Abstract
Biosynthesis of hydrocarbons is a promising approach for the production of alternative sources of energy because of the emerging need to reduce global consumption of fossil fuels. However, the suitability of biogenic hydrocarbons as fuels is limited because their range of the number of carbon atoms is small, and/or they contain unsaturated carbon bonds. Here, we report that a marine phytoplankton, Dicrateria rotunda, collected from the western Arctic Ocean, can synthesize a series of saturated hydrocarbons (n-alkanes) from C10H22 to C38H78, which are categorized as petrol (C10–C15), diesel oils (C16–C20), and fuel oils (C21–C38). The observation that these n-alkanes were also produced by ten other cultivated strains of Dicrateria collected from the Atlantic and Pacific oceans suggests that this capability is a common characteristic of Dicrateria. We also identified that the total contents of the n-alkanes in the Arctic D. rotunda strain increased under dark and nitrogen-deficient conditions. The unique characteristic of D. rotunda could contribute to the development of a new approach for the biosynthesis of n-alkanes.
Similar content being viewed by others
Introduction
Crude oil contains a series of alkanes that are thought to be produced mainly by thermal decomposition of organic compounds but are also produced by abiogenic formation in crustal fluids1 (e.g., polymerization of methane precursors2) and by bacterial biosynthesis3. Biosynthesis of hydrocarbons has been reported in bacteria4,5,6,7, phytoplankton8,9,10,11, and other organisms12,13,14. The biogenic hydrocarbons produced in all organisms are essential components or accidental products of biochemical processes. Phytoplankton are an important source of biogenic hydrocarbons, mainly C15 and C17 straight-chain alkanes and the C21:6 linear alkene10,11,15. Previous studies10,11,15 have reported that 24 species of phytoplankton have the capability to synthesize one or two major linear saturated or unsaturated hydrocarbons (C15, C17, or C21:6) along with trace amounts of other saturated hydrocarbons that have 12–25 carbon atoms. Hydrocarbons synthesized by these species tend to be unstable because the majority are unsaturated and hence vulnerable to attack and easily oxidized. Zooplankton and higher-trophic-level marine organisms also produce hydrocarbons, mainly pristane16,17. Marine bacteria produce mainly linear C17 and C18 alkanes18.
The stability and high diversity of natural marine hydrocarbons make them potentially important sources of fuels. Hydrocarbons used as energy sources are called “fossil hydrocarbons” because fossil hydrocarbons are generally formed or altered by reactions that involve decarbonylation of typically even-numbered fatty aldehydes in sediments or sedimentary rocks under high pressure and temperature over geologic time. However, oil composed mainly of linear alkanes and isoalkanes from C11 to C26 with an age estimated to be less than 1000 years19,20 has been discovered in the Uzon volcano caldera, Kamchatka. The chemical reduction of carbon dioxide by microorganisms has been suggested to be the likely mechanism of formation of this oil seep in the Uzon volcano caldera because the oil field is located 30–35 km from a hydrothermal spring21. However, the suggested mechanism is controversial because the responsible microorganisms have not been identified, and a series of chemical reactions involving microorganisms that would lead to the formation of a variety of linear alkanes with both even and odd numbers of carbons is still unknown, although new pathways for the biosynthesis of alkanes, mainly heptadecane and pentadecane, have been reported in cyanobacteria3.
In this study, we report that a marine phytoplankton, Dicrateria rotunda (D. rotunda) strain ARC1, collected from the western Arctic Ocean, has the novel capability to synthesize a series of linear, saturated hydrocarbons (n-alkanes) ranging from C10H22 to C38H78. This characteristic of D. rotunda is noteworthy because there has been no previous report of any organism with similar capability. We also demonstrate that the production of a series of n-alkanes is a robust characteristic of the Dicrateria genus, and we discuss the possible pathway of production and function of the n-alkanes. We characterize the detailed cellular structure of the ARC1 strain and estimate the environmental conditions that affect the production of n-alkanes. We also discuss the significance from the standpoint of biofuels of the capability of D. rotunda to produce n-alkanes vis-à-vis that of other, previously studied organisms.
Results and discussion
Characteristics of n-alkanes produced by Dicrateria
A drastic reduction of sea ice has recently been observed in the western Arctic Ocean22. We have used the R/V Mirai of the Japan Agency for Marine-Earth Science and Technology23,24,25 to investigate the impacts of the ice reduction on lower-trophic-level organisms in the Arctic Ocean. During the cruise of August–October 2013, we isolated a phytoplankton strain from station 65 (70°00.06'N, 168°44.96'W, 10-m water depth). We found that the organism was a strain of Dicrateria rotunda (D. rotunda), class Haptophyceae, based on phylogenetic analysis of its 18S rRNA sequence (Fig. 1a)26,27. An axenic culture of the Arctic strain (ARC1) of D. rotunda was obtained via antibiotic treatment. The ARC1 strain can swim via two flagella, but it lacks coccoliths and organic lamella on its cell surface (Fig. 1b). The presence of a chloroplast and a lipid body in which various kinds of hydrocarbons might accumulate28 was observed via optical microscopy and fluorescence microscopy with boron-dipyrromethene (BODIPY) fluorescence dye staining29 (Fig. 1b). Additional cellular components such as a nucleus, mitochondria, vacuole, endoplasmic reticulum (ER), and Golgi apparatus were observed by serial block-face scanning electron microscopy (SBF-SEM) (Fig. 1b,c, and Supplementary Video S1).
Phylogenetic and morphogenic characteristics of Dicrateria rotunda (D. rotunda) ARC1 strain. (a) Phylogenetic trees of 18S rRNA genes of the ARC1 strain and other selected haptophyte strains were estimated using the maximum likelihood method. Boot strap values of 1000 trials are shown. (b) Photographs of the ARC1 strain cultured with continuous light at 20 °C. Differential interference contrast microscopy (left upper), fluorescence microscopy with boron-dipyrromethene staining under blue excitation (left lower), and serial block-face scanning electron microscopy (right). Observed cellular structures are shown as colored arrows. (c) Detailed 3D structure of the cell of the ARC1 strain obtained by SBF-SEM. Each organelle has the same color as the corresponding arrow in (b).
