Abstract
Variants in the leucine-rich repeat kinase 2 (LRRK2) gene are associated with increased risk for familial and sporadic Parkinson’s disease (PD). Pathogenic variants in LRRK2, including the common variant G2019S, result in increased LRRK2 kinase activity, supporting the therapeutic potential of LRRK2 kinase inhibitors for PD. To better understand the role of LRRK2 in disease and to support the clinical development of LRRK2 inhibitors, quantitative and high-throughput assays to measure LRRK2 levels and activity are needed. We developed and applied such assays to measure the levels of LRRK2 as well as the phosphorylation of LRRK2 itself or one of its substrates, Rab10 (pT73 Rab10). We observed increased LRRK2 activity in various cellular models of disease, including iPSC-derived microglia, as well as in human subjects carrying the disease-linked variant LRRK2 G2019S. Capitalizing on the high-throughput and sensitive nature of these assays, we detected a significant reduction in LRRK2 activity in subjects carrying missense variants in LRRK2 associated with reduced disease risk. Finally, we optimized these assays to enable analysis of LRRK2 activity following inhibition in human peripheral blood mononuclear cells (PBMCs) and whole blood, demonstrating their potential utility as biomarkers to assess changes in LRRK2 expression and activity in the clinic.
Similar content being viewed by others
Introduction
Variants in the LRRK2 gene are one of the most common genetic risk factors for Parkinson’s disease (PD) and have been shown to modify risk for both familial and sporadic forms of PD as well as immune-related diseases including Crohn’s disease1,2,3. LRRK2 is a multidomain protein that contains a GTPase domain, kinase domain, and several potential protein–protein interaction domains4,5. Disease-associated variants, including the most common pathogenic variant, LRRK2 G2019S, reside within the central catalytic core of LRRK2 and are reported to ultimately lead to increased LRRK2 kinase activity6,7,8. Further, elevated LRRK2 activity has been reported in PD patients carrying variants in other disease-linked genes or with no known genetic cause, suggesting LRRK2 may broadly contribute to the pathogenesis of PD9,10.
Our understanding of how LRRK2 activity is regulated has significantly expanded in recent years through the identification of its direct physiological substrates as well as phosphorylation sites on LRRK2 that are dependent on its kinase activity. LRRK2 phosphorylates a conserved residue on the switch-II domain of a subset of Rab GTPases, including Rab1011. PD-associated variants in LRRK2 increase phosphorylation of these Rab GTPases, and elevated Rab phosphorylation likely impairs their ability to interact with downstream effectors and disrupts various aspects of intracellular trafficking, particularly in the endo-lysosomal system12,13. LRRK2 is also constitutively phosphorylated at a set of serine residues, including Ser935, within the ankyrin and LRR regions of the protein that promotes 14-3-3 binding, and these same sites are dephosphorylated in response to LRRK2 kinase inhibition and have been utilized to assess the extent of LRRK2 inhibition in cellular and in vivo models14,15,16. LRRK2 can undergo autophosphorylation at Ser1292, but analysis of phosphorylation at this site has been of limited utility given its low stoichiometry under endogenous LRRK2 expression8. While analysis of phosphorylation of LRRK2 and its substrates has elucidated many aspects of how LRRK2 levels and activity are modulated, many questions remain unanswered and have been hindered by a lack of highly sensitive and quantitative assays to measure its kinase activity.
We developed high-throughput Meso Scale Discovery (MSD)-based assays to measure the phosphorylation of LRRK2 and one of its direct substrates, Rab10, and used these assays to identify novel aspects of LRRK2 regulation and to support the clinical development of LRRK2 inhibitors by enabling analysis of LRRK2 activity in accessible human samples. We demonstrated that LRRK2 is highly expressed in glial cells, including human iPSC-derived microglia, and that LRRK2 activity is significantly increased in response to lysosomal stress and inflammatory stimuli in these cells. Taking advantage of the sensitive and high-throughput nature of these assays, we were able to detect elevated LRRK2 activity in PBMCs from human subjects that carry the LRRK2 G2019S variant. Importantly, we demonstrated that the LRRK2 N551K R1398H variant associated with reduced risk for PD and Crohn’s disease leads to a reduction in Rab10 phosphorylation in both cellular models as well as in human subjects, suggesting that this variant may reduce disease risk through its effect on LRRK2’s kinase activity. Finally, we used these assays to assess LRRK2 levels and activity in whole blood and PBMCs following LRRK2 kinase inhibition, supporting their use as biomarkers to assess target and pathway engagement following dosing with LRRK2 inhibitors in the clinic.
Results
Development of sensitive, quantitative, and high-throughput assays to measure LRRK2 and pS935 LRRK2
We developed MSD-based assays to detect both total LRRK2 and pS935 LRRK2, a phosphorylated form of LRRK2 that is reduced following LRRK2 kinase inhibition14. Antibody pairs for LRRK2 and pS935 LRRK2 were screened by ELISA assays initially using recombinant LRRK2 protein and cell lysates, and the optimal antibody pairs and orientations for capture and detection were determined based on those that gave the best dynamic range and detection specificity. Increasing levels of recombinant LRRK2 protein were assessed to determine the linearity of the assays, demonstrating a linear range of 0.16–600 ng/mL for pS935 LRRK2 and 0.06–96 ng/mL for total LRRK2 measurement and establishing the lower limits of detection (LLOD) for each assay (Fig. 1A,B; Supplementary Table 1). To assess the specificity of the MSD-based assays in cell lysates, we used these assays to confirm LRRK2 kinase inhibition and deletion using wildtype and LRRK2 KO A549 cells, a cell line chosen based on its high expression of LRRK2. Consistent with effects observed with western blot analysis, LRRK2 was not detected in LRRK2 KO cells and pS935 LRRK2 levels were reduced in wildtype cells following treatment with a selective LRRK2 kinase inhibitor (MLi-2) for 2 h (Fig. 1C–E, Supplementary Fig. S1). These results establish the linearity and specificity of the LRRK2 and pS935 LRRK2 MSD-based assays.
Development of specific, quantitative and high-throughput assays to measure pS935 LRRK2 and total LRRK2. (A,B) Novel MSD assays to measure pS935 LRRK2 and total LRRK2 levels can detect increasing amounts of recombinant LRRK2 protein; n = 3. (C) Specific detection of pS935 LRRK2 and total LRRK2 was confirmed in WT and LRRK2 KO A549 cells with and without LRRK2 kinase inhibitor treatment (MLi-2, 500 nM, 2 h) measured by western blot analysis. (D,E) Consistent with western blot data, the LRRK2 MSD assays specifically detected pS935 LRRK2 and total LRRK2 in A549 cells; n = 3. MSD signals were normalized for protein concentration, and data are shown as mean ± SEM with p values: one-way ANOVA with Tukey’s multiple comparison test; ***p ≤ 0.001, ****p ≤ 0.0001; AU, arbitrary units.
