low additive exposure.
Since 2012, EFSA has started the re-evaluation of all food additives authorized before January 2009. The agency's opinions on an additive are subject to change as evidenced by the update on TiO2, which is no longer considered as safe. EFSA has carried out simulations of exposure, combining average food consumption data from European member states with doses of additives reported by manufacturers, for additives and countries for which such data were available. Overall, when comparing exposure estimates with EFSA's, intakes of the NutriNet-Santé population were relatively lower. For instance, for modified starches, we estimated a mean intake of 24.33 mg/kg bodyweight per day (95th percentile: 66.4 mg/kg), versus 112.0 mg/kg bodyweight/day (95th percentile: 235.5 mg/kg) in EFSA's non-brand-loyal scenario (French population group)76. Similarly for lecithins: 0.83 mg/kg bodyweight per day (95th percentile: 2.6 mg/kg), versus 6.0 mg/kg bodyweight/day (95th percentile: 13.0 mg/kg)77. This overall lower exposure may in part be due to the more health-conscious profiles of NutriNet-Santé participants. However, this may also be due to methodological differences: in the present study, presence/absence of food additives was precisely determined based on the commercial brand and the precise list of ingredients, whereas EFSA stimulations use an average information by product category. Some other studies performed intake estimations for several specific food additives, in particular nitrites/nitrates, colors, monosodium glutamate and sulfites33,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92. Although comparisons of different populations are not straightforward, some similarities in the exposure estimates were observed. For instance, in China, similar mean intakes of monosodium glutamate were found: mean (SD): 2.2 (1.6) g/d33 versus 2.4 (20.2) in the present study. However, as for EFSA simulations and except in rare cases, these studies were based on generic food data (not accounting for the specific brand consumed and thus the precise ingredient list). Besides, these studies focused on one specific additive or a very limited number of additives, which did not permit the investigation of mixtures. A recent study by the French food observatory “Observatoire de l’Alimentation” (Oqali) evaluated the occurrence of certain food additives in a selection of food products of the French market93. The additives most frequently found where consistent with the most consumed in our study (e.g. citric acid, modified starches and lecithins in the top 3; acesulfame K as the most used/consumed sweetener).
For several food additives widely consumed in this study, potential adverse health effects have been suggested by recent in-vivo/in-vitro, and-rarely-epidemiological studies. For instance, an experiment in humans demonstrated that phosphatidylcholine found in lecithin is converted by bacteria in the gut into trimethylamine-N-oxide, which may potentially contribute to hardening of the arteries or atherosclerosis and heart attack94. A potential role in the development of Chron's disease has also been suggested for lecithins95,96,97, and an experimental study among humans suggests a link between lecithins and coronary artery disease through the production of a proatherosclerotic metabolite, trimethylamine-N-oxide (TMAO)94. In a study on ex-vivo models of human microbiota, 20 emulsifiers were tested and a large majority (including carboxymethylcellulose, polysorbate 80, carrageenans, guar/xanthan gums, lecithins), were able to directly modify the gut microbiota in a way that could promote gut inflammation23. Carrageenans have been linked to fasting hyperglycemia and exacerbated glucose intolerance and hyperlipidemia without effect on weight in mice31. Also, sodium nitrite and potassium nitrate intakes have been associated in prospective cohorts with all-cause mortality (nitrates/nitrites from preserved/processed meat)24, and colorectal, gastric and pancreatic cancers25,26,27,98, although their impact remains debated. Phosphates have been associated with vascular effects (e.g. endothelial dysfunction and vascular calcification) in experimental studies among humans41,42. Sulfites have been associated with alteration of the gut and mouth microbiome in-vitro at concentrations close to those found in foods99. The effects of non-nutritive sweeteners such as acesulfame K, sucralose and aspartame on human cardiometabolic health and adiposity are controversial37, and these additives have been linked with hematopoietic neoplasia and gut microbiota alteration in experimental studies on rodents21,38,39,40,40. Sulfite ammonia caramel, present in almost every cola sodas, might carry 4-methylimidazole (4-MEI) defined as possibly carcinogenic to humans by the International Agency for Research on Cancer (IARC). Monosodium glutamate might have patho-physiological and toxicological effects on human health32,34 and was associated with overweight in a prospective cohort33. Carboxymethylcellulose has been associated with changes in microbiota composition, intestinal inflammation and metabolic syndrome (in-vivo)43,100,101,102,102, pro-inflammation (in-vivo, ex-vivo)46,103,104,105,106 and promotion of tumor development (in-vivo)45. In mouse models, it was recently shown that in the presence of intestinal inflammation, the food additive ethylenediaminetetraacetate (EDTA) was capable of exacerbating inflammation and inducing colorectal carcinogenesis at doses presumed to be safe107.