We analyzed the lipid contents of the ARC1 strain via gas chromatography-mass spectrometry (GC–MS) to identify every hydrocarbon based on the mass spectrum. The results of the gas chromatograph analysis revealed a consecutive series of straight-chain alkanes from C10 to C38 (C10H22–C38H78) in the neutral lipid fraction (Fig. 2a). A remarkable feature of the straight-chain alkanes produced by the ARC1 strain was that all straight-chain alkanes from C10 to C38 were present (Figs. 2a, 3a and Supplementary Table S1), and the composition of the alkanes was ideal for fuel oil. In terms of strains NIES1001, 2779, 2780 and NBRC10279, some alkanes were not detected (please see Supplementary Table S1). Another noteworthy characteristic was that the short-chain n-alkanes C10 (decane), C11 (undecane), and C15 (pentadecane) were the major components among all the n-alkanes, and there was no remarkable odd or even carbon number preference (Fig. 3a and Supplementary Table S1). The relatively high abundances of decane, undecane, and pentadecane are qualitatively consistent with the reported characteristics of the hydrocarbons produced by 24 species of algae, including 22 marine phytoplankton belonging to nine algal classes including Bacillariophyceae, Dinophyceae, Cryptophyceae, Haptophyceae, Euglenophyceae, Cyanophyceae, Rhodophyceae, Xanthophyceae, and Chlorophyceae10,11,15,18,30,31,32,33. The analyses of the alkanes produced by those 24 species10,11,15,18,30,31 have indicated that it is typical for the concentrations of one or two hydrocarbons (e.g., n-pentadecane in brown algae and n-heptadecane in red algae) to exceed the concentrations of the other alkanes by orders of magnitude10. Other consecutive n-alkanes from C12 to C25 have also been detected in species belonging to the Cyanophyceae, Rhodophyceae, Xanthophyceae, and Chlorophyceae10,32. However, there are no previous reports of any organisms having a capability like that of D. rotunda to synthesize a consecutive series of straight-chain n-alkanes from a short chain (C10) to a long chain (C38). This capability of D. rotunda to synthesize n-alkanes might enable the production of fuel oil with an ideal composition.
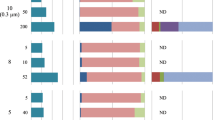
n-alkane concentrations and their compound-specific δ13C in the 11 Dicrateria strains. (a) Content of a series of n-alkanes, ng/mg cell dry weight and (b) their compound-specific δ13C values in the ARC1 strain and 10 other Dicrateria strains collected from ocean sites throughout the world and stored in culture collections. Total amounts of the n-alkanes of each strain are shown in the inset bar graph.
Dicrateria rotunda is found not only in the Arctic Ocean (this study) but also in middle latitudes, according to information in culture collections throughout the world. We examined the capability to produce n-alkanes of four additional strains of D. rotunda (NIES1001, NIES2779, NIES2780, and NBRC102791) and six strains of Dicrateria sp. (RCC3437, RCC4217, RCC4577, RCC4578, RCC5635, and RCC5639). These additional strains were obtained from the culture collections of the National Institute for Environmental Studies (NIES), Japan, the NITE Biological Resource Center (NBRC), Japan, and the Roscoff Culture Collection (RCC), France. Notably, consecutive series of straight-chain alkanes from C10 to C38 were detected in all of the NIES, NBRC, and RCC strains (Fig. 3a and Supplementary Table S1). In contrast, almost no n-alkanes were detected except for small peaks of C14 and C15 in Emiliania huxleyi strain NIES3367 (Fig. 2b and Supplementary Table S1). Emiliania huxleyi is the most cosmopolitan of the species that belong to the algal class Haptophyceae and can live from the equator to subpolar latitudes under a variety of environmental conditions in the world oceans34. Thus, the capability to produce a consecutive series of straight-chain alkanes from short to long chains was a robust and unique characteristic of the genus Dicrateria. The content of total alkanes in the 11 strains of Dicrateria ranged from 12.5 to 79.4 ng/mg dry wt. (Fig. 3 and Supplementary Table S1), i.e., 0.01–0.08% of total cell dry weight. This range was lower than the range of 0.02–0.48% previously reported for 24 species of benthic algae belonging to the Chlorophyceae, Phaeophyceae, and Rhodophyceae10 as well as the range of 0.08–2.9% for six species of soil algae18.
Botryococcus braunii (B. braunii) is one of the most popular organisms used for the production of biodiesel because it has the ability to synthesize a large amount of oil per cell. The total hydrocarbon content of B. braunii varies dramatically, from 0.4% to 76% of cell dry weight, depending on the strain35. The hydrocarbons produced by B. braunii have three characteristics. First, in the odd-number n-alkenes from C25 to C31, there are two or three double bonds in the molecules. The biosynthesis of these compounds begins with oleic acid (C18:1, n-9). Two carbons are added at a time via acetyl-CoA, and odd-number n-alkanes are finally synthesized by decarbonylation. Second, the hydrocarbons include molecules with the stoichiometry CnH2n-10 (n = 30–37) that have a triterpene structure somewhere in the molecule. Third, the hydrocarbons include lycopadiene (C40H70)35. The hydrocarbons synthesized by not only B. braunii but also Botryococcane have been considered as potential sources of the long-chain n-alkanes in crude oil36. However, almost all the hydrocarbons produced by B. braunii are alkenes that are easily oxidized and are therefore not suitable for direct use as fuels. The capability of Dicrateria to produce a series of n-alkanes from C10 to C38 is superior to the hydrocarbon production capability of other known phytoplankton from the standpoint of the suitability of the hydrocarbons as fuels.
Compound-specific carbon isotope ratios of the n-alkanes of Dicrateria
The compound-specific carbon isotope ratio (δ13C) of organic matter reflects the influence of environmental conditions on biological activity, the processes by which compounds are produced, and diagenesis over geological time scales. For example, the compound-specific carbon isotope ratio of organic matter has been used to identify the mechanisms by which n-alkanes are synthesized in petroleum source rocks or in oil shale during diagenesis37,38. Thus, compound-specific δ13C values might be expected to provide important information that could help elucidate the mechanisms by which n-alkanes are synthesized in a cell.
To obtain insight about how n-alkanes are synthesized in Dicrateria, we measured the compound-specific carbon isotope ratio of each alkane. The δ13C values of decane and undecane were relatively light and ranged from − 70 to − 60‰ (Fig. 3b and Supplementary Table S2). The δ13C of other alkanes ranged from − 35 to − 20‰ and were relatively heavy compared with those of decane and undecane (Fig. 3b and Supplementary Table S2). The δ13C composition of phytoplankton is controlled by various environmental parameters, but the immediate products of photosynthesis are generally depleted in 13C compared with the precursor inorganic carbon because of isotope fractionation39. Biosynthesis of a hydrocarbon is considered to involve elongation of a fatty acid followed by loss of the carboxyl carbon40. Fatty aldehydes can serve as the immediate precursors of n-alkanes because Co-porphyrin can catalyze the conversion of aldehydes to the corresponding alkanes with the loss of CO41. Based on the systematic depletion of 13C in products relative to precursors because of isotope fractionation during the biosynthesis of organic compounds38, the patterns of the compound-specific δ13C values of the Dicrateria n-alkanes implies that, the alkanes having heavier δ13C from − 35 to − 20‰ are the precursor of the biosynthesis of the decane and undecane having lighter δ13C from − 70 to − 60‰. Alternatively, decane and undecane may be synthesized through a biosynthesis pathway that is distinct from the biopathway of the other alkanes. In addition to analysis of the compound-specific δ13C values of the n-alkanes, we sought another approach—such as a physiological approach or discovery of the gene that controls production of n-alkanes—to verify our conclusions based on compound-specific isotope data about the mechanism involved in the biosynthesis of n-alkanes in the cell.
Environmental conditions affecting n-alkane production in the ARC1 strain
Changes in the environmental conditions have been reported to promote lipid accumulation in diverse algal strains42. We examined the effects of several key parameters, including light, temperature, and inorganic nitrogen, on n-alkane production in the ARC1 strain. The ARC1 strain was grown under continuous light at 20 °C for two days and then grown for four days under three different conditions of continuous light at 20 °C (light), continuous dark at 20 °C (dark), or continuous light at 4 °C (low temperature) (Fig. 4a). In another experiment, the ARC1 strain was grown in a nitrogen-depleted medium with continuous light at 20 °C for seven days to expose the cells to nitrogen-deficient condition (Fig. 4b). Flow cytometer analysis showed two peaks in the distribution of cell size grown under light condition, which is probably attributed to the two different phases in the cell cycle of the ARC1 strain (Fig. 4c). This analysis revealed decreases in cell size and chlorophyll a (Chl.a) fluorescence and an increase of BODIPY fluorescence under both dark and nitrogen-deficient conditions (Fig. 4c,e). In contrast, increases in cell size and BODIPY fluorescence were observed in the cells under low-temperature conditions, but there was little change of Chl.a fluorescence (Fig. 4c–e). SBF-SEM analysis showed that cells grown under low-temperature conditions formed a more distinct lipid body, and there were increases in the number and total volume of the lipid bodies (Supplementary Fig. S1 and Supplementary Video S2).
Effects of growth conditions on cell size and fluorescence from BODIPY and Chl.a. (a,b) Fluorescence from Chl.a was monitored in the ARC1 strain grown under four different culture conditions: continuous light at 20 °C (red); continuous dark at 20 °C (green); continuous light at 4 °C (yellow); and continuous light under nitrogen-deficient conditions at 20 °C (blue). Error bars indicate standard deviations of triplicate experiments. (c–e) Flow cytometer analysis of cell size (c), and intensity of BODIPY (d) and Chl.a (e) fluorescence of the ARC1 strain. (f) Light (upper) and fluorescence (lower) microscopic observations of the ARC1 strain under four different growth conditions. Red and green fluorescence from Chl.a and BODIPY, respectively were detected under blue light excitation.
However, GC analysis showed that the behavior of the n-alkane contents differed from that of the lipid bodies. Total content of n-alkanes per dry cell weight was 45.1 ng/mg for light condition, 242 ng/mg for dark condition, 65.9 ng/mg for low-temperature condition, and 209 ng/mg for nitrogen-deficient condition (Supplementary Table S1). The range of the total n-alkane content of the ARC1 strain per dry cell weight under the four different conditions (0.005–0.024%) was lower than those of 0.02–2.9% previously reported for benthic and soil algae10,18. The total content of n-alkanes increased 5.6-fold (Dunnett’s test; p < 0.05) under dark condition and 4.8-fold (Dunnett’s test; p < 0.05) under nitrogen-deficient condition compared with continuous-light conditions (Fig. 5a inset and Supplementary Table S1). The increases in most of the carbon numbers of n-alkanes were significant (Dunnett’s test; p < 0.05) (Fig. 5a, filled boxes). The total n-alkane content was 1.5-fold larger under low-temperature conditions than under continuous-light conditions, but the difference was not significant (Fig. 5a, inset). These results demonstrate that the n-alkanes of the ARC1 strain accumulated under dark and nitrogen-deficient conditions, but they did not accumulate to a significant extent under low-temperature condition, even though the numbers and volumes of the lipid bodies increased. The fact that we did not observe a substantial treatment effect on the fold change of the n-alkanes versus the treatment effect on their carbon numbers (Fig. 5a) implies that the composition of the n-alkanes was not affected. The dark-induced accumulation of the alkanes in the ARC1 strain implies that the alkanes did not serve as an energy source in the absence of photosynthesis. The increase of the n-alkane content under nitrogen-deficient condition, during which the growth of the cells was suppressed, suggests that n-alkanes were produced by a growth-independent mechanism. We also measured the δ13C of the n-alkanes of the ARC1 strain under the four different culture conditions, but the fact that most of the δ13C values did not change significantly (Fig. 5b and Supplementary Table S2) implies that the pathway of biosynthesis of the n-alkanes was not changed by the different growth conditions.