Generation of a phospho-specific Rab10 antibody and development of assays to measure total and pT73 Rab10
LRRK2-dependent Rab phosphorylation has been shown to be elevated by variants in LRRK2 that increase its kinase activity and reduced following LRRK2 kinase inhibition, supporting its potential utility as a readout of LRRK2 activity11,17. Of the multiple Rabs known to undergo LRRK2-dependent phosphorylation, Rab10 has emerged as an attractive LRRK2-dependent candidate biomarker given its high expression in many cell types, including peripheral immune cells18,19. We generated a monoclonal phospho-specific antibody against pT73 Rab10, the LRRK2 phosphorylation site in Rab10, and confirmed that our antibody selectively detected phosphorylation of Rab10 and failed to detect phosphorylation of other LRRK2 Rab substrates (Fig. 2A, Supplementary Fig. S2A–C). Using our pT73 Rab10 antibody and commercial antibodies against Rab10, we developed MSD-based assays to enable quantitative and high-throughput detection of LRRK2-dependent Rab phosphorylation as well as total levels of Rab10. The linearity and LLOD for both assays were established using recombinant human Rab10 protein that is phosphorylated at T73, with linear ranges of 65.2–8350 ng/mL and LLOD of 8.36 ng/mL for pT73 Rab10 assay and linear ranges of 0.6–45.7 ng/mL and LLOD of 0.02 ng/mL for Rab10 assay (Fig. 2B,C; Supplementary Table 2). We confirmed that these assays selectively detect LRRK2-dependent phosphorylation of Rab10 in A549 cells and matched with data obtained via western blot analysis, detecting robust reduction of pT73 Rab10 levels in wildtype cells following LRRK2 kinase inhibition and a lack of signal in RAB10 and LRRK2 KO cells (Fig. 2D–F; Supplementary Figs. S2D,E and S3A,B). pT73 Rab10 and total Rab10 signals were maintained in cells lacking a related LRRK2-Rab substrate (RAB8A KO), confirming the specificity of the assay for detecting endogenous Rab10 and Rab10 phosphorylation (Fig. 2D–F and Supplementary Fig. S2D,E).
Antibody generation against pT73 Rab10 and development of MSD assays to measure pT73 Rab10 and total Rab10 in cells and in vivo. To measure LRRK2 kinase activity, we generated a phospho-specific antibody against the LRRK2 phosphorylation site in Rab10 (pT73 Rab10). (A) The monoclonal pT73 Rab10 antibody DNLI 19-4 is highly selective for phosphorylated Rab10 as shown by assessing detected signal in HEK293 cells overexpressing different mCherry-Rab proteins and FLAG-LRRK2 G2019S. (B) The pT73 Rab10 MSD-based assay specifically detects recombinant phosphorylated Rab10 protein but not non-phosphorylated Rab10; n = 3. (C) The total Rab10 MSD-based assay specifically measures recombinant Rab10 protein but not Rab8a protein; n = 3. (D) Specific detection of LRRK2 kinase dependent phosphorylation of Rab10 was shown using wildtype, RAB8A KO, RAB10 KO and LRRK2 KO A549 cells with and without LRRK2 kinase inhibitor treatment (500 nM MLi-2 for 2 h) using western blot analysis. (E,F) The pT73 Rab10 and total Rab10 MSD assays specifically measure LRRK2-dependent phosphorylation of Rab10 and total Rab10, respectively, in A549 cells; n = 3. Data are shown as mean ± SEM with p values: one-way ANOVA with Sidak’s multiple comparison test. (G) Loss of pS935 LRRK2 and total LRRK2 signals was confirmed in both striatum and lung from LRRK2 KO rats; n = 11 animals for each group. (H) LRRK2 KO rats had a 30% reduction of pT73 Rab10 levels in striatum and complete loss of pT73 Rab10 signal in lung; n = 11 animals for each group. Given that different lysis buffers were used for the lysis of striatum and lung, direct comparison of MSD values between tissues should be interpreted with caution. Data are reported as MSD signal (for recombinant protein) or MSD signal normalized for protein concentration for studies performed using cell or tissue lysates and are shown as mean ± SEM with p values: two-way ANOVA with Sidak’s multiple comparison test; **p ≤ 0.01 ***p ≤ 0.001, ****p ≤ 0.0001.
We next wanted to assess the extent of LRRK2-dependent phosphorylation of Rab10 in the brain and periphery in vivo. The levels of pT73 and total Rab10 were measured using our MSD-based assays in the striatum and lung of wildtype and LRRK2 KO rats, tissues selected based on their abundant LRRK2 expression20,21. Rab10 phosphorylation was abolished in the lung of LRRK2 KO rats compared to wildtype animals, demonstrating that phosphorylation at T73 is mediated by LRRK2 in lung (Fig. 2G,H). pT73 Rab10 levels were reduced by approximately 30% in brain with LRRK2 deletion, suggesting that other kinase(s) may phosphorylate Rab10 in brain in addition to LRRK2 (Fig. 2H). Together, these data demonstrate that our MSD-based assays support the selective detection of LRRK2-dependent phosphorylation of Rab10 in cells and in vivo.
High LRRK2 expression and activity are observed in mouse glia cells and human iPSC-derived microglia cells
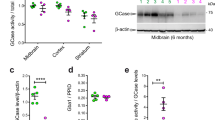
While LRRK2 has been previously shown to be highly expressed in immune cells, it remains unclear which specific cell type(s) within the brain show highest levels of LRRK2 activity. To assess this, we measured the levels of LRRK2 and pT73 Rab10 in primary cortical neurons, astrocytes, and microglia cultured from wildtype mice. Consistent with previous data, LRRK2 levels were highest in mouse astrocytes and microglia and lower levels were observed in primary cortical neurons (Supplementary Fig. S4A,C,D)22. Glial cells also showed the highest LRRK2 activity, with mouse astrocytes showing the greatest levels of pT73 Rab10 (Fig. 3A). Treatment with a selective LRRK2 kinase inhibitor, MLi-2, significantly reduced levels of pT73 Rab10 across these CNS cell types, suggesting that phosphorylation at T73 of Rab10 in these cells is largely LRRK2 dependent. We next assessed the extent to which endogenous expression of the LRRK2 G2019S variant modulated Rab10 phosphorylation in CNS cells, focusing on cultured astrocytes from LRRK2 G2019S knock-in (KI) mice given their abundant levels of LRRK2 and pT73 Rab10. pT73 Rab10 levels were increased by nearly twofold in primary astrocytes from LRRK2 G2019S KI mice compared to wildtype controls, demonstrating that endogenous expression of the LRRK2 G2019S variant increases Rab phosphorylation in this cell type (Fig. 3B and Supplementary Table 3). While total LRRK2 levels were comparable between wildtype and LRRK2 G2019S cells, we observed a significant reduction in pS935 LRRK2 levels.