The NMF procedure followed by k-means clustering allowed us to describe profiles of exposure to mixtures of food additives, which corresponded to specific socio-demographic profiles and dietary behaviors. Although about half of the population study pertained to cluster 6 and tended to have a relatively limited exposure to food additives overall. The other half of the study population was exposed to different additive mixtures (5 main mixtures identified). In a previous work consisting in the exploration of the Open Food Facts database, we identified clusters of additives found in food products of the French market30. The mixtures of food additives identified in the present work resulted 1) from the co-occurrence of several additives in a same industrial product (as shown previously30) and 2) from the co-consumption of various food products within usual dietary patterns. For instance, participants of cluster 1 were notably the highest consumers of sweet cakes and cookies, thus, they were particularly exposed to food additives of a specific cluster in our previous work ("stabilizers and emulsifiers mostly used in biscuits and cakes").
So far, detailed information on potential cocktail effects of food additives is lacking. However, several studies started to suggest potential interactions and synergies. For instance, mixture of colorings with sodium benzoate were associated with increased hyperactivity in children108. Neurotoxic effects were also observed between combinations of brilliant blue with L-glutamic acid and quinoline yellow with aspartame in-vitro109 and a mixture of food coloring additives increased oxidative stress in rats110. Future prospective studies and experimental research should investigate the health effects of chronic exposure to these mixtures of food additives, as they are consumed in real life.
Strengths of this study included the large sample size and the accuracy of dietary intake data used to estimate additive exposure at the individual level, which is necessary for future etiological studies (population-based simulations are not appropriate for this purpose). Indeed, repeated 24 h records allowed us to collect detailed information on > 3500 generic foods/beverages, each declined in dozens of commercial brands, which is a strength compared to previous nutritional studies. Three complementary databases were used to determine qualitative additive composition and thousands of assays were performed and complemented by EFSA and GSFA data to retrieve information on quantitative doses. However, some limitations should be acknowledged. First, not all food additives could be covered due to a lack of quantitative data for some additives. However, the latter were not the most relevant in terms of potential public health impact since they were mostly consumed by less than 10% of the population. Second, as it is generally the case for cohorts with a primary etiological focus, the recruitment method was based on a voluntary participation and the study population was not intended to be representative of the French population. Thus, the individuals included in the cohort were more often women, with "healthier" behaviors, a higher socioeconomic status and a higher level of education than the general French population111,112. However, even if lowest socioeconomic statuses were under-represented, the cohort still included about 6% of unemployed citizens or state aid recipients, which is lower than the national ≈10%, but higher than in other health studies that are not Internet-based. Moreover, the geographical distribution of the cohort was close to that of metropolitan France113. Also, the proportion of energy intake brought by ultra-processed foods (i.e. the main sources of food additives) among the participants of the cohort was 30–35%, consistent with the 31% assessed in two French nationally representative surveys114,115. Besides, a potential selection bias has been minimized since all analyses were weighted according to the characteristics of the French population (INSEE 2016 census). Last, industrial products may be reformulated across time by choice of manufacturers or regulation requirements, thereby complicating exposure assessment. However, bias linked to this aspect was limited in the present study since 1) the composition and consumption data were matched taking into account the year (dynamic matching), accounting for different compositions for a same product/brand consumed several years apart; and 2) the top 50 of most consumed food additives computed for 3 different periods of time in the 2009–2020 time-frame marginally changed, which illustrates the relative stability of additive exposure. In future etiological studies, it will be possible to study food additive exposure as time-dependent variables.
This large population-based study provided for the first time a comprehensive overview of intakes for a wide range of additives, highlighting a widespread consumption of food additives for which health concerns are currently debated, and identified mixtures of food additives that were associated to consumer and food consumption profiles. Their health impact and potential cocktail effects should be explored in future epidemiological and experimental studies. In the meantime, and following the precautionary principle, several public health authorities worldwide recently started to recommend limiting the consumption of ultra-processed foods and, in practice, choosing food products of better nutritional quality (according to the Nutri-Score116) and without or with as few additives as possible117,118.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval. Researchers from public institutions can submit a collaboration request including information on the institution and a brief description of the project to collaboration@etude-nutrinet-sante.fr. All requests will be reviewed by the steering committee of the NutriNet-Santé study. A financial contribution may be requested. If the collaboration is accepted, a data access agreement will be necessary and appropriate authorizations from the competent administrative authorities may be needed. In accordance with existing regulations, no personal data will be accessible.
Abbreviations
- ADI:
-
Acceptable Daily Intake
- EFSA:
-
European Food Safety Authority
- GNPD:
-
Global New Products Database
- IPAQ:
-
International Physical Activity Questionnaire
- GSFA:
-
General Standard for Food Additives
- NMF:
-
Non-negative Matrix Factorization
- OQALI:
-
Observatoire de la Qualité de l'Alimentation
References
Blekas, G. A. Food Additives: Classification, Uses and Regulation. in Encyclopedia of Food and Health (eds. Caballero, B., Finglas, P. M. & Toldrá, F.) 731–736 (Academic Press, 2016). https://doi.org/10.1016/B978-0-12-384947-2.00304-4.
Codex General Standard for Food Additives (GSFA, Codex STAN 192–1995). http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCODEX%2BSTAN%2B192-1995%252FCXS_192e.pdf (2018).
Monteiro, C. A. et al. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. https://doi.org/10.1017/S1368980017000234 (2017).
Pagliai, G. et al. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br. J. Nutr. https://doi.org/10.1017/S0007114520002688 (2020).
Lane, M. M. et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obesity Rev. n/a, (2020).
Askari, M., Heshmati, J., Shahinfar, H., Tripathi, N. & Daneshzad, E. Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int. J. Obes. (Lond) 44, 2080–2091 (2020).
Fiolet, T. et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ k322 https://doi.org/10.1136/bmj.k322 (2018).
Srour, B. et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 365, l1451 (2019).
Srour, B. et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Santé prospective cohort. JAMA Intern. Med. 180, 283 (2020).
Adjibade, M. et al. Prospective association between ultra-processed food consumption and incident depressive symptoms in the French NutriNet-Santé cohort. BMC Med. 17, 78 (2019).
Schnabel, L. et al. Association between ultra-processed food consumption and functional gastrointestinal disorders: results from the French NutriNet-Santé cohort. Am. J. Gastroenterol. 113, 1217–1228 (2018).
Schnabel, L. et al. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern. Med. https://doi.org/10.1001/jamainternmed.2018.7289 (2019).
Beslay, M. et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Santé cohort. PLoS Med. 17, e1003256 (2020).
Cediel, G. et al. Ultra-processed foods and added sugars in the Chilean diet (2010). Public Health Nutr. 21, 125–133 (2018).
Moubarac, J. C., Batal, M., Louzada, M. L., Martinez, S. E. & Monteiro, C. A. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 108, 512–520 (2017).
Luiten, C. M., Steenhuis, I. H., Eyles, H., Ni Mhurchu, C. & Waterlander, W. E. Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets–CORRIGENDUM. Public Health Nutr. 19, 539 (2016).
Poti, J. M., Mendez, M. A., Ng, S. W. & Popkin, B. M. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households?. Am. J. Clin. Nutr. 101, 1251–1262 (2015).
Soffritti, M., Belpoggi, F., Esposti, D. D. & Lambertini, L. Aspartame induces lymphomas and leukaemias in rats. Eur. J. Oncol. 10, 107–116 (2005).
Soffritti, M. et al. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environ. Health Perspect. 114, 379–385 (2006).
Soffritti, M., Belpoggi, F., Tibaldi, E., Esposti, D. D. & Lauriola, M. Life-span exposure to low doses of aspartame beginning during prenatal life increases cancer effects in rats. Environ. Health Perspect. 115, 1293–1297 (2007).
Bandyopadhyay, A., Ghoshal, S. & Mukherjee, A. Genotoxicity testing of low-calorie sweeteners: aspartame, acesulfame-K, and saccharin. Drug Chem. Toxicol. 31, 447–457 (2008).
Titanium dioxide: E171 no longer considered safe when used as a food additive. European Food Safety Authority https://www.efsa.europa.eu/en/news/titanium-dioxide-e171-no-longer-considered-safe-when-used-food-additive.
Naimi, S., Viennois, E., Gewirtz, A. T. & Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 9, 148 (2021).
Etemadi, A. et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ 357, j1957 (2017).
Song, P., Wu, L. & Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients 7, 9872–9895 (2015).
Quist, A. J. L. et al. Ingested nitrate and nitrite, disinfection by-products, and pancreatic cancer risk in postmenopausal women. Int. J. Cancer 142, 251–261 (2018).
Hosseini, F. et al. Nitrate-nitrite exposure through drinking water and diet and risk of colorectal cancer: a systematic review and meta-analysis of observational studies. Clin. Nutr. https://doi.org/10.1016/j.clnu.2020.11.010 (2020).
Niu, X. et al. Small Laccase from Streptomyces coelicolor catalyzed chitosan–pectin blending film for hazardous gas removal. Environ. Technol. Innov. 23, 101690 (2021).
What is ultra-processed food? And why do people disagree about its utility as a concept? | TABLE Debates. https://www.tabledebates.org/building-blocks/what-ultra-processed-food-and-why-do-people-disagree-about-its-utility-concept.
Chazelas, E. et al. Food additives: distribution and co-occurrence in 126,000 food products of the French market. Sci. Rep. 10, 3980 (2020).
Bhattacharyya, S., O-Sullivan, I., Katyal, S., Unterman, T. & Tobacman, J. K. Exposure to the common food additive carrageenan leads to glucose intolerance, insulin resistance and inhibition of insulin signalling in HepG2 cells and C57BL/6J mice. Diabetologia. 55, 194–203 (2012).
Ataseven, N., Yüzbaşıoğlu, D., Keskin, A. Ç. & Ünal, F. Genotoxicity of monosodium glutamate. Food Chem. Toxicol. 91, 8–18 (2016).
He, K. et al. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am. J. Clin. Nutr. 93, 1328–1336 (2011).
Chakraborty, S. P. Patho-physiological and toxicological aspects of monosodium glutamate. Toxicol. Mech. Methods https://doi.org/10.1080/15376516.2018.1528649 (2018).
Hagiwara, A. et al. A thirteen-week oral toxicity study of annatto extract (norbixin), a natural food color extracted from the seed coat of annatto (Bixa orellana L.), in Sprague-Dawley rats. Food Chem. Toxicol. 41, 1157–1164 (2003).
Uysal, H., Semerdöken, S., Çolak, D. A. & Ayar, A. The hazardous effects of three natural food dyes on developmental stages and longevity of Drosophila melanogaster. Toxicol. Ind. Health. 31, 624–629 (2015).
Azad, M. B. et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 189, E929–E939 (2017).
Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
Soffritti, M. et al. Sucralose administered in feed, beginning prenatally through lifespan, induces hematopoietic neoplasias in male swiss mice. Int. J. Occup. Environ. Health 22, 7–17 (2016).
Abou-Donia, M. B., El-Masry, E. M., Abdel-Rahman, A. A., McLendon, R. E. & Schiffman, S. S. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J. Toxicol. Environ. Health Part A 71, 1415–1429 (2008).
Ritz, E., Hahn, K., Ketteler, M., Kuhlmann, M. K. & Mann, J. Phosphate additives in food-a health risk. Dtsch. Arztebl. Int. 109, 49–55 (2012).
McCarty, M. F. & DiNicolantonio, J. J. Bioavailable dietary phosphate, a mediator of cardiovascular disease, may be decreased with plant-based diets, phosphate binders, niacin, and avoidance of phosphate additives. Nutrition 30, 739–747 (2014).
Chassaing, B. et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96 (2015).
Viennois, E., Merlin, D., Gewirtz, A. T. & Chassaing, B. Dietary Emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 77, 27–40 (2017).
Viennois, E. & Chassaing, B. First victim, later aggressor: How the intestinal microbiota drives the pro-inflammatory effects of dietary emulsifiers?. Gut. Microbes. 9, 289–291 (2018).
Viennois, E. et al. Dietary emulsifiers directly impact adherent-invasive E. coli gene expression to drive chronic intestinal inflammation. Cell Reports 33, 108229 (2020).
Smith, T. J. S. et al. Caramel color in soft drinks and exposure to 4-methylimidazole: a quantitative risk assessment. PLoS One 10, 706 (2015).
Sasaki, Y. F. et al. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat. Res. 519, 103–119 (2002).
Leo, L. et al. Occurrence of azo food dyes and their effects on cellular inflammatory responses. Nutrition 46, 36–40 (2018).
Bettini, S. et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 7, 40373 (2017).
Jovanović, B. et al. The effects of a human food additive, titanium dioxide nanoparticles E171, on Drosophila melanogaster-a 20 generation dietary exposure experiment. Sci. Rep. 8, 17922 (2018).
Papa, G., Di Prisco, G., Spini, G., Puglisi, E. & Negri, I. Acute and chronic effects of Titanium dioxide (TiO 2) PM 1 on honey bee gut microbiota under laboratory conditions. Sci. Rep. 11, 5946 (2021).
Hercberg, S. et al. The Nutrinet-Santé Study: a web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health 10, 242 (2010).
Mejean, C. et al. Motives for participating in a web-based nutrition cohort according to sociodemographic, lifestyle, and health characteristics: the NutriNet-Sante cohort study. J Med. Internet. Res. 16, e189 (2014).
Vergnaud, A. C. et al. Agreement between web-based and paper versions of a socio-demographic questionnaire in the NutriNet-Sante study. Int. J. Public Health. https://doi.org/10.1007/s00038-011-0257-5 (2011).
Touvier, M. et al. Comparison between web-based and paper versions of a self-administered anthropometric questionnaire. Eur. J. Epidemiol. 25, 287–296 (2010).
Lassale, C. et al. Validity of web-based self-reported weight and height: results of the Nutrinet-Sante study. J. Med. Int. Res. 15, 152 (2013).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395 (2003).
Touvier, M. et al. Comparison between an interactive web-based self-administered 24 h dietary record and an interview by a dietitian for large-scale epidemiological studies. Br. J. Nutr. 105, 1055–1064 (2011).
Lassale, C. et al. Validation of a Web-based, self-administered, non-consecutive-day dietary record tool against urinary biomarkers. Br. J. Nutr. 113, 953–962 (2015).
Lassale, C. et al. Correlations between fruit, vegetables, fish, vitamins, and fatty acids estimated by web-based nonconsecutive dietary records and respective biomarkers of nutritional status. J. Acad. Nutr. Diet. 116, 427–438 (2016).
Le Moullec, N. et al. Validation du Manuel-photos utilisé pour l’enquête alimentaire de l’étude SU.VI.MAX. Cah. Nutr. Diet 31, 158–164 (1996).
Unité de recherche en épidémiologie nutritionnelle (Bobigny). Table de composition des aliments, Etude NutriNet-Santé. [Food composition table, NutriNet-Santé study] (in French). (Les éditions INSERM/Economica, 2013).
Black, A. E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord. 24, 1119–1130 (2000).
OQALI-Home page. https://www.oqali.fr/oqali_eng/.
Open Food Facts. https://world.openfoodfacts.org/discover.
GNPD-Banque de données mondiale de nouveaux produits, suivi des tendances nouveaux produits et innovations. https://www.gnpd.com/sinatra/anonymous_frontpage/.
INSEE. French National Census Data. Inst Natl Stat Etudes Econ (2016).
Food additives. European Food Safety Authority https://www.efsa.europa.eu/en/topics/topic/food-additives.
Lee, D. D. & Seung, H. S. Algorithms for Non-negative Matrix Factorization. in Advances in Neural Information Processing Systems 13 (eds. Leen, T. K., Dietterich, T. G. & Tresp, V.) 556–562 (MIT Press, 2001).
Hoyer, P. O. Non-negative matrix factorization with sparseness constraints. arXiv:cs/0408058 (2004).
Brunet, J.-P., Tamayo, P., Golub, T. R. & Mesirov, J. P. Metagenes and molecular pattern discovery using matrix factorization. PNAS 101, 4164–4169 (2004).
Gaujoux, R. & Seoighe, C. A flexible R package for nonnegative matrix factorization. BMC Bioinf. 11, 367 (2010).
Friedman, J., Hastie, T. & Tibshirani, R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics 9, 432–441 (2008).
Mortensen, A. et al. Re-evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydroxypropyl starch (E 1440), hydroxypropyl distarch phosphate (E 1442), starch sodium octenyl succinate (E 1450), acetylated oxidised starch (E 1451) and starch aluminium octenyl succinate (E 1452) as food additives. EFSA J. 15, e04911 (2017).
Mortensen, A. et al. Re-evaluation of lecithins (E 322) as a food additive. EFSA J. 15, e04742 (2017).
Mischek, D. & Krapfenbauer-Cermak, C. Exposure assessment of food preservatives (sulphites, benzoic and sorbic acid) in Austria. Food Addit. Contam. Part A 29, 371–382 (2012).
Leclercq, C. et al. Dietary intake exposure to sulphites in Italy-analytical determination of sulphite-containing foods and their combination into standard meals for adults and children. Food Addit. Contam. 17, 979–989 (2000).
Bemrah, N., Leblanc, J.-C. & Volatier, J.-L. Assessment of dietary exposure in the French population to 13 selected food colours, preservatives, antioxidants, stabilizers, emulsifiers and sweeteners. Food Addit. Contam. Part B 1, 2–14 (2008).
Bemrah, N. et al. Assessment of dietary exposure to annatto (E160b), nitrites (E249–250), sulphites (E220–228) and tartaric acid (E334) in the French population: the second French total diet study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 29, 875–885 (2012).
Botterweck, A. A., Verhagen, H., Goldbohm, R. A., Kleinjans, J. & van den Brandt, P. A. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: results from analyses in the Netherlands Cohort Study. Food Chem. Toxicol. 38, 599–605 (2000).
Ha, M.-S., Ha, S.-D., Choi, S.-H. & Bae, D.-H. Exposure assessment of synthetic colours approved in Korea. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 30, 643–653 (2013).
Knekt, P., Järvinen, R., Dich, J. & Hakulinen, T. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: a follow-up study. Int. J. Cancer 80, 852–856 (1999).
Larsson, K., Darnerud, P. O., Ilbäck, N.-G. & Merino, L. Estimated dietary intake of nitrite and nitrate in Swedish children. Food Addit. Contam. Part A 28, 659–666 (2011).
Leth, T., Fagt, S., Nielsen, S. & Andersen, R. Nitrite and nitrate content in meat products and estimated intake in Denmark from 1998 to 2006. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 25, 1237–1245 (2008).
Lien, K.-W. et al. Food safety risk assessment for estimating dietary intake of sulfites in the Taiwanese population. Toxicol. Rep. 3, 544–551 (2016).
Menard, C., Heraud, F., Volatier, J.-L. & Leblanc, J.-C. Assessment of dietary exposure of nitrate and nitrite in France. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 25, 971–988 (2008).
Nagata, C. et al. Dietary intakes of glutamic acid and glycine are associated with stroke mortality in Japanese adults. J. Nutr. 145, 720–728 (2015).
Soubra, L., Sarkis, D., Hilan, C. & Verger, P. Dietary exposure of children and teenagers to benzoates, sulphites, butylhydroxyanisol (BHA) and butylhydroxytoluen (BHT) in Beirut (Lebanon). Regul. Toxicol. Pharmacol. 47, 68–77 (2007).
Shah, R., Kolanos, R., DiNovi, M. J., Mattia, A. & Kaneko, K. J. Dietary exposures for the safety assessment of seven emulsifiers commonly added to foods in the United States and implications for safety. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 34, 905–917 (2017).
Mancini, F. R. et al. Dietary exposure to benzoates (E210–E213), parabens (E214–E219), nitrites (E249–E250), nitrates (E251–E252), BHA (E320), BHT (E321) and aspartame (E951) in children less than 3 years old in France. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 32, 293–306 (2015).
Oqali. Bilan et évolution de l’utilisation des additifs dans les produits transformés. (2019).
Tang, W. H. W. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 (2013).
Marion-Letellier, R., Amamou, A., Savoye, G. & Ghosh, S. Inflammatory bowel diseases and food additives: to add fuel on the flames! Nutrients 11, 1111 (2019).
Roberts, C. L., Rushworth, S. L., Richman, E. & Rhodes, J. M. Hypothesis: increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J. Crohns. Colitis 7, 338–341 (2013).
Sandall, A. M. et al. Emulsifiers impact colonic length in mice and emulsifier restriction is feasible in people with Crohn’s disease. Nutrients 12, 2827 (2020).
Crowe, W., Elliott, C. T. & Green, B. D. A review of the in vivo evidence investigating the role of nitrite exposure from processed meat consumption in the development of colorectal cancer. Nutrients 11, 2673 (2019).
Irwin, S. V., Fisher, P., Graham, E., Malek, A. & Robidoux, A. Sulfites inhibit the growth of four species of beneficial gut bacteria at concentrations regarded as safe for food. PLOS ONE 12, e0186629 (2017).
Holder, M. K. & Chassaing, B. Impact of food additives on the gut-brain axis. Physiol. Behav. 192, 173–176 (2018).
Zinöcker, M. K. & Lindseth, I. A. The Western Diet-microbiome-host interaction and its role in metabolic disease. Nutrients 10, 19 (2018).
Roca-Saavedra, P. et al. Food additives, contaminants and other minor components: effects on human gut microbiota-a review. J. Physiol. Biochem. 74, 69–83 (2018).
Chassaing, B., Van de Wiele, T., De, B. J., Marzorati, M. & Gewirtz, A. T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 66, 1414–1427 (2017).
Viennois, E. & Chassaing, B. First victim, later aggressor: How the intestinal microbiota drives the pro-inflammatory effects of dietary emulsifiers?. Gut Microbes https://doi.org/10.1080/19490976.2017.1421885 (2018).
Swidsinski, A. et al. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm. Bowel Dis. 15, 359–364 (2009).
Martino, J. V., Van Limbergen, J. & Cahill, L. E. The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front Pediatr 5, 96 (2017).
Evstatiev, R. et al. The food additive EDTA aggravates colitis and colon carcinogenesis in mouse models. Sci. Rep. 11, 5188 (2021).
McCann, D. et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet 370, 1560–1567 (2007).
Lau, K., McLean, W. G., Williams, D. P. & Howard, C. V. Synergistic interactions between commonly used food additives in a developmental neurotoxicity test. Toxicol. Sci. 90, 178–187 (2006).
Başak, K. et al. Does maternal exposure to artificial food coloring additives increase oxidative stress in the skin of rats?. Hum. Exp. Toxicol. 36, 1023–1030 (2017).
Andreeva, V. A. et al. Comparison of the sociodemographic characteristics of the large NutriNet-Sante e-cohort with French Census data: the issue of volunteer bias revisited. J. Epidemiol. Community Health 69, 893–898 (2015).
Andreeva, V. A. et al. Comparison of dietary intakes between a large online cohort study (Etude NutriNet-Santé) and a nationally representative cross-sectional study (Etude Nationale Nutrition Santé) in France: addressing the issue of generalizability in E-epidemiology. Am. J. Epidemiol. 184, 660–669 (2016).
Andreeva, V. A. et al. Comparison of the sociodemographic characteristics of the large NutriNet-Santé e-cohort with French Census data: the issue of volunteer bias revisited. J. Epidemiol. Community Health 69, 893–898 (2015).
Calixto Andrade, G. et al. Consumption of ultra-processed food and its association with sociodemographic characteristics and diet quality in a representative sample of French adults. Nutrients 13, 682 (2021).
Salomé, M. et al. Contrary to ultra-processed foods, the consumption of unprocessed or minimally processed foods is associated with favorable patterns of protein intake, diet quality and lower cardiometabolic risk in French adults (INCA3). Eur. J. Nutr. https://doi.org/10.1007/s00394-021-02576-2 (2021).
Egnell, M., Hercberg, S. & Julia, C. Development and validation of the Nutri-Score A colour-coded summary front-of-pack nutrition label. eFOOD Lab_International (2019).
Santé Publique France. Recommandations sur l’alimentation, l’activité physique & la sédentarité pour les adultes. 20 (2019).
Monteiro, C. A., Cannon, G., Lawrence, M., da Costa Louzada, M. L. & Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health using the NOVA Classification System (FAO, 2019).
Acknowledgements
The authors warmly thank all the volunteers of the NutriNet-Santé cohort. We also thank Thi Hong Van Duong, Régis Gatibelza, and Jagatjit Mohinder (computer scientists), Julien Allègre, Nathalie Arnault, Laurent Bourhis (data-managers/biostatisticians), and Sandrine Kamdem, MD (physician), for their technical contribution to the NutriNet-Santé study.
IARC disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
Funding
The NutriNet-Santé study was supported by the following public institutions: Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut National de la Recherche Agronomique (INRAE), Conservatoire National des Arts et Métiers (CNAM) and University Sorbonne Paris Nord. EC was supported by a Doctoral Funding from University Sorbonne Paris Nord—Galilée Doctoral School. CD was supported by a grant from the French National Cancer Institute (INCa). This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 864219), the French National Cancer Institute (INCa_14059), the French Ministry of Health (arrêté 29.11.19) and the IdEx Université de Paris (ANR-18-IDEX-0001). This project was awarded the NACRe (French network for Nutrition And Cancer Research) Partnership Label. This work only reflects the authors' view and the funders are not responsible for any use that may be made of the information it contains. Researchers were independent from funders. Funders had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
The authors’ contributions were as follows – E.C., N.D.P., Y.E., F.S.E., C.A., A.D.S., R.L. developed the additives composition database and performed the matches with composition databases to the data match with the other databases. N.D.P. Y.E. E.C., M.T. supervised this technical work. E.C. and M.T.: designed the research; E.C.: performed statistical analysis; M.T.: supervised statistical analysis; E.C. drafted the manuscript; MT supervised the writing; E.C., N.D.P., Y.E., F.S.E., C.A., A.D.S., R.L., P.R., B.S., C.D., G.W.F., I.H., F.P., X.C., C.J., E.K.G., B.A., P.G., S.H., M.D.T. and M.T.: contributed to the data interpretation and revised each draft for important intellectual content. All authors read and approved the final manuscript. MT had primary responsibility for the final content, she is the guarantor. None of the authors reported a conflict of interest related to the study. The funders had no role in the design, implementation, analysis, or interpretation of the data. The corresponding author (EC) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chazelas, E., Druesne-Pecollo, N., Esseddik, Y. et al. Exposure to food additive mixtures in 106,000 French adults from the NutriNet-Santé cohort. Sci Rep 11, 19680 (2021). https://doi.org/10.1038/s41598-021-98496-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98496-6
This article is cited by
-
Ultra-processed foods and food additives in gut health and disease
Nature Reviews Gastroenterology & Hepatology (2024)
-
Ultra-processed foods, adiposity and risk of head and neck cancer and oesophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study: a mediation analysis
European Journal of Nutrition (2024)
-
From ultra-processed foods to ultra-processed dietary patterns
Nature Food (2022)
-
Nutrition research challenges for processed food and health
Nature Food (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.