Effect of environmental conditions on the concentrations and compound-specific δ13C of n-alkanes in the ARC1 strain. (a) Concentration of a series of n-alkanes per dry weight; (b) n-alkane compound-specific δ13C composition. Both were quantified in the ARC1 strain grown under four different culture conditions: continuous light at 20 °C (light); continuous dark at 20 °C (dark); continuous light at 4 °C (low temp); and continuous light under nitrogen-deficient conditions at 20 °C (N def). Error bars indicate standard deviations (n = 3 for dark, low temp, and N def conditions; n = 6 for light condition). Significant differences in the total amount of the series of n-alkane concentrations between light and low temp, dark, or N def conditions were detected by Dunnett’s test (asterisks show p < 0.05 in the inset figure). For each alkane compound, environmental conditions showing the significant difference in the Dunnett’s test (p < 0.05) are shown as filled boxes. Lines, bars, and boxes are colored to reflect growth conditions.
Some strains of the order Isochrysidales of Haptophyte algae, including Emiliania huxleyi, (Fig. 1a) produce unique long-chain unsaturated methyl- or ethyl-ketones called alkenones43. The alkenones are thought to serve as metabolic storage molecules44. The capability to produce alkenones has not been observed in other genera of the order Pryminesiales45. Chrysochromulina tobin, which belongs to the order Pryminesiales of the phylum Haptophyte (Fig. 1a), contains high concentrations of saturated and unsaturated fatty acids ranging from C14 to C22 in the lipid bodies, and it uses those fatty acids for energy storage during a light/dark cycle46. These observations suggest that substantial diversity exists in the kinds of hydrocarbons produced by each genus of Haptophyte. Culture experiments have found that some cyanobacteria synthesize C15–C19 saturated hydrocarbons and accumulate them in their membranes47. In an experiment using cyanobacteria that were deficient in n-alkanes, the sizes of the cells increased because the membranes were unstable47. It has been suggested that n-alkanes are needed in membranes to reduce membrane permeability, to control cell size, to enable the cell to grow, and to give the cell membranes the flexibility needed for optimal cell division47. Lea-Smith et al.47 have also suggested that it is essential that hydrocarbons accumulate in a cell membrane and that such accumulation enables microalgae to minimize risks and to grow rapidly under optimal growth conditions. One possible function of the n-alkanes in Dicrateria might be keeping the membrane system in optimal condition during the shrinking of cell volumes under the stress of dark and nitrogen-deficient growth conditions (Fig. 4c). Further analysis is required to elucidate the physiological roles and the location of the n-alkanes in a Dicrateria cell.
Materials and methods
Samples
Surface water was collected at St.65 (70° 0.06′ N, 168° 44.96′ W) in the Chukchi Sea, western Arctic Ocean, during cruise MR13-06 of the R/V Mirai. Basic feature of surface water sample was as follows; water temperature: 2.56 °C, salinity: 31.4, oxygen concentration: 334.7 µmol/kg, nitrate concentration: 0.05 µmol/kg, nitrite concentration: 0.02 µmol/kg, silicate concentration: 0.41 µmol/kg, phosphate concentration: 0.395 µmol/kg. An aliquot of seawater was separated into two batch culture bottles containing natural seawater enriched with nutrients as specified for 1/10 MNK medium48. Cultures were incubated at 4 °C on a 16-h light:8-h dark cycle of illumination on board the ship. An aliquot (20 mL) of the seawater cultured on board the ship was transferred to MA-ESM medium49, and the cells were further cultured at 5 °C and 10 °C under a photoperiod illumination of 20 µmol quanta m−2 s−1 on a 16-h:8-h L:D cycle. We succeeded in isolating an axenic Arctic strain (ARC1) by treatment with the antibiotics G-418 (200 mg/mL), kanamycin (20 mg/mL), spectinomycin (20 mg/mL), and ampicillin (100 mg/mL) and by single-cell isolation under a microscope. The axenicity was checked by microscopic observation of the cells of the ARC1 strain that had been cultured on a Marine Broth 2216 agar plate (BD Bioscience) for 1 week at 25 °C. The axenic culture of the D. rotunda ARC1 was deposited in the culture collection of the National Institute of Technology and Evaluation (NITE), Japan with accession number FERM BP-22332. We also obtained other Dicrateria strains from culture collections. Those strains included D. rotunda from the National Institute for Environmental Studies (NIES), Japan (http://mcc.nies.go.jp/index_en.html) and Roscoff Culture Collection (RCC), France (http://www.roscoff-culture-collection.org/). The strains were D. rotunda Reynolds emend. J. C. Green & Pienaar (NIES1001, NIES2779, NIES2780, and NBRC102791 collected from the North Pacific Ocean) and Dicrateria sp. (RCC3437, RCC4577, and RCC4578 collected from the South Atlantic Ocean; RCC5635 and RCC5639 collected from the South Pacific Ocean; and RCC4214 collected from North Atlantic Ocean). The ARC1 strain was maintained in f/2 medium50 under aeration, aseptic conditions, 20 °C, and 10 µmol quanta m−2 s−1 of continuous illumination. The other strains were grown under the same light and temperature conditions in K51, K/251, or ESM52 liquid media as described in Supplementary Table S1. The culture in stationary phase was used for measurements of the concentration of n-alkanes and their compound-specific stable carbon isotope ratios.
Phylogenetic analysis
The 18S rRNA sequence of D. rotunda (GenBank accession LC519889) was obtained from a draft genome assembly using the Pacbio RSII sequencer (Hirose et al., in preparation). Representative haptophytes and their 18S rRNA sequences are listed in Supplementary Table S1 as follows: Pavlova lutheri (AF102369), Rebecca salina DHmm3W3 (KU561125), Exanthemachrysis gayraliae YP15 (AF106060), Phaeocystis globose CCMP 627 (AJ278035), Isochrysis galbana CCMP1323 (HM149540), Emiliania huxleyi CCMP 1516 (AHAL01000301), Cruciplacolithus neohelis CCMP 298 (AJ246262), Pleurochrysis dentata CCAP 944/2 (KJ756811), Imantonia sp. CCMP1404 (AM491015), Pseudohaptolina arctica CCMP1204 (AM491016), Braarudosphaera bigelowii Funahama-T3 (AB478412), Haptolina brevifila Kawachi (AM490995), Chrysochromulina sp. CCMP291 (JWZX01002122), and Chrysochromulina sp. RCC 390 (KT878670). In addition, Guillardia theta CCMP2712 (AEIE01003291), a member of the Cryptophyceae, was used as an outgroup. These sequences were aligned with MAFFT version 7 online with default parameters53. The phylogenetic tree was estimated by the maximum likelihood method using RAxML (version 8.2.11)54. The GTRGAMMA54 model was used with 1000 bootstrap iterations (options -f a -m GTRGAMMA -# 1000).
Scanning electron microscopy
For serial block-face scanning electron microscopy (SBF-SEM), cells were grown at 20 °C or 4 °C in a 400 mL flask, fixed with 3% glutaraldehyde by volume for 30 min, and collected by centrifugation at 3000 × g at room temperature for 5 min. The cells were washed with phosphate-buffered saline solution (PBS, pH 7.5) to remove glutaraldehyde and then post-fixed with 2% osmium tetroxide and 1.5% potassium ferrocyanide in PBS for one hour. The cells were washed with distilled water and incubated in 1% thiocarbohydrazide solution for 20 min. They were then washed with distilled water and fixed again with 2% osmium tetroxide in distilled water at room temperature for 30 min. After washing with distilled water, the fixed cells were stained en bloc with 1% uranyl acetate in distilled water at 4 °C overnight and with 0.2 mol/L lead aspartate (pH 5.5) at 60 °C for 30 min. The cells were washed with distilled water and dehydrated with a series of ethanol washes (50–100%) at 4 °C and dehydrated with acetone at room temperature. Finally, they were infiltrated with Durcupan (Sigma-Aldrich, St Louis, Missouri, USA) resin and polymerized at 60 °C for three days. The resin block containing the sample was trimmed and glued onto an aluminum rivet with a conductive epoxy resin (SPI Conductive Silver Epoxy; SPI Supplies and Structure Probe, Inc., West Chester, PA, USA) and then coated with gold using an ion coater. A serial block-face scanning electron microscope (MERLIN; Carl Zeiss Microscopy, Jena, Germany) equipped with a OnPoint back-scattered electron detector (Gatan Inc., Pleasanton, CA, USA) was used to slice and image the specimens. The scanning electron microscope was operated at a low accelerating voltage of 1.2 kV. The serial images were automatically acquired by using Gatan Digital Micrograph software. All images were taken at an image size of 8192 × 8192 pixels (pixel size = 4 nm) with a thickness of 50 nm. The image series was automatically aligned using Fiji (http://fiji.sc/Fiji) and cropped around each single cell. Segmentation of the volume data was performed with Amira version 5.4.5 (Thermo Fisher Scientific, Waltham, MA, USA).
Effect of growth environment and flow cytometric characterization
To investigate the effects of growth conditions on n-alkane production, 40 mL of the culture of the ARC1 strain of D. rotunda at stationary phase was transferred to 400 mL of fresh f/2 medium and cultured in 1 L Erlenmeyer flasks containing 400 mL of f/2 medium. The ARC1 strain was cultured under continuous light (flasks were aerated; the culture aseptic; and continuous light was supplied by a white fluorescent lamp at an intensity of 10 µmol quanta m−2 s−1) at 20 °C. Dicrateria rotunda was cultured under these conditions for two days, and the ARC1 strain was then transferred and grown under four different conditions for four days: (1) continuous light at 20 °C; (2) continuous darkness at 20 °C; (3) continuous light at 4 °C and; (4) continuous light with nitrogen deficiency at 20 °C. Because the optimum cell growth of the ARC1 strain was observed at a low cell density, the growth of the cells was estimated by the measurement of red fluorescence of Chl.a using a Qubit 4 fluorometer (Thermo Fisher Scientific) once each day instead of by measurement of optical density using a spectrophotometer. Each of the four different culture experiments was run in triplicate (total of 12 flasks). After cultivation for a total of six days, cells were collected by centrifugation for 10 min at 3780×g and frozen at −80 °C for gas chromatography-mass spectrometry and gas chromatography analyses of the n-alkane compositions and their contents, respectively. About 30 mL of culture was centrifuged for 5 min at 3000×g, resuspended in 500 µL of f/2 medium, and subjected to flow cytometry and microscopy. The fluorescence intensity of Chl.a, BODIPY, and cell size were measured as R-fluorescence, G-fluorescence, and forward-scattering light, respectively, via 488-nm blue laser excitation using a flow cytometer (CytoFLEX, Beckman Coulter, Inc., Indianapolis, IN, USA). A Flow Cytometry Size Calibration Kit (Invitrogen) was used as the standard maker for the forward-scattering analysis. The cells of the NIES and RCC strains were prepared for n-alkane quantification as described for the ARC1 strain under continuous illumination at 20 °C. For differential interference contrast microscopy and fluorescence microscopy, a 1/100 volume dilution of BODIPY 505/515 solution (Thermo Fischer Scientific, 10 µg/mL in DMSO) was added to the cell solution and observed using an IX-73 microscope (Olympus Corporation, Japan) equipped with a DP-80 camera (Olympus Corporation, Japan). Green BODIPY fluorescence and red Chl.a fluorescence were observed with blue light excitation (460–495 nm) using a U-FBW mirror unit (Olympus Corporation, Japan).
Alkane analysis
A sample of 20–30 mg of dry cells of D. rotunda was placed in a stainless-steel cell, and all lipids were extracted with a mixed solvent of dichloromethane and methanol (9:1 by volume) using an accelerated solvent extractor (ASE-200, DIONEX Japan Ltd., Japan) under 100 °C and 1500 psi (= 10,343 kPa) for 20 min. The solvent mixture of dichloromethane and methanol was concentrated to a volume of less than 1 mL with nitrogen gas. Ten milliliters of a mixture of 0.5 mol/L potassium hydroxide in methanol was added to the concentrated solvent mixture of dichloromethane and methanol to saponify the mixture at 80 °C for 2 h. After saponification, an aliquot of hexane was added to the cooled, saponified solution, and the solution was shaken to dissolve the lipid fraction into the hexane layer. The hexane layer was taken up and transferred into a glass tube. The hexane fraction was concentrated under nitrogen gas to an approximate volume of 1 mL. This concentrated hexane fraction was transferred to a silica gel column, and the n-alkane fraction was extracted with hexane by silica gel column chromatography (Rapid Trace SPE Workstation, Biotage Japan Ltd., Tokyo, Japan). The series of n-alkanes was identified with a gas chromatograph-mass spectrometer (Agilent 7200GC/Q-TOF mass spectrometer, Agilent Technologies Japan Ltd., Tokyo, Japan) equipped with a glass column (DB-5 ms: 30 m long, 0.25 mm inner size, 0.25 µm thickness). A series of n-alkanes was also analyzed with a gas chromatograph (Agilent 6890 N, Agilent Technologies Japan Ltd., Tokyo, Japan) equipped with a glass column (HP-5MS, Agilent: 60 m long, 0.32 mm inner-diameter, and 0.25 µm thickness) and flame-ionization detector (FID). The oven program started at 60 °C, increased at 10 °C/min up to 120 °C, 5 °C/min up to 310 °C, and was then held constant for 22 min at 310 °C. The drifting baseline was subtracted from the peak area when each FID detector response (peak area) was calculated. A series of n-alkanes was quantified via calibration curves estimated from the relationship between the concentration of each C10–C38 alkane standard material (GL Science Inc., Tokyo, Japan) and the FID detector response. The yield of C10–C38 alkanes over all steps from extraction of lipids to the GC measurement was 60–70% for C10–C14 alkanes and more than 95% for alkanes larger than C15. The yield of short-chain n-alkanes was lower than that of long-chain alkanes because of the high volatility of the former. Precision of replicate measurements of n-alkane concentrations was within 3% for C10–C38 alkane concentrations (n ≥ 3). Dunnett’s test was performed using multicomp ver.1.4.14. of the R package (version 4.0.3, 2020, https://www.R-project.org).
Compound-specific stable carbon isotope analysis of alkanes
Compound-specific stable carbon isotope analysis of n-alkanes was carried out with a gas chromatograph/combustion/isotope ratio mass spectrometer (GC/C/IRMS, DeltaPlus XP, Thermo Fisher Scientific, K.K., Tokyo, Japan) equipped with a glass column (HP-1MS, Agilent: 60 m long, 0.32 mm inner-diameter, 0.25 µm thickness). The oven program of the GC started at 60 °C for 1 min, increased at 2.5 °C/min up to 80 °C, 40 °C/min up to 120 °C, 5 °C/min up to 310 °C, and was then held for 20 min at 310 °C. The temperature of combustion was 950 °C. Precision for replicate measurements of individual compounds in standard C10–C38 hydrocarbons (GL Science Inc., Tokyo, Japan) using this configuration ranged between 0.2‰ and 0.5‰ for n-alkanes. Prior to the carbon isotope analysis, the CO2 reference gas was calibrated relative to Pee Dee Belemnite (PDB) using the primary NBS 19 and 22 standards. Instrument performance was routinely checked using the previously prepared n-alkane standard solutions of n-eicosane (GL Science Inc., Tokyo, Japan; its δ13C was determined to be − 31.9‰ by SI Science Ltd., Japan) and n-pentatriacontane (GL Science Inc., Tokyo, Japan; its δ13C was determined to be − 30.7‰ by SHOKO Science CO. Ltd., Kanagawa, Japan). Isotopic compositions of n-alkanes were reported relative to PDB, and δ13C values were averaged over replicate measurements (n ≥ 3).
References
Welhan, J. A. & Craig, H. Methane and hydrogen in East Pacific Rise hydrothermal fluids. Geophys. Res. Lett. 6, 829–831. https://doi.org/10.1029/GL006i011p00829 (1979).
Sherwood Lollar, B., Westgate, T. D., Ward, J. A., Slater, G. F. & Lacrampe-Couloume, G. Abiogenic formation of alkanes in the Earth’s crust as a minor source for global hydrocarbon reservoirs. Nature 416, 522–524. https://doi.org/10.1038/416522a (2002).
Schirmer, A., Rude, M. A., Li, X., Popova, E. & del Cardayre, S. B. Microbial biosynthesis of alkanes. Science (New York, N.Y.) 329, 559–562. https://doi.org/10.1126/science.1187936 (2010).
Fehler, S. W. & Light, R. J. Biosynthesis of hydrocarbons in Anabaena variabilis. Incorporation of [methyl-14C]- and [methyl-2H3]methionine into 7- and 8-methylheptadecanes. Biochemistry 9, 418–422. https://doi.org/10.1021/bi00804a032 (1970).
Lea-Smith, D. J. et al. Contribution of cyanobacterial alkane production to the ocean hydrocarbon cycle. Proc. Natl. Acad. Sci. USA 112, 13591–13596. https://doi.org/10.1073/pnas.1507274112 (2015).
Love, C. R. et al. Microbial production and consumption of hydrocarbons in the global ocean. Nat. Microbiol. 6, 489–498. https://doi.org/10.1038/s41564-020-00859-8 (2021).
McGenity, T. J., McKew, B. A. & Lea-Smith, D. J. Cryptic microbial hydrocarbon cycling. Nat. Microbiol. 6, 419–420. https://doi.org/10.1038/s41564-021-00881-4 (2021).
Sorigué, D. et al. Microalgae synthesize hydrocarbons from long-chain fatty acids via a light dependent pathway. Plant Physiol. 171, 2393–2405. https://doi.org/10.1104/pp.16.00462 (2016).
Winters, K., Parker, P. L. & Van Baalen, C. Hydrocarbons of blue-green algae: Geochemical significance. Science 163, 467–468. https://doi.org/10.1126/science.163.3866.467 (1969).
Youngblood, W. W., Blumer, M., Guillard, R. L. & Fiore, F. Saturated and unsaturated hydrocarbons in marine benthic algae. Mar. Biol. 8, 190–201. https://doi.org/10.1007/BF00355215 (1971).
Blumer, M., Guillard, R. R. L. & Chase, T. Hydrocarbons of marine phytoplankton. Mar. Biol. 8, 183–189. https://doi.org/10.1007/BF00355214 (1971).
Tillman, J. A., Seybold, S. J., Jurenka, R. A. & Blomquist, G. J. Insect pheromones—An overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 29, 481–514. https://doi.org/10.1016/s0965-1748(99)00016-8 (1999).
Strobel, G. A. et al. The production of myco-diesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072). Microbiology (Reading, England) 154, 3319–3328. https://doi.org/10.1099/mic.0.2008/022186-0 (2008).
Samuels, L., Kunst, L. & Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 59, 683–707. https://doi.org/10.1146/annurev.arplant.59.103006.093219 (2008).
Lee, R. F. & Loeblich, A. R. Distribution of 21:6 hydrocarbon and its relationship to 22:6 fatty acid in algae. Phytochemistry 10, 593–602. https://doi.org/10.1016/S0031-9422(00)94703-4 (1971).
Blumer, M. & Thomas, D. W. Phytadienes in zooplankton. Science (New York, N.Y.) 147, 1148. https://doi.org/10.1126/science.147.3662.1148 (1965).
Gershbein, L. L. & Singh, E. J. Hydrocarbons of dogfish and cod livers and herring oil. J. Am. Oil Chem. Soc. 46, 554–557. https://doi.org/10.1007/BF02633182 (1969).
Jones, J. G. Studies on lipids of soil micro-organisms with particular reference to hydrocarbons. J. Gen. Microbiol. 59, 145–152. https://doi.org/10.1099/00221287-59-2-145 (1969).
Simoneit, B. R. T., Deamer, D. W. & Kompanichenko, V. Characterization of hydrothermally generated oil from the Uzon caldera, Kamchatka. Appl. Geochem. 24, 303–309. https://doi.org/10.1016/j.apgeochem.2008.10.007 (2009).
Varfolomeev, S. D., Karpov, G. A., Synal, H. A., Lomakin, S. M. & Nikolaev, E. N. The youngest natural oil on earth. Dokl. Chem. 438, 144–147. https://doi.org/10.1134/S0012500811050053 (2011).
Maryutina, T. A., Karpov, G. A. & Varfolomeev, S. D. Basic hydrocarbon components and chemical composition of the environmental medium of the youngest oil on earth. Dokl. Chem. 449, 77–80. https://doi.org/10.1134/S0012500813030026 (2013).
Timmermans, M.-L., Toole, J. & Krishfield, R. Warming of the interior Arctic Ocean linked to sea ice losses at the basin margins. Sci. Adv. 4, eaat6773. https://doi.org/10.1126/sciadv.aat6773 (2018).
Watanabe, E. et al. Enhanced role of eddies in the Arctic marine biological pump. Nat. Commun. 5, 3950. https://doi.org/10.1038/ncomms4950 (2014).
Watanabe, E. et al. Wind-driven interannual variability of sea ice algal production in the western Arctic Chukchi Borderland. Biogeosciences 12, 6147–6168. https://doi.org/10.5194/bg-12-6147-2015 (2015).
Nishino, S. et al. Biogeochemical anatomy of a cyclonic warm-core eddy in the Arctic Ocean. Geophys. Res. Lett. 45, 11284–11292. https://doi.org/10.1029/2018GL079659 (2018).
Edvardsen, B. et al. Ribosomal DNA phylogenies and a morphological revision provide the basis for a revised taxonomy of the Prymnesiales (Haptophyta). Eur. J. Phycol. 46, 202–228. https://doi.org/10.1080/09670262.2011.594095 (2011).
Bendif, E. M., Probert, I., Schroeder, D. C. & de Vargas, C. On the description of Tisochrysis lutea gen. nov. sp. Nov. and Isochrysis nuda sp. Nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J. Appl. Phycol. 25, 1763–1776. https://doi.org/10.1007/s10811-013-0037-0 (2013).
Shishlyannikov, S. M., Nikonova, A. A., Klimenkov, I. V. & Gorshkov, A. G. Accumulation of petroleum hydrocarbons in intracellular lipid bodies of the freshwater diatom Synedra acus subsp. radians. Environ. Sci. Pollut. Res. Int. 24, 275–283. https://doi.org/10.1007/s11356-016-7782-y (2017).
Gocze, P. M. & Freeman, D. A. Factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry 17, 151–158. https://doi.org/10.1002/cyto.990170207 (1994).
Sargent, J. R. & Whittle, K. J. Lipids and hydrocarbons in the marine food web. In Analysis of Marine Ecosystems (ed. Longhurst, A. R.) 491–533 (Academic Press, London, 1981).
Metzger, P. & Casadevall, E. Aldehydes, very long chain alkenylphenols, epoxides and other lipids from an alkadiene-producing strain of Botryococcus braunii. Phytochemistry 28, 2097–2104. https://doi.org/10.1016/S0031-9422(00)97927-5 (1989).
Clark, R. C. & Blumer, M. Distribution of n-paraffins in marine organisms and sediment. Limnol. Oceanogr. 12, 79–87. https://doi.org/10.4319/lo.1967.12.1.0079 (1967).
Oro, J., Tornabene, T. G., Nooner, D. W. & Gelpi, E. Aliphatic hydrocarbons and fatty acids of some marine and freshwater microorganisms. J. Bacteriol. 93, 1811–1818 (1967).
Read, B. A. et al. Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 499, 209–213. https://doi.org/10.1038/nature12221 (2013).
Metzger, P. & Casadevall, E. Lycopadiene, a tetraterpenoid hydrocarbon from new strains of the green alga Botryococcus braunii. Tetrahedron Lett. 28, 3931–3934. https://doi.org/10.1016/S0040-4039(00)96423-2 (1987).