LRRK2 levels and activity are abundant in mouse glia cells and human iPSC-derived microglia cells and are regulated by lysosomal stress and inflammatory stimuli. (A) LRRK2-dependent phosphorylation of Rab10 occurs in primary cultured cortical neurons, astrocytes, and microglia and is highest in astrocytes. Inhibition of LRRK2 kinase activity by MLi-2 (500 nM, 2 h) significantly reduced phosphorylation of Rab10 across primary cultured cortical brain cells as assessed by western blot analysis; n = 3. Data are shown as mean ± SEM with p values: two-way ANOVA with Sidak’s multiple comparison test. (B) pT73 Rab10 levels were increased and pS935 LRRK2 levels were decreased in primary mouse astrocytes from G2019S KI mice; n = 3. Data were normalized for protein concentration and normalized to the median within each batch and then to the control group, shown as mean ± SEM with p values based on paired t-test. (C) Human iPSC-derived microglia expressed high levels of LRRK2 and pT73 Rab10, and LRRK2 inhibition (MLi-2, 500 nM, 2 h) significantly reduced pT73 Rab10 levels; n = 4. Data are shown as MSD signals normalized for protein concentration and displayed as mean ± SEM with p values: paired t-test. (D,E) iMicroglia treated with pharmacological agents that induce lysosomal stress (50 µM chloroquine (CQ) for 24 h and 100 nM Bafilomycin A1 (Baf) for 24 h) resulted in increased pT73 Rab10 levels. LRRK2 levels were largely not affected, with only a mild increase observed with Bafilomycin A1 treatment; n = 5–8. (F,G) IFN-\(\gamma\) treatment (20 ng/mL, 24 h) induced a significant increase in both LRRK2 and pT73 Rab10 levels in iMicroglia cells; n = 5–8. (D–G) MSD signals were normalized for protein concentration, and data were then normalized to the median within each batch and to the control group, shown as mean ± SEM with p values based on one-way ANOVA with Sidak’ multiple comparison test on log transformed data. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Transcriptome analysis of CNS cells from human cortex samples showed enrichment of LRRK2 transcript in microglia, highlighting the potential of microglia as a relevant human CNS cell model to study mechanisms regulating LRRK2 expression and activity23. We generated human iPSC-derived microglia (iMicroglia) using an established differentiation protocol and assessed the levels of LRRK2 and pT73 Rab10 basally and following LRRK2 inhibition24. Strong MSD signal was observed for LRRK2, pT73 Rab10 and total Rab10 in human iMicroglia (Fig. 3C, Supplementary Fig. S4B). Rab10 phosphorylation was largely LRRK2-dependent in these cells, as pT73 Rab10 levels were significantly reduced after a 2 h treatment with MLi-2. Interestingly, LRRK2 levels were also reduced at this time point, suggesting that degradation of LRRK2 might occur rapidly in human microglia upon LRRK2 inhibition. Taken together, these data show that LRRK2 is highly active in glial cells and suggest that Rab10 phosphorylation can serve as a useful readout of LRRK2 activity in these cell types.
While the expression and activity of LRRK2 are highly responsive to perturbations in cellular homeostasis in some cell types, it remains unclear whether such regulation occurs in the CNS. Previous studies have shown that pharmacological manipulations that induce lysosomal damage trigger LRRK2 translocation to lysosomes and lead to an increase in Rab phosphorylation25,26. Further, LRRK2 expression in immune cells is elevated in response to various inflammatory stimuli such as IFN-\(\gamma\)27,28,29. We next assessed the extent to which lysosomotropic agents and inflammatory stimulation modulate LRRK2 levels and activity in human iMicroglia. iMicroglia were treated with Bafilomycin A1(BafA1) or chloroquine (CQ), pharmacological agents that disrupt the lysosomal pH gradient and perturb lysosomal function, and the effects on LRRK2 and pT73 Rab10 were assessed. CQ and BafA1 increased pT73 Rab10 levels by three- and eightfold, respectively, as early as 6 h post-treatment, and the effects on LRRK2 activity were sustained up to 24 h after treatment (Fig. 3D,E and Supplementary Fig. S5A,B; Supplementary Table 3). Both lysosomotropic compounds increased Rab10 phosphorylation in a LRRK2-dependent fashion, as treatment with a LRRK2 kinase inhibitor significantly reduced pT73 Rab10 levels in iMicroglia (Fig. 3D and Supplementary Fig. S5A). LRRK2 levels were minimally impacted following treatment with these lysosomotropic agents, with a slight, but significant, increase in LRRK2 levels observed with BafA1 treatment (Fig. 3E and Supplementary Fig. S5B). These data add to growing evidence that LRRK2 activity is significantly elevated in response to lysosomal stress and demonstrate that LRRK2-dependent responses to damage occur in CNS cells.
To understand whether LRRK2 levels or activity were altered in these cells in response to inflammatory stimuli, iMicroglia were stimulated with IFN-\(\gamma\). Consistent with published data in peripheral immune cells27,28,29, LRRK2 expression was dramatically elevated by approximately sevenfold in iMicroglia after 24 h stimulation with IFN-\(\gamma\) (Fig. 3F and Supplementary Table 3). Elevation of LRRK2 translated to an approximately 13-fold increase in pT73 Rab10 levels, suggesting that IFN-\(\gamma\) treatment might affect both LRRK2 levels and activity (Fig. 3G). In support of a direct effect on LRRK2 activity, Rab10 phosphorylation was significantly, albeit more modestly, increased as early as 6 h after IFN-\(\gamma\) stimulation with no significant effect on LRRK2 levels themselves (Supplementary Fig. S5C,D). The observed increase in pT73 Rab10 levels was dependent on LRRK2 kinase activity, as LRRK2 kinase inhibition prevented the elevation in Rab10 phosphorylation in response to IFN-\(\gamma\) treatment. These data demonstrate that LRRK2 levels and activity can both be elevated in response to inflammatory stimuli in human microglia and suggest that perturbed immune responses observed in PD patient brain might upregulate the LRRK2 signaling pathway.
Analysis of LRRK2 and pT73 Rab10 as human blood-based biomarkers
We next applied these assays to enable the analysis of the levels of total and pS935 LRRK2 and total and pT73 Rab10 as peripheral biomarkers for use in human samples. Increasing amounts of PBMC lysates were tested to assess the linearity of the LRRK2 and Rab10 assays in this matrix. The linear ranges of detection for pS935 and total LRRK2 were from 12 to 3070 µg/mL of PBMC lysate (3070 µg/mL was the highest concentration tested; Fig. 4A and Supplementary Table 4). Measurements of total and pT73 Rab10 were slightly less sensitive, with a linear range of 191.9–3070 µg/mL of PBMC lysates for pT73 Rab10 and 3–12 µg/mL for total Rab10 (Fig. 4B and Supplementary Table 5). To confirm these assays could be employed to monitor LRRK2 inhibition, we treated PBMCs from three healthy donors with increasing concentrations of MLi-2 ex vivo and measured the effects of treatment on pS935 LRRK2 and pT73 Rab10 levels (Fig. 4C). LRRK2 inhibition reduced the levels of pS935 LRRK2 and pT73 Rab10 in a comparable dose-dependent manner, with IC50s of 3.8 nM for pS935 LRRK2 and 5.3 nM for pT73 Rab10.
Development of LRRK2 and pT73 Rab10 assays for human blood-based biomarker analysis. (A) pS935 LRRK2 and total LRRK2 levels were measured with serial dilutions of lysates from human PBMCs. Each run denotes an independent experiment with a pooled sample from 4 to 5 donors. (B) pT73 Rab10 and total Rab10 levels were measured with serial dilutions of lysates from human PBMCs. (C) Inhibition of LRRK2 kinase activity decreased pS935 LRRK2 and pT73 Rab10 levels in a dose-dependent manner in PBMCs with ex vivo treatment of MLi-2 (1 h). PBMCs were from n = 3 donors. The MSD signals were normalized to the response to the lowest concentration of MLi2 (0.003 nM) as 100% within each donor. (D) pS935 and total LRRK2 levels measured with serial dilutions of samples of human whole blood. Each run denotes an independent experiment with a pooled sample from 4 to 5 donors. (E,F) Whole blood specimens from 12 Parkinson’s Disease patients (3 Female, 9 Male) and 6 Non-Parkinson’s controls (2 Female, 4 Male) from the 24-h Biofluid Sampling Study (provided by the Michael J. Fox Foundation) were analyzed. Biospecimens were donated by each subject at 11 time points over 26 h. (E) Total LRRK2 levels in whole blood from healthy subjects and PD patients were stable within subjects over the course of 24 h (average coefficient of variation (CV) = 7%; average maximum to minimum value within-subject fold change of 1.25), while displaying high variability between subjects (66% CV, 11.6-fold difference in maximum to minimum value). (F) pS935 LRRK2 levels measured in human whole blood samples varied to a greater extent than total LRRK2 within subjects (average CV = 17%, average within-subject maximum to minimum value fold change of 1.76), with less variability between subjects than that observed for total LRRK2 (36% CV, 3.8-fold difference in maximum to minimum value).
Given the sensitivity of the pS935 and LRRK2 MSD-based assays, we evaluated whether these assays could be used to directly measure LRRK2 levels and inhibition from whole blood to enable simpler analysis in a clinical setting. Blood samples from healthy donors were serially diluted, and the levels of pS935 and total LRRK2 were assessed (Fig. 4D). Both measures showed a linear increase from the range of 2- to 512-fold dilution (Supplementary Table 6). We confirmed the specificity of detection of these assays by measuring the levels of pS935 and total LRRK2 in PBMCs and whole blood collected from wildtype or LRRK2 KO rats following dosing with a tool LRRK2 kinase inhibitor developed by Denali, DN1388, for 10 days. The pS935 LRRK2 signal was completely abolished in both PBMCs and whole blood from LRRK2 KO rats or those dosed with 100 mg/kg DN1388 (Supplementary Fig. S6). LRRK2 levels were not detected in PBMCs or whole blood from LRRK2 KO rats. These data demonstrate the specificity of these measurements and validate the use of the whole blood assay to measure pS935 and total LRRK2.
To further assess the potential utility of LRRK2 and pS935 LRRK2 as biomarkers in future clinical studies, we characterized the intra- and inter-subject variability of these analytes in blood. Whole blood samples from healthy controls and PD subjects were obtained from the 24-h Biofluid Sampling Study sponsored by the Michael J. Fox Foundation in which samples were collected at 11 time points over a 24-h period, and the levels of pS935 and total LRRK2 were measured. LRRK2 levels were stable within subjects over the course of 24 h (average coefficient of variation (CV) = 7%), while displaying high variability between subjects (66% CV) (Fig. 4E). pS935 LRRK2 levels varied to a greater extent than LRRK2 within subjects (average CV = 17%) but the variability was less than that of total LRRK2 between subjects (36% CV) (Fig. 4F). The relatively low intra-subject variability and detectability of the analytes in human whole blood support the potential of these assays to assess LRRK2 levels and inhibition in blood in a clinical setting.
LRRK2 and pT73 Rab10 levels in LRRK2 G2019S carriers compared to healthy controls
We next wanted to better define the extent to which variants in LRRK2 associated with increased risk for developing disease affect the levels of LRRK2 and its kinase activity. Variants in LRRK2 within the ROC, COR, kinase, and WD40 domains have been linked to increased risk for PD or Crohn’s disease and have been reported to lead to increased Rab10 phosphorylation1,30. Disease-linked LRRK2 variants were expressed in HEK293T cells, and the levels of pT73 Rab10 were measured and normalized to total levels of LRRK2 to control for any differences in LRRK2 expression. Consistent with previous findings, all variants assessed led to an increase in pT73 Rab10 levels when normalized to LRRK2, with the LRRK2 G2019S variant showing an approximately threefold increase in pT73 Rab10 levels (Supplementary Fig. S7A and Supplementary Table 3).
We exploited the high-throughput and quantitative nature of our assays to determine whether the levels or kinase activity of LRRK2 were altered in PD patients that were heterozygous for the LRRK2 G2019S variant or in those with sporadic disease. We measured LRRK2, pS935 LRRK2, and pT73 Rab10 levels in a large collection of PBMC samples obtained from healthy subjects and PD patients with and without the LRRK2 G2019S variant. pS935 LRRK2 and total LRRK2 levels were reduced in subjects with PD carrying the G2019S variant compared to sporadic PD patients and healthy LRRK2 G2019S carriers (Fig. 5A,B and Supplementary Figure S8). This same sample set has been recently analyzed for total and pS935 LRRK2 levels via an orthogonal and analytically validated assay, with substantially similar results, as well as via a sandwich ELISA-based assay with a noted non-significant trend toward reduced pS935 LRRK2 normalized to total LRRK2 levels, providing independent validation of these results31,32. pT73 Rab10 levels were not significantly different in any group, but when normalized for variations in total LRRK2 levels, we observed a significant elevation in LRRK2 G2019S carriers with PD compared to sporadic PD patients and in healthy LRRK2 G2019S carriers compared to non-carriers (Fig. 5C,D; Supplementary Table 3). Further, there was a non-significant trend toward increased pT73 Rab10 normalized to total LRRK2 in sporadic PD patients compared to healthy subjects (Fig. 5D). These data support that LRRK2 G2019S carriers with PD have elevated LRRK2 kinase activity that can be measured in a clinical setting.
LRRK2 and pT73 Rab10 levels assessed in PD patients and healthy subjects. LRRK2 levels and activities were measured in PBMCs from PD patients and healthy subjects with and without LRRK2 G2019S variant provided by the LRRK2 Detection in PBMC Consortium32; n = 16 non-PD-LRRK2 G2019S carriers, n = 22 non-PD non-G2019S carriers, n = 46 PD non-G2019S carriers, n = 33 PD and G2019S carriers. pS935 and total LRRK2 levels were shown as ng/ml and were calculated based on a standard curve of recombinant LRRK2 protein that was analyzed along with the PBMC lysates as in-plate reference material. For each variable, an ANCOVA model was fit, with log2 (value) as response and terms for Cohort, G2019S status (and their interaction), Gender and Age. For each variable, a forward model selection step was performed to assess the usefulness of adjusting for Age or including an interaction term; p values were based on pairwise comparison between groups. (A) For pS935 LRRK2, p = 0.0002 between G2019S carriers and non-carriers in PD (Relative Geometric mean ± 95% confidence interval = 0.651 (0.524–0.810)); p = 0.0134 between PD and non-PD in G2019S carriers (relative geometric mean ± 95% confidence interval = 0.673 (0.492–0.920)); p = 0.0233 between G2019S carriers and non-carriers (Relative Geometric mean ± 95% confidence interval = 0.805 (0.660–0.970)). (B) For LRRK2, p = 0.0028 between G2019S carriers and non-carriers in PD (relative geometric mean ± 95% confidence interval = 0.688 (0.540–0.877)); p = 0.0396 between PD and non-PD in G2019S carriers (relative geometric mean ± 95% confidence interval = 0.693 (0.489–0.982)). (C,D) For pT73 Rab10/LRRK2, p = 0.0171 between G2019S carriers and non-carriers in PD and in non-PD (relative geometric mean ± 95% confidence interval = 1.44 (1.07–1.95)). Relative geometric means given are adjusted for age and sex. Data are shown as geometric mean ± 95% confidence interval (CI). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
LRRK2 N551K R1398H protective haplotype is associated with reduced pT73 Rab10 levels
We assessed whether variants in LRRK2 associated with reduced risk for developing disease affect the levels of LRRK2 and its kinase activity. The N551K R1398H haplotype is associated with protection from developing PD and Crohn’s disease30,33,34,35. Previous in vitro studies have suggested that the R1398H variant enhances the GTPase activity of LRRK236. However, it remains unclear how this variant affects LRRK2 activity in cells. We expressed various LRRK2 variants that possessed the N551K R1398H variant on its own or in the presence of additional variants that are associated with increased risk for PD or Crohn’s disease (Fig. 6A). Expression of the LRRK2 N551K R1398H variant on its own or together with disease-associated LRRK2 variants within its kinase domain or WD40 domain led to reduced total levels of LRRK2 (Fig. 6B). The decrease in LRRK2 levels observed with the LRRK2 N551K R1398H variant singly or together with several disease-linked variants correlated with a reduction in levels of pT73 Rab10 (Fig. 6C; Supplementary Figure S7B–D and Supplementary Table 3). The protective LRRK2 variant in combination with variants in the ROC or COR domains did not impact LRRK2 levels or activity. To confirm that the LRRK2 protective haplotype modulates LRRK2 levels and activity under endogenous expression conditions, we generated LRRK2 N551K R1398H KI A549 cells and observed a reduction in LRRK2 levels and concomitant decrease in pT73 Rab10 levels in LRRK2 N551K R1398H KI cells compared to wildtype cells (Fig. 6D,E; Supplementary Figure S7E–G and Supplementary Table 3). These data suggest that the N551K R1398H variant ultimately leads to reduced LRRK2 activity and may mediate its effects on LRRK2 stability through interactions within the GTPase domains.
The LRRK2 protective variant reduces LRRK2 levels and activity in cellular models. (A) Schematic overview of LRRK2 risk and protective variants associated with PD and Crohn’s disease (CD). N551K R1398H: protective variant associated with both PD and CD (in blue). N2081D: a risk variant associated with CD (in orange). (B,C) In HEK293T cells overexpressing different LRRK2 variants and Rab10, total LRRK2 and pT73 Rab10 levels were reduced with the LRRK2 N551K R1398H variant on its own or with PD-linked LRRK2 variants in the kinase and WD40 domains; n = 5–6. Data were normalized for protein concentration and then normalized to the median within the batch and to the wildtype group; shown as mean ± SEM with p values based on paired t-tests on log transformed data, without adjustment for multiple comparisons. (D,E) LRRK2 and pT73 Rab10 levels were reduced in LRRK2 N551K R1398H KI A549 cells. Three wildtype pooled cells and three clones of LRRK2 N551K R1398H KI A549 cells were used; n = 4 experiments. Data are displayed as MSD signals normalized for protein concentration and then normalized to the median within the batch and to the wildtype group and are shown as mean ± SEM with p values: one-way ANOVA with Sidak’s multiple comparison test. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
To expand upon our observations in cellular models and capitalize on our high-throughput assays, we assessed whether the LRRK2 protective haplotype modulates LRRK2 levels or activity in PBMCs obtained from healthy subjects and PD patients with and without the LRRK2 N551K R1398H variant. Levels of total and pS935 LRRK2 were unchanged in LRRK2 N551K R1398H carriers compared to non-carriers regardless of disease status (Fig. 7A,B). These data differ from effects observed on total LRRK2 in LRRK2 N551K R1398H cell models, suggesting the protective haplotype may affect LRRK2 levels in a cell-type dependent manner. Importantly, Rab10 phosphorylation was significantly reduced in LRRK2 N551K R1398H carriers compared to non-carriers, an effect that was maintained when normalized for total LRRK2 levels and in sensitivity analyses that additionally adjusted for disease status and/or LRRK2 G2019S status (Fig. 7C,D; Supplementary Tables 3 and 7). These data provide the first evidence, to our knowledge, that the LRRK2 protective haplotype is associated with reduced LRRK2 activity in human subjects.
LRRK2 and pT73 Rab10 levels in N551K R1398H haplotype carriers and non-carriers. LRRK2, pS935 LRRK2 (A,B) and pT73 Rab10 levels (C,D) were measured in PBMCs isolated from PD and healthy subjects, and the N551K R1398H haplotype status was derived from genotype array data (n = 62 healthy control N551K R1398H non-carriers, n = 64 for PD N551K R1398H non-carriers, n = 11 for healthy control N551K R1398H carriers, and n = 3 for PD N551K R1398H carriers). Raw protein levels were natural log transformed and fit in a linear model against age, sex, and the first two principle components derived from measured genotype data to account for genetic ancestry. Residuals were then inverse normal transformed and tested for association with the N551K R1398H haplotype. Statistical analysis was performed using R version 3.6.1. (A,B) Levels of total and pS935 LRRK2 were comparable in N551K R1398H carriers and non-carriers; for total LRRK2, p = 0.485 and for pS935 LRRK2, p = 0.7129. (C,D) pT73 Rab10 (0.66 standard deviation decrease in carriers vs non-carriers) and the pT73 Rab10/LRRK2 ratio (0.66 standard deviation decrease in carriers vs non-carriers) were reduced in N551K R1398H carriers compared to non-carriers; for pT73 Rab10, p = 0.0182 and for pT73 Rab10/LRRK2, p = 0.0172. pT73 Rab10 and pT73 Rab10/LRRK2 were also significantly reduced in N551K R1398H carriers relative to non-carriers in sensitivity analyses that additionally adjusted for disease status and/or LRRK2 G2019S status; all p-values were < 0.05 with exception of pT73 Rab10/LRRK2 phenotype in "Model 4" (see “Materials and methods”), which had p = 0.053. Data shown as mean ± SEM. *p ≤ 0.05.
Discussion
In the current study, we describe the development of high-throughput and sensitive assays to quantify the levels and phosphorylation of LRRK2 and Rab10 and apply these assays to better understand how LRRK2 is regulated in cellular models and in human subjects that carry LRRK2 variants. Using these assays, we observed an approximately one and a half to twofold increase in Rab10 phosphorylation with the LRRK2 G2019S variant under endogenous expression conditions in mouse astrocytes and in PBMCs from LRRK2 G2019S variant carriers after normalization for variations in levels of LRRK2. Beyond effects observed with variants in LRRK2 itself, we found Rab10 phosphorylation was significantly increased in response to pharmacological agents that perturb PD-relevant pathways in iPSC-derived microglia and demonstrated that this was dependent on the kinase activity of LRRK2. Finally, we showed that missense variants in LRRK2 associated with reduced risk for PD and Crohn’s disease lead to lower levels of Rab10 phosphorylation, suggesting that these variants may lower disease risk by reducing the activity of the LRRK2 signaling pathway. Given their successful application in human PBMCs and whole blood, these assays have the potential to enable quantitative analysis of LRRK2 levels and activity following dosing with LRRK2 inhibitors in the clinic.
An understanding of the function of LRRK2 in different cell types throughout the brain has been hindered by a lack of quantitative tools to measure LRRK2 levels and activity across CNS cell types. Previous studies have reported varying levels of LRRK2 expression in neurons and glial cells, including transcriptome analysis of neurons, glia, and vascular cells from the cortex of mouse and human samples which showed enrichment of LRRK2 transcript in astrocytes and microglia, respectively22,23,37,38,39,40. Further, recent work to identify specific variants that are likely causal for PD suggests that non-coding variants impact LRRK2 expression by modulating microglia-specific regulatory elements, highlighting the potential relevance of this cell type for interrogating LRRK2 biology41,42. We analyzed LRRK2 levels and activity across primary mouse cortical neurons, astrocytes, and microglia and confirmed high expression of LRRK2 in glial cells with weaker expression observed in neurons. Additionally, we showed that LRRK2 inhibition results in a near complete reduction in pT73 Rab10 levels across all three cell types, demonstrating that LRRK2-dependent Rab10 phosphorylation is relevant in brain. Our analysis of LRRK2 levels and activity in iPSC-derived microglia yielded similar findings and suggested that LRRK2-dependent phosphorylation of Rab10 also occurs in human microglia. Rab10 phosphorylation was not completely abolished in brain lysates from LRRK2 KO mice, suggesting the LRRK2-dependence of Rab10 phosphorylation may be cell type or brain region specific or depend on the activation state of glial cells within the brain. Emerging data suggest that LRRK2 expression or kinase activity are modulated by inflammatory stimuli or lysosomal damage25,26,43,44. We demonstrated that Rab10 phosphorylation was significantly increased in iPSC-derived microglia following treatment with pharmacological agents that dissipate the endo-lysosomal pH gradient, providing support that lysosomal dysfunction can impact LRRK2 activity in human microglia and highlighting the potential of this model to better understand the mechanisms governing LRRK2 regulation. We cannot exclude the possibility that in vitro culture conditions may impact LRRK2 activity following lysosomal damage, and additional studies are warranted to understand whether similar regulation occurs in glial cells from PD patient brains with evidence of lysosomal dysfunction. While our studies focused on LRRK2-dependent phosphorylation of Rab10, expanded analysis of the effects of LRRK2 activity on phosphorylation of other LRRK2-linked Rab GTPases in brain would be informative and could identify additional relevant biomarkers of LRRK2 activity in the CNS.
To enable the clinical development of LRRK2 inhibitors and analysis of alterations in LRRK2 pathway activity in PD patient populations, assays are needed that can be used to reliably quantify LRRK2 levels and phosphorylation of the kinase itself and its direct substrates in samples easily obtained from human subjects. We optimized our MSD-based assays and demonstrated linear quantification of the levels of total and pS935 LRRK2 as well as total and pT73 Rab10 in human PBMCs. Ex vivo treatment of PBMCs with the LRRK2 inhibitor MLi-2 resulted in dose-dependent reduction of pS935 LRRK2 and pT73 Rab10 levels with an equivalent cellular potency, confirming the utility of both readouts as measures of LRRK2 inhibition in accessible human samples. We further optimized the pS935 and total LRRK2 assays to allow the quantification of both analytes in whole blood, providing a critical advance to measure the extent of LRRK2 inhibition reliably across multiple locations and with multiple handlers for use in a clinical setting. Although we applied our assays here specifically to assess LRRK2 levels and activity in PBMCs and whole blood, analysis of LRRK2 and Rab10 phosphorylation in peripheral samples can potentially be used to estimate the inhibition of signaling downstream of LRRK2 in the CNS if the distribution of drug (e.g. LRRK2 kinase inhibitor) tested is equivalent between the periphery and CNS. Additional in vivo studies are required to determine how closely peripheral LRRK2 kinase inhibition reflects CNS inhibition and would be aided by the identification of other Rabs in brain whose phosphorylation are highly sensitive to LRRK2 kinase inhibition. Future work will focus on using these assays to demonstrate target engagement and modulation of downstream pathway activity following dosing with LRRK2 inhibitors in human subjects to help assess the potential of LRRK2 inhibitors in the clinic.
While pathogenic variants in LRRK2 lead to increased kinase activity and elevated phosphorylation of its Rab substrates, the extent to which these variants, including the most common variant G2019S, increase LRRK2 kinase activity and the broader relevance of increased LRRK2 activity to sporadic PD remain controversial. Defining the degree to which LRRK2 activity is elevated in PD patients with and without variants in LRRK2 is paramount for the clinical development of LRRK2 inhibitors and can help determine how much LRRK2 inhibition should be targeted as well as better identify which patient populations might benefit most from LRRK2 inhibition. The LRRK2 G2019S variant has been reported to elevate its kinase activity by approximately two- to tenfold depending on the model system used and method employed to quantify autophosphorylation or phosphorylation of its direct substrates8,11,45,46,47. As many previous studies have relied on cellular models overexpressing LRRK2 or peripheral tissues from in vivo models, it remains unclear the degree to which variants in LRRK2 affect its kinase activity in the CNS or the extent to which its activity is increased in PD patients. By assessing the levels of pT73 Rab10, we demonstrate that LRRK2 activity is increased nearly twofold in primary astrocytes derived from mice expressing the LRRK2 G2019S variant at endogenous levels. Further, we showed that PBMCs from heterozygous LRRK2 G2019S variant carriers also had a significant increase in Rab10 phosphorylation of approximately 1.5-fold compared to non-carriers after normalizing for variations in LRRK2 levels. Our results are largely consistent with findings from other studies that show a mild increase in LRRK2 activity in G2019S carriers compared to non-carriers, including recent data showing an approximately 1.9-fold increase in pT73 Rab10 levels in neutrophils obtained from LRRK2 G2019S carriers compared to healthy controls using a mass-spectrometry based approach18,19,48. Together, these data add further evidence that LRRK2 G2019S activity is elevated approximately one-and-a-half- to twofold under endogenous conditions in relevant cell types, including CNS cells, and suggest a small (< twofold) increase in phosphorylated Rab10 is sufficient to confer an elevated lifetime risk of Parkinson’s Disease. These results can serve as a useful guide to inform the level of LRRK2 kinase inhibition required to normalize LRRK2 activity in the clinic. We also observed a non-significant trend of increased Rab10 phosphorylation in PBMCs from sporadic PD patients, and additional analysis of LRRK2 activity is warranted in larger cohorts and across broader populations to better understand the range of increase in kinase activity conferred by other pathogenic LRRK2 variants or in sporadic PD more broadly.
While pathogenic variants in LRRK2 ultimately lead to an increase in its kinase activity, the functional consequences of a protective LRRK2 haplotype remained poorly understood. The N551K-R1398H-K1423K LRRK2 haplotype is associated with reduced risk for developing PD, REM behavior sleep disorder, and Crohn’s disease, with a combined odds ratio for PD of approximately 0.8230,33,34,35,49. The R1398H variant has been hypothesized to increase the amount of LRRK2 in an “off” state by reducing the amount of GTP-bound LRRK2 and increasing GTP hydrolysis, in contrast to effects observed with pathogenic variants in the ROC-COR domain of LRRK230,36. We show that the LRRK2 N551K R1398H variant reduces the levels of pT73 Rab10 on its own and in combination with disease-associated variants in the LRRK2 kinase domain using a combination of cellular models and PBMCs obtained from human carriers with and without the protective haplotype. These data are in contrast to a previous report that showed no effect of this variant on pT73 Rab10 levels and may be explained by the more quantitative and sensitive assays and larger sample size employed in the current study30. The LRRK2 protective haplotype was associated with reduced levels of total LRRK2 in cellular models, suggesting this variant might regulate the stability of LRRK2 in certain cell types. As the ROC-COR domain plays a key role in LRRK2 dimerization and the N551K R1398H variant did not impact LRRK2 levels in the presence of PD-associated variants in the ROC-COR domain, the protective variant may mediate its effects on LRRK2 levels by interfering with intermolecular interactions. LRRK2 variants that attenuate its kinase activity and LRRK2 kinase inhibition have both been shown to lead to reduced levels of LRRK2, and it remains unclear whether the reduction of LRRK2 levels observed here is a consequence of the effects of the protective haplotype on LRRK2 kinase activity50,51. Additional work is needed to better define how the protective LRRK2 haplotype leads to reduced kinase activity and to determine the consequences of these variants on the stability of LRRK2 itself. Together, our data suggest that the protective LRRK2 variant may be associated with lower PD risk by reducing LRRK2 activity and support the potential of therapeutic approaches that recapitulate these effects.
Materials and methods
pT73 Rab10 monoclonal antibody generation
To generate antibodies against phospho-T73-Rab10, peptide C-AGQERFH(pT)ITTSYYR (conjugated with either -KLH or -OVA) was used to inject 4 rabbits (New Zealand White rabbits) by Abcam. Rabbit bleeds/antisera were collected and screened by peptide ELISA assay and western blot. Two rabbits were selected for monoclonal antibody generation. Briefly, lymphocytes from rabbit spleen were fused with 240E-W2 cells to generate hybridomas. The multiclone hybridomas were screened by peptide ELISA, western blot and sandwich ELISA with cell lysate to identify antibodies for potential ELISA-based applications. 9 multiclones were selected and further diluted to generate single clones. 18 monoclonal antibodies were further selected, and 8 of them were sequenced. The monoclonal antibody 19-4 was selected for the pT73 Rab10 MSD assay described here.
MSD assay
Capture antibodies were biotinylated using EZ-Link™ NHS-LC-LC-Biotin (Thermo Fisher, #21343), and detection antibodies were conjugated using Sulfo-TAG NHS-Ester (MSD, R31AA-1). 96-well (or 384-well) MSD GOLD Small Spot Streptavidin plates (MSD L45SSA-1) were coated with 25 µl (or 15 µl for 384 well plates) of capture antibody diluted in Diluent 100 (MSD, R50AA-2) for 1 h at room temperature with 700 rpm shaking (1000 rpm for 384-well). After three washes with TBST, 25 µl samples were added each well (10 µl for 384-well) and incubated at 4 °C overnight with agitation at 700 rpm. After three additional washes with TBST, 25 µl of detection antibodies (15 µl for 384-well) were added to each well diluted in TBST containing 25% MSD blocker A (MSD R93AA-1) together with rabbit (Rockland Antibodies D610-1000) and mouse gamma globin fraction (D609-0100). After 1 h incubation at room temperature at 700 rpm and three washes with TBST, 150 µl MSD read buffer (MSD R92TC, 1:1 diluted with water) was added (35 µl for 384-well), and plates were read on the MSD Sector S 600. For pT73 Rab10 MSD assays, three detection antibodies (listed below) were tested with comparable performance. The detection antibody (Abcam, ab181367) was mainly used for data generation. For the assay performance tests, the results were plotted with MSD arbitrary units (A.U.) after background subtraction. For the MSD analysis with cellular and tissue samples, the MSD signals (A.U.) were normalized to protein concentration of sample lysates measured by BCA assay.
Antibodies used for the MSD assay
Assay | Antibody type | Targets | Vendor | Cat no. | Concentration (µg/mL) |
|---|---|---|---|---|---|
pS935 LRRK2 | Capture | pS935 LRRK2 | Abcam | ab133450 | 0.5 |
Detection | Total LRRK2 | BioLegend | 808201 | 1 | |
Total LRRK2 | Capture | Total LRRK2 | BioLegend | 844401 | 0.5 |
Detection | Total LRRK2 | BioLegend | 808201 | 1 | |
pT73 Rab10 | Capture | pT73 Rab10 | Denali | 19-4 | 1 |
Detection | Total Rab10 | Abcam | ab181367 | 2 | |
Detection | Total Rab10 | Abcam | ab104859 | 2 | |
Detection | Total Rab10 | Creative diagnostics | DCABH-13141 | 2 | |
Total Rab10 | Capture | Total Rab10 | Creative diagnostics | DCABH-13141 | 1 |
Detection | Total Rab10 | Abcam | ab181367 | 4 |
MSD assay performance test with recombinant proteins, human PBMC and whole blood
The assay parameters (linear range and CV) were analyzed based on 384-well plates. Recombinant LRRK2 protein was purchased from ThermoFisher (#A15197). For pT73 Rab10 assay, recombinant Rab10 protein (DU62271) and in vitro phosphorylated pT73 Rab10 protein (estimated phosphorylation of 93% based on Phos-tag gel analysis) were from the University of Dundee (https://mrcppureagents.dundee.ac.uk/). For the total Rab10 assay, recombinant Rab10 protein (LSBio, LS-G2178) and Rab8 protein (LS-G29310) were used. Human cryopreserved PBMCs (AllCells, PB005F) were thawed and cultured in RPMI 1640 media supplemented with 10% FBS and penicillin/streptomycin. Resuspended cells were filtered through 100 µm cell strainer, cultured in 96-well round bottom plates, and treated with MLi-2 (MedChemExpress LLC) for 1 h. Frozen human whole blood samples were from the Denali Employee Blood donation program, thawed, and directly lysed in 96-well plates (100 µl whole blood sample with 100 µl lysis buffer). Samples were spun down (2500×g for 20 min at 4 °C) before MSD analysis.
Cell culture, transfection and treatment
RAB8A KO and RAB10 KO A549 cells were generously provided by Dr. Dario Alessi11,52. HEK293 cells were transiently transfected with FLAG-tagged LRRK2 plasmids or HA-tagged Rab10 plasmids or mCherry-tagged Rab plasmids using Lipofectamine 3000 and lysed 48 h after transfection. Cells were lysed in lysis buffer (Cell Signaling, #9803) supplemented with cOmplete tablet (Roche #04693159001), phosSTOP (Roche #04906837001) and Benzonase nuclease (Sigma, E1014).
Western blot
The primary antibodies used were anti-pS935-LRRK2 (1:500, Abcam, ab133450), anti-LRRK2 (1:500, N241A/34, UC Davis/NIH NeuroMab Facility), anti-vinculin (1:500, Abcam, ab129002), anti-pT73 Rab10 (1:1000, Denali #19-4), anti-Rab10 (1:1000, Abcam, ab104859), anti-mCherry (1:1000, ab213511) and anti-actin (1:5000, Sigma, A2228). Additional details can be found in the Supplementary Methods.
Animal study approval
All procedures in animals were conducted in accordance with the ARRIVE guidelines 2.0 and performed in adherence to ethical regulations and protocols approved by the Institutional Animal Care and Use Committee at Denali Therapeutics. Animals were housed under a 12-h light/dark cycle and provided access to rodent chow and water ad libitum.
Mouse strains and study design
LRRK2 G2019S KI mice (C57BL/6-Lrrk2tm4.1Arte) were maintained on a C57BL/6NTac genetic background at Taconic Biosciences.
Male LRRK2 KO and age-matched wild-type control rats (Long Evan, HsdSage:LE-Lrrktm1Sage) were obtained from Envigo. LRRK2 KO (n = 11) and WT (n = 11) rats were administered daily doses of 100 mg/kg of LRRK2 kinase inhibitor (DN1388) via oral gavage for 10 consecutive days and sacrificed 6 h following the last dose. Vehicle treated LRRK2 KO (n = 11) and WT (n = 11) rats served as the respective comparator groups. Sample sizes were determined based on prior studies and past experiences with the rat model and end points used.
Animal tissue processing
For sample collection at necropsy, animals were deeply anesthetized via intraperitoneal injection of 2.5% Avertin. Blood was collected via cardiac puncture, transferred into EDTA tubes, immediately frozen on dry ice, and stored at − 80 °C. For PMBC collection, whole blood was transferred to Heparin tubes and kept on a rocker at room temperature until further processed to PBMCs. Animals were transcardially perfused with ice-cold PBS and tissues were extracted, weighed and collected in 1.5 mL Eppendorf tubes, and immediately frozen on dry ice and stored at − 80 °C. Tissues were homogenized in lysis buffer (10× of tissue weigh (mg) in µl) using the Qiagen TissueLyzer II for 2 × 3 min at 30 Hz. Lung samples were lysed in CST (#9803) lysis buffer, and brain samples were lysed in RIPA buffer, and neat lysates were used for pT73 Rab10 MSD assay while tenfold diluted lysates were used for LRRK2 and pS935 LRRK2 MSD assays. Homogenates were spun at 14,000 rpm for 30 min at 4 °C, and the supernatants were used for subsequent analysis.
iPSC-derived microglia cells
Human iPSCs (Thermo Fisher #A18945) were maintained in mTeSR1 medium (StemCell, #85857) and routinely passaged as clumps onto Geltrex (Thermo Fisher, #A1413301)-coated plates. The differentiation protocol used was established previously24. Briefly, iPSCs were first differentiated into hematopoietic progenitor cells (HPCs) using the STEMdiff Hematopoietic Kit (StemCell, #05310). HPCs that were positive for identity markers CD34, CD43, and CD45 were transferred to plates containing primary human astrocytes and co-cultured in Media C adapted from Pandya et al.53 for 14–21 days, during which time HPCs were progressively removed. The media included IMDM (Thermo Fisher), FBS, PenStrep, 20 ng/mL each of IL3, GM-CSF, M-CSF (Peprotech) . Once non-adherent cells in co-culture were predominantly mature microglia (> 80%), the microglia were collected and plated for subsequent analysis.
Generation of LRRK2 KO and N551K R1398H KI A549 cells with CRISPR
A549 cells were electroporated by AMAXA with guide-RNA Cas9-NLS ribonuclear protein (RNP) complexes. The exon 1 of the LRRK2 gene was targeted to generate LRRK2 KO cells, and the guides 5ʹ-tggctagtggcagctgtcagggg and 5ʹ-agaaacgctggtccaaatcctgg were used.
To introduce p. N551K (AAC > AAG) SNP modification, the guide ggtcctagcagctttgaaca was used. To introduce p. R1398H (CGT > CAT), the guide taatctttatttaggtcgtg was used. Individual clones were isolated from CRISPR pooled cells by limiting dilution. For LRRK2 KO cells, single clones were sequenced by PCR-TOPO cloning and further verified by western blot analysis. For N551K R1398H KI cells, individual clones were screened and confirmed by Sanger sequencing and next generation sequencing.
LRRK2 and pT73 Rab10 measurements in healthy subjects and PD patients
Whole blood specimens from 12 Parkinson’s Disease patients (3 Female, 9 Male) and 6 Non-Parkinson’s controls (2 Female, 4 Male) were obtained from the 24-h Biofluid Sampling Study, in which biospecimens were donated by each subject at 11 time points over 26 h54. These specimens are a subset of a large Michael J. Fox Foundation biospecimen bank available to the community (https://www.michaeljfox.org/biospecimens). All relevant study documentation was reviewed and approved by the responsible Institutional Review Board (IRB; Aspire IRB, 11491 Woodside Avenue, Santee, California 92071, USA). All subjects were informed of the nature and purpose of the study and provided written informed consent during pre-study screening. Samples were processed and analyzed for pS935 LRRK2 and total LRRK2 levels according to methods established in this manuscript. PBMC lysates from Parkinson’s Disease patients and healthy controls with and without a LRRK2 mutation (n = 16 non-PD-LRRK2 G2019S carrier, n = 22 non-PD non-G2019S, n = 46 PD non-G2019S carrier, n = 33 PD and G2019S carrier) were obtained from the LRRK2 Detection in PBMC Consortium32. The study was approved by the CUIMC Institutional Review Board. Samples were thawed and analyzed according to methods established in this manuscript. All research was performed in accordance to accredited Independent Review Boards meeting all applicable guidelines and regulations for human research, and informed consent was obtained from all participants and/or their legal guardians.
Isolation of PBMCs contributing to analysis of N551K R1398H relationship to LRRK2 and pT73 Rab10 levels
Subjects with and without a self-reported diagnosis of Parkinson’s Disease were recruited from the surrounding area (within a 4-h drive) as part of three biomarker studies conducted from 2016 to 2017 (Study IDs SAN-BB-01 (approved by Quorum Review IRB), PLMDEN201609v1 (approved by New England IRB), and US-IRB-13-2016.1 (approved by E&I Review Services)). All research was performed in accordance to accredited Independent Review Boards meeting all applicable guidelines and regulations for human research, and informed consent was obtained from all participants and/or their legal guardians. Blood was collected into CPT-sodium heparin tubes (BD BDAM362780) and PBMCs were then isolated following the manufacturer’s protocol. PBMCs were pelleted by centrifugation at maximum speed and then resuspended in PBMC lysis buffer (1× cell lysis buffer [CST #9803] with PhosSTOP phosphatase inhibitor [Roche 04906837001], complete protease inhibitor [Roche 04693159001], and Benzonase [Sigma E8263]). The lysates were held on ice for 20 min, followed by centrifugation at maximum speed at 4 °C for 20 min. Supernatants were aliquoted and stored at − 80 °C for later immunoassay analysis.
Statistical analysis
Data have either been expressed as means ± SEM or geometric means with 95% CI as indicated in figure legends. All statistical analysis was performed in GraphPad Prism 9 or R version 3.6.1. Analysis was performed using paired t test, one-way analysis of variance (ANOVA) with Tukey’s multiple comparison, one-way ANOVA with Sidak’s multiple comparison test, or two-way ANOVA with Sidak’s test, as indicated in figure legends. Criterion for differences to be considered significant was p < 0.05. The methods by which statistical analysis was performed to assess the effects of the G2019S variant and the N551K R1398H variant on LRRK2 and pT73 Rab10 levels in human samples are described in detail in the Supplementary Information.
Data availability
The data that support the findings of this study and the pT73 Rab10 antibody used in these studies are available from the corresponding authors upon reasonable request.
References
Kluss, J. H., Mamais, A. & Cookson, M. R. LRRK2 links genetic and sporadic Parkinson’s disease. Biochem Soc Trans 47, 651–661 (2019).
Li, J.-Q., Tan, L. & Yu, J.-T. The role of the LRRK2 gene in Parkinsonism. Mol. Neurodegener. 9, 47 (2014).
Wallings, R. L., Herrick, M. K. & Tansey, M. G. LRRK2 at the interface between peripheral and central immune function in Parkinson’s. Front. Neurosci. 14, 443 (2020).
Bernd, G., Marita, E. & Christian Johannes, G. Regulation of LRRK2: Insights from structural and biochemical analysis. Biol. Chem. 399, 637–642 (2018).
Cookson, M. R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 11, 791–797 (2010).
Alessi, D. R. & Sammler, E. LRRK2 kinase in Parkinson’s disease. Science 360, 36–37 (2018).
Di Fonzo, A. et al. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson’s disease. Lancet 365, 412–415 (2005).
Sheng, Z. et al. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci. Transl. Med. 4, 164ra161 (2012).
Di Maio, R. et al. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med. 10, eaar5429 (2018).
Mir, R. et al. The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem. J. 475, 1861–1883 (2018).
Steger, M. et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5, e12813 (2016).
Gomez, R. C., Wawro, P., Lis, P., Alessi, D. R. & Pfeffer, S. R. Membrane association but not identity is required for LRRK2 activation and phosphorylation of Rab GTPases. J. Cell Biol. 218, 4157–4170 (2019).
Steger, M. et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife 6, e31012 (2017).
Dzamko, N. et al. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem J 430, 405–413 (2010).
Fuji, R. N. et al. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci. Transl. Med. 7, 273ra215 (2015).
Manschwetus, J. T. et al. Binding of the human 14-3-3 isoforms to distinct sites in the leucine-rich repeat kinase 2. Front. Neurosci. 14, 302 (2020).
Thirstrup, K. et al. Selective LRRK2 kinase inhibition reduces phosphorylation of endogenous Rab10 and Rab12 in human peripheral mononuclear blood cells. Sci. Rep. 7, 10300 (2017).
Atashrazm, F. et al. LRRK2-mediated Rab10 phosphorylation in immune cells from Parkinson’s disease patients. Mov. Disord. 34, 406–415 (2019).
Karayel, Ö. et al. Accurate MS-based Rab10 phosphorylation stoichiometry determination as readout for LRRK2 activity in Parkinson’s disease. Mol. Cell. Proteomics 19, 1546–1560 (2020).
Davies, P. et al. