Abstract
mTOR inhibitors offer advantages after kidney transplantation including antiviral and antitumor activity besides facilitating low calcineurin inhibitor exposure to reduce nephrotoxicity. Concerns about adverse effects due to antiproliferative and antiangiogenic properties have limited their clinical use particularly early after transplantation. Interference with vascular endothelial growth factor (VEGF)-A, important for physiologic functioning of renal endothelial cells and tubular epithelium, has been implicated in detrimental renal effects of mTOR inhibitors. Low doses of Rapamycin (loading dose 3 mg/kg bodyweight, daily doses 1.5 mg/kg bodyweight) were administered in an allogenic rat kidney transplantation model resulting in a mean through concentration of 4.30 ng/mL. Glomerular and peritubular capillaries, tubular cell proliferation, or functional recovery from preservation/reperfusion injury were not compromised in comparison to vehicle treated animals. VEGF-A, VEGF receptor 2, and the co-receptor Neuropilin-1 were upregulated by Rapamycin within 7 days. Rat proximal tubular cells (RPTC) responded in vitro to hypoxia with increased VEGF-A and VEGF-R1 expression that was not suppressed by Rapamycin at therapeutic concentrations. Rapamycin did not impair proliferation of RPTC under hypoxic conditions. Low-dose Rapamycin early posttransplant does not negatively influence the VEGF network crucial for recovery from preservation/reperfusion injury. Enhancement of VEGF signaling peritransplant holds potential to further improve outcomes.
Similar content being viewed by others
Introduction
Although rapamycin (Rapa), the first-in-class inhibitor of the mechanistic target of rapamycin (mTOR), had been approved for immunosuppression after kidney transplantation more than 20 years ago the debate on the most appropriate use of mTOR inhibitors (mTORi) is still ongoing1,2. During the last decades it became clear that beyond immunosuppression mTORi exert not only specific anti-viral activity resulting in reduced incidents of cytomegalovirus3,4,5 and BK virus4 infections but also provide some defense against malignant tumors5. Moreover, it had been speculated that mTORi could protect from cardiovascular events and chronic allograft dysfunction through intrinsic mechanisms and by enabling avoidance or at least reduction of nephrotoxic calcineurin inhibitors (CNI) with the final goal of improved long-term graft and patient survival.
Despite these important advantages, mTORi are frequently withheld or withdrawn since they are perceived as being associated with an unfavorable safety profile limiting their use in many transplant recipients. In particular, delayed wound healing, formation of lymphoceles, increased proteinuria, higher rejection rates, cytopenias, an adverse metabolic profile and increased mortality have been of concern6,7. Studies yielded heterogeneous results depending on timing of mTORi administration (de novo transplants, early conversion, late conversion) and choice of concomitant immunosuppression (mycophenolate, CNI at standard or recued doses, steroids). Of note, recent trials and meta-analyses clearly demonstrate non-inferiority of mTORi based regimes that acknowledge drug-drug interactions and combine mTORi and CNI at reduced doses8,9,10 indicating a learning curve that may not have reached its climax yet.
mTOR, by integrating nutrient availability and growth factor signaling, functions as a ubiquitous central regulator of proliferation, protein synthesis and other important cell functions such as autophagy11. Many of the advantageous but also of the adverse non-immune effects of mTORi have been attributed to their inherent antiproliferative properties and their complex context dependent impact on cellular growth, differentiation, and metabolism11. One example is the antitumor effect of mTORi that has been ascribed not only to general inhibition of proliferation but also to a specific antiangiogenic activity due to reduced production of and response to vascular endothelial growth factor (VEGF)-A resulting in limited blood supply to the tumor12,13.
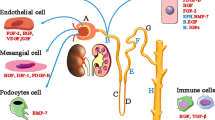
Importantly, expression of VEGF-A and its receptors is also found with distinctive patterns at multiple sites in healthy kidneys such as endothelial cells of glomeruli and peritubular capillaries, podocytes, mesangial cells and tubular epithelial cells14. The VEGF network is involved in maintaining physiologic functioning of glomeruli and peritubular capillaries14,15 and has been found to be dysregulated in a large variety of kidney diseases including diabetic nephropathy, glomerulonephritis, thrombotic microangiopathies and chronic allograft nephropathy (CAN) after kidney transplantation14,15. Remarkably, also tubular epithelial cells feature a functional VEGF network14,16 connecting them to vascular maintenance under stable conditions and initiation of angiogenesis when oxygen supply does not meet the demand15. Moreover, VEGF-A has been implicated in proliferation and protection of tubular cells from apoptosis rendering it a survival factor for tubular epithelium16 that might act in an autocrine or paracrine manner.
To further improve mTORi containing immunosuppressive regimes for de novo kidney transplants, we aimed to study the impact of mTOR inhibition with Rapa on vascular integrity and tubular regeneration in correlation to the VEGF network during the immediate posttransplant period in a life-supporting rat kidney transplantation model. We found intact vascular structures, adequate tubular cell proliferation, and rapid functional recovery from preservation/reperfusion injury until day 2 in the face of Rapa treatment. VEGF-A, VEGF receptor 2 (VEGF-R2) and the VEGF co-receptor Neuropilin-1 were upregulated by Rapa within 7 days. In addition, rat proximal tubular cells (RPTC) responded to hypoxia in vitro with increased VEGF-A and VEGF-R1 expression that was not suppressed by Rapa at therapeutic concentrations. These findings support the introduction of mTORi immediately after transplantation and provide insight into the early dynamics of the VEGF network opening a perspective for targeted interventions to further improve outcomes.
Results
We took advantage of our rat kidney transplantation model with a low-responder strain combination (Fischer to Lewis)20 to study the effect of mTOR inhibition with Rapa on vascular integrity as well as tubular and functional recovery during the immediate posttranplantation phase.
Rapa does not interfere with vascular integrity during recovery from posttransplantation preservation injury
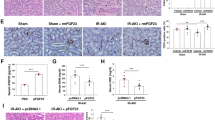
As shown by immunostaining of endothelial cells, mTOR inhibition did not compromise the integrity of glomerular or peritubular capillaries neither in cortex (Fig. 1a, c) nor in outer medulla (Fig. 1b) that is particularly susceptible to hypoxic injury. Furthermore, expression of eNOS was even increased on day 2 in Rapa treated animals compared to vehicle on protein (Fig. 1d) and mRNA levels (Fig. 1e) indicating endothelial protection in the post-immediate period. On days 5 and 7, representing the late regeneration phase, eNOS mRNA but not protein was upregulated in vehicle treated animals in comparison to the early phase. However, no significant difference was detected between both treatment groups (Fig. 1d, e).
Effect of Rapa on vascular integrity early after kidney transplantation. (a, b) Positive area in the cortex (a) and medulla (b) of transplanted kidneys from vehicle or Rapa treated rats stained by immunohistochemistry for rat endothelial cell antigen (RECA)-1. n = 4. (c) Representative photomicrographs for RECA-1 immunohistochemistry 7 days posttransplant with vehicle or Rapa treatment. (d) Total positive area in kidney transplants from vehicle or Rapa treated rats stained by immunohistochemistry for endothelial nitric oxide synthase (eNOS). n = 4–5. (e) Quantification of eNOS mRNA in whole transplanted kidneys by real-time PCR after treatment with vehicle or Rapa. n = 4–5. *P < 0.05, **P < 0.01.
On a physiologic level, kidney function was mildly impaired in Rapa treated animals on day 1 but completely recovered as early as on day 2 as reflected by plasma creatinine (Fig. 2a) and urea measurements (Fig. 2b). There was moderate albuminuria on day 1 that completely resolved until day 5 independently of treatment (Fig. 2c). Control rats merely developed slight albuminuria on day 7 not significantly different from rats on Rapa (Fig. 2c). These findings suggest rapid resolution of functional changes induced by preservation/reperfusion injury in recipient rats despite mTOR blockade with Rapa.
Renal function and albuminuria during the first week after kidney transplantation. Plasma creatinine (a) and urea (b) concentrations in transplanted rats receiving vehicle or Rapa. n = 5–7. (c) Albumin concentrations measured in urine samples collected over 24 h in metabolic cages. n = 4–5. *P < 0.05, **P < 0.01, ***P < 0.001.
The intrarenal VEGF network is activated by Rapa early posttransplant
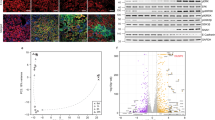
Hypoxia inducible factor-1α (HIF-1α) is stabilized under hypoxic conditions as found during transplantation associated ischemia and has been shown to be activated by mTOR21. As a transcription factor, HIF-1α promotes expression of VEGF-A, the decisive mediator of vascular homeostasis and driver of angiogenesis. Thus, we analyzed the VEGF network to decipher autocrine and paracrine cues with the ability to maintain peritransplant vascular integrity found in our model. VEGF-A mRNA transcripts were 2.3 times more abundant in Rapa treated rats compared to vehicle controls on day 7 and were already increased by trend on days 1 and 2 (Fig. 3a). Remarkably, VEGF-A transcripts in vehicle treated rats increased significantly from day 2 to day 5 to equal those of Rapa treated rats only on day 5 (Fig. 3a). VEGF expression was predominantly found in epithelial cells of distal tubules as demonstrated by immunohistology (Fig. 3b). VEGF-R1 mRNA was neither altered by treatment nor time (Fig. 3c). Of note, expression of VEGF-R2 paralleled that of VEGF-A (Fig. 3d): Rapa was associated with nonsignificant higher levels on days 1 and 2 and 2.7-fold more transcripts on day 7 in comparison to vehicle. On day 5, there were significantly more VEGF-R2 transcripts in vehicle treated animals than at the other time points reaching the same level as found with Rapa. A similar pattern was observed for neuropilin-1, a co-receptor for VEGF-A (Fig. 3e). Rapa in comparison to vehicle resulted in significantly increased transcription of neuropilin-1 on days 1 and 7 and by trend on day 2 while expression of neuropilin-1 was upregulated on day 5 in the vehicle group (Fig. 3e).
The vascular endothelial growth factor (VEGF) network in kidney grafts from rats receiving vehicle or Rapa. Quantitative real-time PCR on days 1–7 (a) and immunohistochemistry on day 7 (b) for VEGF-A. Representative photomicrographs are shown. mRNA expression of the VEGF receptors 1 (VEGF-R1; c) and 2 (VEGF-R2; d) and the VEGF co-receptor neuropilin-1 (e) as assessed with quantitative real-time PCR. n = 4–6. *P < 0.05, **P < 0.01, ***P < 0.001.
Stimulation of VEGF expression by hypoxia overrides inhibitory Rapa mediated effects at therapeutic concentrations
To analyze the relative contribution of hypoxia and mTOR inhibition – both operative in our transplant model—on the VEGF network, proliferation, and metabolism in renal tubular epithelial cells, we performed a series of in vitro experiments with RPTC. VEGF-A mRNA was strongly upregulated 4 h after induction of hypoxia (Fig. 4a). Rapa slightly reduced VEGF-A only at the suprapharmacological dose of 100 nM (Fig. 4a). Relative expression waned after 8 h but was still highly significantly amplified under hypoxic conditions compared to normoxia (Fig. 4b). Elevated mRNA levels translated into increased secretion of VEGF-A into the supernatant as determined after 24 h by ELISA (Fig. 4c). Again, the amount of VEGF-A protein was reduced only at 20 nM and 100 nM (Fig. 4c), concentrations exceeding those found in patient plasma. There was no detectable effect of mTOR inhibition by Rapa on VEGF-A expression under normoxic conditions both on mRNA and protein levels (Fig. 4a-c). VEGF-R1 mRNA was markedly upregulated by hypoxia (Fig. 4d). Rapa did not influence mRNA levels independently of oxygen saturation (Fig. 4d). In accordance with reports on animal studies14, we did not detect VEGF-R2 mRNA transcripts in RPTC (not shown).
Influence of hypoxia and mTOR inhibition by Rapa on the vascular endothelial growth factor (VEGF) network in rat renal proximal tubular cells (RPTC). RPTC were incubated with Rapa under normoxic or hypoxic conditions. Expression of VEGF-A mRNA was analyzed by quantitative real-time PCR after 4 h (a) and after 8 h (b). Results were normalized to vehicle control (0 nM Rapa) at normoxia (set to 1.00). Normoxia n = 7–8, hypoxia n = 4. (c) VEGF-A protein measured with an enzyme-linked immunosorbent assay (ELISA) in cell culture supernatants after 24 h normalized to total protein content. n = 9. (d) Expression of VEGF receptor 1 (VEGF-R1) mRNA quantified by real-time PCR after 8 h normalized to vehicle control (0 nM Rapa) at normoxia (set to 1.00). Normoxia n = 6, hypoxia n = 5. *P < 0.05, **P < 0.01, ***P < 0.001.
Proliferation of tubular epithelial cells is preserved despite mTOR inhibition by Rapa during posttransplant repair and under hypoxia
More than 30% of tubular cells in cortex and medulla exhibited proliferative activity as determined by immunostaining for proliferating cell nuclear antigen (PCNA) on day 1 after transplantation independently of mTOR inhibition (Fig. 5a–c). On day 2, proliferation remained high in the cortex in both groups (Fig. 5a, b). However, Rapa diminished proliferation of tubular epithelial cells located in the medulla (Fig. 5b). At later time points, proliferation was largely decreased in cortex and medulla in both treatment groups (Fig. 5a–c). Cell culture experiments revealed that proliferation of RPTC as measured by BrdU incorporation was decreased by Rapa only under normoxic conditions whereas mTOR inhibition had no effect when cells were exposed to hypoxia (Fig. 6a). Similarly, overall metabolic activity of RPTC when determined with the MTT assay declined with increasing doses of Rapa at normoxia, but not hypoxia (Fig. 6b).
Effect of Rapa on proliferation of tubular cells after kidney transplantation. Percentage of tubular cells positive for proliferating cell nuclear antigen (PCNA) in cortex (a) and medulla (b) during the first week after kidney transplantation as seen with immunohistochemistry. n = 3. (c) Representative photomicrographs for PCNA immunohistochemistry 2 days posttransplant with vehicle or Rapa treatment.
Effect of Rapa on proliferation and metabolic activity of rat renal tubular cells in vitro under normoxic and hypoxic conditions. Rat renal proximal tubular cells (RPTC) were incubated with Rapa under normoxic or hypoxic conditions for 24 h. (a) Proliferation was assessed as incorporation of BrdU. Results are normalized to vehicle control (0 nM Rapa) for each experiment. Normoxia n = 6, hypoxia n = 4. (b) Metabolic activity was measured as conversion of MTT to formazan. Results are normalized to vehicle control (0 nM Rapa) for each experiment. Normoxia n = 4, hypoxia n = 3. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In our low-responder life-supporting rat renal transplantation model, the mTORi Rapa did not exert adverse effects in the early posttransplant period up to day 7. This is in contrast to early reports that linked induction immunosuppression with Rapa to increased rates of delayed graft function (DGF)22 and prolonged recovery from this important complication23,24. Since DGF is associated with unfavorable short- and long-term outcomes25, it was concluded that Rapa might not be the best choice for transplants at risk for DGF23 although 1-year graft function was not compromised24. More recent studies did not find an association of mTORi use with DGF5,26. The most important differences to earlier protocols are reduced target trough levels for mTORi, e.g. for Rapa instead of 10–15 ng/mL23 only 4–8 ng/mL as established in the ELITE-Symphony study27, no combination with mycophenolate as an additional anti-proliferative agent9, and reduced CNI exposure28. Hence, we aimed to elucidate the effect of low-dose Rapa on regenerative mechanisms operative early after transplantation that might be involved in protection from DGF.
A major objection put forward against the use of mTORi in the immediate posttransplant period is a possible negative impact of their well-known antiproliferative potential on graft regeneration from preservation injury resulting in DGF. However, we did not find reduced proliferation with Rapa in tubular epithelial cells in cortex and medulla with the exception of a 30% reduction on day 2 in the medulla at low Rapa concentrations. In addition, inhibition of the VEGF axis by Rapa12,13 might be particularly harmful for transplanted kidneys with preservation/reperfusion damage since VEGF-A is not only required for maintaining integrity and functionality of glomerular structures14,29 but has also been established as a growth supporter for tubular epithelium16, the renal compartment with the highest susceptibility to hypoxic damage. Rapa and other mTORi have been studied in various models relevant for kidney transplantation. However, to our knowledge, none of the previously published works examined the effect of mTOR inhibition on allograft regeneration and the VEGF network in the early posttransplant period.
As an example, Ko et al. used the same low-responder Fischer-to-Lewis kidney transplantation model as us to study the impact of Rapa on chronic allograft dysfunction30. After 24 weeks, they did not observe reduced expression of VEGF-A and VEGF-R1 in whole kidney grafts with Rapa in comparison to Cyclosporine A (CsA) while VEGF-R2 mRNA was almost doubled in CsA treated rats30. Tubuli were not evaluated separately, but mRNA and protein levels of the VEGF axis components were strikingly reduced in glomerular structures and intrarenal arteries with Rapa treatment30. Glomerular and vascular impairment of the VEGF system was associated with increased proteinuria on one hand and protection from vasculopathy on the other30, illustrating both sides of the coin with potential detrimental and beneficial consequences of mTORi use after transplantation.
In our study, glomerular and peritubular capillaries were not structurally altered with intact eNOS expression indicating endothelial functioning during the first week after transplantation. Correspondingly, albuminuria resolved as quickly in Rapa treated animals as in those receiving vehicle. Thus, there was no evidence for adverse effects of mTOR inhibition on restoration of glomerular function and vascular integrity immediately after transplantation. Moreover, our in vitro experiments with RPTC demonstrate neutralization of any inhibiting effect of Rapa at clinically relevant concentrations on proliferation and metabolism when cells were exposed to hypoxic conditions. Remarkably, these findings on structural and functional levels were related to preservation or even amplification of the VEGF network in vivo and in vitro despite the presence of Rapa. A possible explanation is the activation of some defense mechanism during cold ischemia overriding potentially negative influences of mTOR inhibition on VEGF production and function as described in other contexts12,13,30.
The hypoxia inducible factors (HIF) 1 and 2 have been identified as central players in the protection of tubular epithelial cells during ischemia–reperfusion injury (IRI)31. As oxygen-sensitive transcription factors, they promote the expression of proteins related to the adoptive response to hypoxia such as erythropoietin (EPO) and VEGF-A21. In rat kidneys, activation of the cellular master switch for adaptation to low oxygen tension, HIF-1α, has been observed not only during ischemia but also on days 3, 5 and 7 of reperfusion without evidence of persistent tissue hypoxia32. The HIF-1α target genes EPO and VEGF-A were upregulated 3 days after ischemia and negative genetic interference with HIF-1α directed siRNA exacerbated tubular injury and dramatically worsened renal function on day 332. Thus, induction of HIF-1α and its target genes including VEGF-A appears to be necessary in the immediate and early posttransplant period to ensure timely recovery from preservation/reperfusion injury. Besides hypoxia, oxygen-independent pathways such as growth factor signaling, heat shock protein 90, and others can activate HIF-1α dependent transcription21. In proximal tubular cells, such pathways have been shown to be induced independently of oxygen levels by nutrient depletion and replenishment mimicking transplantation associated ischemia32. Together with our findings, this evidence suggests that hypoxia in addition to hypoxia independent mechanisms operative in preservation/reperfusion injury act as powerful inducers of the VEGF network that is not suppressed by Rapa at low concentrations.
Following evidence from animal studies, HIF-1α targets have been evaluated as therapeutic options to reduce DGF. Disappointingly, EPO failed to improve recovery from preservation/reperfusion injury and to protect from DGF in transplant patients33,34. Different means of HIF-1α induction other than hypoxia such as peritransplant recipient treatment with carbon monoxide35 or donor pretreatment with a prolyl-hydroxylase inhibitor36 are still experimental. Amplification of VEGF signaling during the vulnerable peritransplant period could be an alternative approach. Application of highly specific VEGF-R2 activating aptamers37 directly to the allograft during cold storage or immediately prior to graft implantation would be a reasonable approach. This strategy holds promise to be particularly beneficial since it would specifically target intrarenal vascular, glomerular, and tubular structures at risk for preservation/reperfusion injury with good responsiveness to VEGF within a short window of opportunity while avoiding off-target effects in other tissues.
In human kidney transplant recipients, introduction of mTORi bears a risk for detrimental consequences predominantly in already structurally altered grafts with low glomerular filtration rate and preexisting proteinuria as a consequence of severely damaged glomerula in CAN38. Thus, early use of mTORi might be prudent to avoid the development of calcineurin inhibitor toxicity and CAN and to derive the maximum benefit with regard to viral infections, cardiovascular events, and malignant tumors5, although we did not evaluate the effect of Rapa in combination with a CNI on the VEGF network. There is a growing body of evidence that combining mTORi with reduced CNI exposure in de novo kidney transplants has an acceptable side effect profile and is immunologically safe4,8,9,10,39. Particularly, there was no indication to increased rates of DGF associated with early use of mTORi5,26.
Taken together, our study does not raise a safety signal that mTOR inhibition with Rapa at doses used in modern immunosuppressive protocols after kidney transplantation negatively interferes with the VEGF network that is crucial for successful recovery from preservation/reperfusion injury. Moreover, we provide a rational to evaluate a possible role for therapeutic enhancement of VEGF signaling peritransplant to further improve outcomes. In the light of recent clinical trials, mTORi may be considered in all transplant recipients with low to moderate immunologic risk.
Methods
Animals and transplantation surgery
All surgical and experimental procedures were approved by local authorities (Landesamt für Gesundheit und Soziales, LaGeSo, Berlin, Germany) and were in accordance with the guidelines of the American Physiological Society. The ARRIVE guidelines were met. 10-weeks old inbred male Fischer (F344) and Lewis (Lew, RT1) rats (Harlan-Winkelman, Sulzbach, Germany) weighing 150–200 g were kept at 24 °C with regular lighting conditions (lights on 6:00–18:00) with free access to tap water and standard rat diet (C-1000, Altromin, Lage, Germany). Fischer rats served as kidney donors and were prepared as described previously17. After in situ perfusion with 5 mL pre-cooled University of Wisconsin (UW) solution through the cannulated aorta, the explanted kidney was placed in cold UW (4 °C) for 2 h. The Lewis recipients were anesthetized with isoflurane and underwent bilateral nephrectomy followed by orthotopic implantation of the left donor kidney. Anastomoses of the artery, vein and ureter were done end-to-end with 10–0 polypropylene (Prolene, Ethicon, Norderstedt, Germany) sutures within 30 min18.
Rapa (LC Laboratories, Woburn, MA, USA) was administered by gavage from a 1 mg/mL stock solution. A loading dose of 3 mg/kg was applied followed by daily maintenance doses of 1.5 mg/kg. Rapa trough levels of 4.30 ± 0.64 ng/mL were achieved.
As previously described19, rats were placed in metabolic cages for collection of 24-h urine samples. When animals were killed at the indicated time points venous blood was collected for automated measurements of creatinine and urea in the university’s central laboratory facility and kidney grafts were harvested.
Histology, immunohistochemistry, and morphometric quantification
Histological and immunohistochemistry techniques followed previously described protocols18. We used the alkaline phosphatase/anti-alkaline phosphatase (APAAP) complex method (DakoCytomation, Hamburg, Germany) for immunostaining. Acetone-fixed cryosections (6 µm) were used for analysis with antibodies directed against rat endothelial cell antigen (RECA; Abcam, Cambridge, UK), endothelial NO-synthase (eNOS; Thermo Fisher Scientific, Waltham, MA, USA), and VEGF-A (R&D Systems, Minneapolis, MN, USA) using the neufuchsin-naphtol-As-Bi-phosphate substrate (Merck, Darmstadt, Germany). Proliferating cell nuclear antigen (PCNA; Zymed Laboratories Inc., San Francisco, CA, USA) was detected in paraffin Sects. (4 µm) of formalin fixed tissue with amino ethyl carbazole (AEC) as the chromogen (DakoCytomation). Negative control staining was performed by incubation with corresponding isotype controls instead of primary antibody. Hematoxylin counterstain was applied to all sections after development of the antibody signal.
For all parameters, 10 randomly chosen fields of view (FOV) at 400× magnification were evaluated and summarized to obtain a single mean value for each individual rat. The area stained by RECA and eNOS antibodies was measured using a computer-assisted morphometry unit (axiocam HR with axiovision 4.4 software, Zeiss/Kontron, Göttingen, Germany) and expressed as the percentage of the total area. PCNA positive tubular cells were assessed as percentage of all tubular cells.
Cell culture
Immortalized rat renal proximal tubular cells (RPTC) were generously provided by Julie Ingelfinger (Pediatric Nephrology Laboratory, Harvard Medical School, Boston, MA, USA). RPTC were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mmol/L glutamine at 37 °C with 5% CO2 in a humidified atmosphere. Cells were passaged before reaching confluence.
RPTC were seeded at a density of 250,000 cells per well in 6-well plates for RNA extraction or 10,000 cells per well in 96-well plates for BrdU and MTT assays in complete DMEM and were allowed to adhere overnight. After cells were exposed to serum-free medium for 24 h they were treated with Rapa at the indicated concentrations or with ethanol as a vehicle control for 4, 8, or 24 h at normoxia and hypoxia in parallel.
To create hypoxic conditions, culture dishes were placed in an air-tight hypoxia chamber with constant 95% N2/5% CO2 in a humidified atmosphere at 37 °C.
Rapa (LC Laboratories) was dissolved in ethanol and stock solutions with concentrations of 2 µmol/L, 12 µmol/L, and 100 µmol/L were prepared.
Quantitative real-time PCR
Total RNA was extracted from deep frozen graft tissue samples or cultured cells with TRIzol (Invitrogen, Carlsbad, CA, USA) and purified following standard procedures. Isolated RNA was checked for integrity by agarose gel electrophoresis with ethidium bromide staining and spectrometrically quantified. Complementary DNA (cDNA) was obtained from 1 µg of RNA using the PCR Core Kit and random hexamer primers (Applied Biosystems, Foster City, CA, USA) following to the manufacturer's protocol.
The Light Cycler PCR and detection system (Roche, Mannheim, Germany) was used for amplification and online quantification. Specific primer pairs (TIB Molbiol, Berlin, Germany) were designed to detect the following target transcripts: eNOS, forward 5′-TGA CCC TCA CCG ATA CAA CA, reverse 5′-CTG GCC TTC TGC TCA TTT TC; VEGF-A, forward 5′-TGC ACC CAC GAC AGA AGG GGA, reverse 5′-TCA CCG CCT TGG CTT GTC ACA T; VEGF-R1, forward 5′-CAA GGG ACT CTA CAC TTG TC, reverse 5′-CCG AAT AGC GAG CAG ATT TC; VEGF-R2, forward 5′-GCC AAT GAA GGG GAA CTG AAG AC, reverse 5′-TCT GAC TGC TGG TGA TGC TGT C; neuropilin-1, forward 5′-GAT TCC CTG AAG TTG GCC CT, reverse 5′-TCT CCT GGT GTC CAC CCG TT. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard (forward 5′-CCA TCT TCC AGG AGC GAG AT, reverse 5′-GAT GAC CTT GCC CAC AGC CT). The PCR mixture consisted of H2O, Taq polymerase, 3 mM magnesium chloride, Master Sybr Green Mix® (Roche), and specific primers. cDNA corresponding to 0.1 µg RNA was analyzed per reaction. Melting curve analyses were performed to verify the specificity of the reactions. Run data were analyzed with the quantification program Quant V3·0 using the delta-CT method.
VEGF-A enzyme immunoassay (EIA)
Cell culture supernatants were cleared by centrifugation for 3 min at 100×g. Concentration of VEGF-A was determined with a commercial human VEGF EIA that crossreacts with rat VEGF-A (PromoKine C-64407; PromoCell, Heidelberg, Germany) following the manufacturer′s protocol.
BrdU incorporation for assessment of cell proliferation
RPTC in 96-well were exposed to the indicated Rapa concentrations or ethanol as vehicle control in serum-free DMEM for 24 h. 5-Bromo-2′-deoxy-uridine (BrdU) was added 1:1,000 for the last 2 h. BrdU incorporation into newly synthetized DNA was measured as a surrogate for proliferation using the BrdU cell proliferation kit (Roche) according to the manufacturer’s instructions. Each measurement consisted of three replicates.
MTT assay for metabolic activity
RPTC in 96-well plates cells were exposed to the indicated concentrations of Rapa or ethanol as the vehicle control for 24 h. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazoliumbromid (MTT; Merck) at a concentration of 5 g/L in sterile 0.9% NaCl solution was added to a final concentration of 1.5 mmol/L for the last 60 min. After washing with PBS, formazan crystals formed by metabolically active cells were solubilized in 100 µl isopropanol/4 mol/L HCl. Absorbance was measured in a microplate reader at 570 nm. Background absorbance was subtracted and means of five replicates were calculated.
Statistical analysis
Quantitative results are expressed as means ± SEM. Numbers of animals or independent replicates are given in each figure legend. Treatment groups and different time points or normoxic versus hypoxic conditions and different rapa concentrations were compared with the two-way analysis of variance (ANOVA). Bonferroni’s multiple comparisons test was used for post-testing. Statistical significance was considered at a two-sided P value of < 0.05. GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) for Windows was used for all analyses.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Flechner, S. M. mTOR inhibition and clinical transplantation: kidney. Transplantation 102, S17–S18. https://doi.org/10.1097/TP.0000000000001692 (2018).
Pascual, J. et al. Evidence-based practice: Guidance for using everolimus in combination with low-exposure calcineurin inhibitors as initial immunosuppression in kidney transplant patients. Transplant. Rev. (Orlando) 33, 191–199. https://doi.org/10.1016/j.trre.2019.07.001 (2019).
Mallat, S. G. et al. CMV and BKPyV infections in renal transplant recipients receiving an mTOR inhibitor-based regimen versus a CNI-based regimen: a systematic review and meta-analysis of randomized, controlled trials. Clin. J. Am. Soc. Nephrol. 12, 1321–1336. https://doi.org/10.2215/CJN.13221216 (2017).
Sommerer, C. et al. An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int 96, 231–244. https://doi.org/10.1016/j.kint.2019.01.041 (2019).
Montero, N. et al. Mammalian target of rapamycin inhibitors combined with calcineurin inhibitors as initial immunosuppression in renal transplantation: a meta-analysis. Transplantation 103, 2031–2056. https://doi.org/10.1097/TP.0000000000002769 (2019).
Ponticelli, C. The pros and the cons of mTOR inhibitors in kidney transplantation. Expert Rev. Clin. Immunol. 10, 295–305. https://doi.org/10.1586/1744666X.2014.872562 (2014).
Ventura-Aguiar, P., Campistol, J. M. & Diekmann, F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opin. Drug Saf. 15, 303–319. https://doi.org/10.1517/14740338.2016.1132698 (2016).
Andrade, L. G. & Tedesco-Silva, H. Critical analysis of graft loss and death in kidney transplant recipients treated with mTOR inhibitors. J. Bras. Nefrol. 39, 70–78. https://doi.org/10.5935/0101-2800.20170012 (2017).
Tedesco Silva, H., Rosso Felipe, C. & Medina Pestana, J. O. Reviewing 15 years of experience with sirolimus. Transplant. Res. 4, 6. https://doi.org/10.1186/s13737-015-0028-6 (2015).
Berger, S. P. et al. Two-year outcomes in de novo renal transplant recipients receiving everolimus-facilitated calcineurin inhibitor reduction regimen from the TRANSFORM study. Am. J. Transplant. 19, 3018–3034. https://doi.org/10.1111/ajt.15480 (2019).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371. https://doi.org/10.1016/j.cell.2017.03.035 (2017).
Guba, M. et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat. Med. 8, 128–135. https://doi.org/10.1038/nm0202-128 (2002).
Faes, S., Santoro, T., Demartines, N. & Dormond, O. Evolving significance and future relevance of anti-angiogenic activity of mTOR inhibitors in cancer therapy. Cancers (Basel) https://doi.org/10.3390/cancers9110152 (2017).
Schrijvers, B. F., Flyvbjerg, A. & De Vriese, A. S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 65, 2003–2017. https://doi.org/10.1111/j.1523-1755.2004.00621.x (2004).
Tanaka, S., Tanaka, T. & Nangaku, M. Hypoxia and dysregulated angiogenesis in kidney disease. Kidney Dis. (Basel) 1, 80–89. https://doi.org/10.1159/000381515 (2015).
Kanellis, J., Fraser, S., Katerelos, M. & Power, D. A. Vascular endothelial growth factor is a survival factor for renal tubular epithelial cells. Am. J. Physiol. Renal. Physiol. 278, F905-915. https://doi.org/10.1152/ajprenal.2000.278.6.F905 (2000).
Dragun, D. et al. Ischemia-reperfusion injury in renal transplantation is independent of the immunologic background. Kidney Int. 58, 2166–2177. https://doi.org/10.1111/j.1523-1755.2000.00390.x (2000).
Fuller, T. F. et al. Cytoprotective actions of FTY720 modulate severe preservation reperfusion injury in rat renal transplants. Transplantation 89, 402–408. https://doi.org/10.1097/TP.0b013e3181caa499 (2010).
Brasen, J. H. et al. Lectin-like oxidized low-density lipoprotein (LDL) receptor (LOX-1)-mediated pathway and vascular oxidative injury in older-age rat renal transplants. Kidney Int. 67, 1583–1594. https://doi.org/10.1111/j.1523-1755.2005.00240.x (2005).
White, E., Hildemann, W. H. & Mullen, Y. Chronic kidney allograft reactions in rats. Transplantation 8, 602–617. https://doi.org/10.1097/00007890-196911000-00007 (1969).
Masoud, G. N. & Li, W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 5, 378–389. https://doi.org/10.1016/j.apsb.2015.05.007 (2015).
Simon, J. F. et al. Induction sirolimus and delayed graft function after deceased donor kidney transplantation in the United States. Am. J. Nephrol. 24, 393–401. https://doi.org/10.1159/000079734 (2004).
McTaggart, R. A. et al. Sirolimus prolongs recovery from delayed graft function after cadaveric renal transplantation. Am. J. Transplant. 3, 416–423. https://doi.org/10.1034/j.1600-6143.2003.00078.x (2003).
Stallone, G. et al. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1-year graft function. J. Am. Soc. Nephrol. 15, 228–233. https://doi.org/10.1097/01.asn.0000102469.32182.8c (2004).
Mannon, R. B. Delayed graft function: the AKI of kidney transplantation. Nephron 140, 94–98. https://doi.org/10.1159/000491558 (2018).
Albano, L. et al. Incidence of delayed graft function and wound healing complications after deceased-donor kidney transplantation is not affected by de novo everolimus. Transplantation 88, 69–76. https://doi.org/10.1097/TP.0b013e3181aa7d87 (2009).
Ekberg, H. et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N. Engl. J. Med. 357, 2562–2575. https://doi.org/10.1056/NEJMoa067411 (2007).
Dantal, J. et al. Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-month results of a randomized, multicenter trial. Transpl. Int. 23, 1084–1093. https://doi.org/10.1111/j.1432-2277.2010.01094.x (2010).
Shye, M. et al. Worsening proteinuria and renal function after intravitreal vascular endothelial growth factor blockade for diabetic proliferative retinopathy. Clin. Kidney J. 13, 969–980. https://doi.org/10.1093/ckj/sfaa049 (2020).
Ko, H. T. et al. Sirolimus reduces vasculopathy but exacerbates proteinuria in association with inhibition of VEGF and VEGFR in a rat kidney model of chronic allograft dysfunction. Nephrol. Dial. Transplant. 28, 327–336. https://doi.org/10.1093/ndt/gfs453 (2013).
Smith, S. F., Hosgood, S. A. & Nicholson, M. L. Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int. 95, 50–56. https://doi.org/10.1016/j.kint.2018.10.009 (2019).
Conde, E. et al. Hypoxia inducible factor 1-alpha (HIF-1 alpha) is induced during reperfusion after renal ischemia and is critical for proximal tubule cell survival. PLoS ONE 7, e33258. https://doi.org/10.1371/journal.pone.0033258 (2012).
Pallet, N., Rabant, M., Legendre, C., Martinez, F. & Choukroun, G. The nephroprotective properties of recombinant human erythropoietin in kidney transplantation: experimental facts and clinical proofs. Am. J. Transplant. 12, 3184–3190. https://doi.org/10.1111/j.1600-6143.2012.04287.x (2012).
Zhou, J., Lu, J. & Cai, D. Recombinant human erythropoietin for kidney transplantation: a systematic review and meta-analysis. Urol. J. 17, 217–223. https://doi.org/10.22037/uj.v0i0.5399 (2020).
Faleo, G. et al. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation 85, 1833–1840. https://doi.org/10.1097/TP.0b013e31817c6f63 (2008).
Bernhardt, W. M. et al. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc. Natl. Acad. Sci. USA 106, 21276–21281. https://doi.org/10.1073/pnas.0903978106 (2009).
Ramaswamy, V. et al. DNA aptamer assembly as a vascular endothelial growth factor receptor agonist. Nucleic Acid Ther. 25, 227–234. https://doi.org/10.1089/nat.2014.0519 (2015).
Boratynska, M. et al. Conversion to sirolimus from cyclosporine may induce nephrotic proteinuria and progressive deterioration of renal function in chronic allograft nephropathy patients. Transplant. Proc. 38, 101–104. https://doi.org/10.1016/j.transproceed.2005.12.023 (2006).
Pascual, J. et al. Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J. Am. Soc. Nephrol. 29, 1979–1991. https://doi.org/10.1681/ASN.2018010009 (2018).
Acknowledgements
We dedicate this paper to the commemoration of Prof. Duska Dragun who deceased in December 2020. She initiated this study and contributed with the wealth of her ideas and analytic thoughts substantially to its successful completion.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Wyeth Pharma.
Author information
Authors and Affiliations
Contributions
U.H., M.N.-K., K.B., and B.H. designed the research. U.H., D.M., M.N.-K., and B.H. performed the experiments. U.H., D.M., K.B., and B.H. analyzed the data. B.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Klemens Budde has received research funds and/or honoraria from Abbvie, Alexion, Astellas, Bristol-Myers Squibb, Chiesi, CSL Behring, Fresenius, Hexal, Hookipa Biotech, Merck Sharp & Dohme, Novartis, Otsuka, Pfizer, Roche, Shire, Siemens, Takeda, Veloxis and Vitaeris. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoff, U., Markmann, D., Nieminen-Kelhä, M. et al. Low-dose rapamycin does not impair vascular integrity and tubular regeneration after kidney transplantation in rats. Sci Rep 11, 16270 (2021). https://doi.org/10.1038/s41598-021-95790-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-95790-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.