McKidry, D., Cox, R., Volkman, J. & Howell, V. Botryococcane in a new class of Australian non-marine crude. Nature 320, 57–59. https://doi.org/10.1038/320057a0 (1986).
Bjorфy, M., Hall, K., Gillyon, P. & Jumeau, J. Carbon isotope variations in n-alkanes and isoprenoids of whole oils. Chem. Geol. 93, 13–20 (1991).
Sinninghe Damstè, J. S., van Duin, A. C. T., Hollander, D., Kohnen, M. E. L. & de Leeuw, J. W. Early diagenesis of bacteriohopanepolyol derivatives: Formation of fossil homohopanoids. Geochim. Cosmochim. Acta 59, 5141–5155 (1995).
Libes, S. M. An Introduction to Marine Biogeochemistry Vol. 72(3) (Wiley, 1992).
Kolattukudy, P. E. Biosynthesis of wax in Brassica oleracea. Relation of fatty acids to wax. Biochemistry 5, 2265–2275. https://doi.org/10.1021/bi00871a015 (1966).
Dennis, M. & Kolattukudy, P. E. A cobalt-porphyrin enzyme converts a fatty aldehyde to a hydrocarbon and CO. Proc. Natl. Acad. Sci. USA 89, 5306–5310. https://doi.org/10.1073/pnas.89.12.5306 (1992).
Hu, Q. et al. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 54, 621–639. https://doi.org/10.1111/j.1365-313X.2008.03492.x (2008).
Marlowe, I. T. et al. Long chain (n-C37–C39) alkenones in the Prymnesiophyceae. Distribution of alkenones and other lipids and their taxonomic significance. Br. Phycol. J. 19, 203–216. https://doi.org/10.1080/00071618400650221 (1984).
Volkman, J. K., Eglinton, G., Corner, E. D. S. & Sargent, J. R. Novel unsaturated straight-chain C37–C39 methyl and ethyl ketones in marine sediments and a coccolithophore Emiliania huxleyi. in Advances in Organic Geochemistry 1979 (eds Douglas, A. G. & Maxwell, J. J.) 219–227. https://doi.org/10.1016/0079-1946(79)90106-X (Pergamon Press, Oxford, 1980).
Epstein, B. L., D’Hondt, S. & Hargraves, P. E. The possible metabolic role of C37 alkenones in Emiliania huxleyi. Org. Geochem. 32, 867–875. https://doi.org/10.1016/S0146-6380(01)00026-2 (2001).
Hovde, B. T. et al. Genome sequence and transcriptome analyses of Chrysochromulina tobin: Metabolic tools for enhanced algal fitness in the prominent order Prymnesiales (Haptophyceae). PLoS Genet. 11, e1005469 (2015).
Lea-Smith, D. J. et al. Hydrocarbons are essential for optimal cell size, division, and growth of cyanobacteria. Plant Physiol. 172, 1928–1940 (2016).
Noël, M.-H., Kawachi, M. & Inouye, I. Induced dimorphic life cycle of a coccolithophorid, Calyptrosphaera sphaeroidea (Prymnesiophyceae, Haptophyta). J. Phycol. 40, 112–129. https://doi.org/10.1046/j.1529-8817.2004.03053.x (2004).
Danbara, A. & Shiraiwa, Y. The requirement of selenium for the growth of marine coccolithophorids, Emiliania huxleyi, Gehphyrocapsa oceanica and Helladosphaera sp. Plant Cell Physiology 40, 762–766. https://doi.org/10.1093/oxfordjournals.pcp.a029603 (1999).
Guillard, R. R. & Ryther, J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 8, 229–239. https://doi.org/10.1139/m62-029 (1962).
Keller, M. D. & Guillard, R. R. L. Factors significant to marine diatom culture. In Toxic Dinoflagellates (eds Anderson, D. M. et al.) 113–116 (Elsevier, New York, 1985).
Okaichi, T., Nishio, S. & Imatomi, Y. Collection and mass culture (Shiryô no saisyu to baiyô). In Toxic Phytoplankton—Occurrence, Mode of Action, and Toxins (eds Yûdoku, P.-H. & Sayôkikô, D.) 22–34 (Jpn. Fish. Soc., Kôseisya-Kôseikaku, Tokyo, 1982) (in Japanese without English title).
Katoh, K., Rozewicki, J. & Yamada, K. D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166. https://doi.org/10.1093/bib/bbx108 (2019).
Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033 (2014).
Acknowledgements
We gratefully thank Professor Iwane Suzuki and Professor Emeritus Yoshihiro Shiraiwa of the University of Tsukuba for their support of the deck work by students from the University of Tsukuba during the R/V Mirai cruise in the Arctic Ocean, and Ms. Sachiko Yamada for helping segment the SBF-SEM data. NH was funded by two Grants-in-Aid for Scientific Research (S) from the Japan Society for the Promotion of Science FY2010–2014, No. 22221003 and FY2015–2019, 15H05712. YH was funded by the Cooperative Study Program of the National Institute for Physiological Sciences.
Author information
Authors and Affiliations
Contributions
N.H. coordinated the research strategy and interpreted the hydrocarbon data and hydrocarbon stable carbon isotope data. N.H. and Y.H. wrote the manuscript. Y.H. contributed to the phylogenetic analysis of D. rotunda and to all culture experiments using Arctic and non-Arctic strains. S.C. and K.M. contributed to analysis of the SBF-SEM images of D. rotunda. Y.H. and H.K. collected data from the cell analyzer. J.O. and M.S. worked on the hydrocasts to take water samples and also succeeded in isolating the ARC1 strain on board. M.S. also contributed to the extraction and detection of the alkanes from all Dicrateria strains and to the identification and quantification of a series of n-alkane concentrations and their stable isotope data. F.I. contributed to the isolation of the ARC1 strain of D. rotunda from water samples and to growth of the single clone on board the research vessel.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harada, N., Hirose, Y., Chihong, S. et al. A novel characteristic of a phytoplankton as a potential source of straight-chain alkanes. Sci Rep 11, 14190 (2021). https://doi.org/10.1038/s41598-021-93204-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93204-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.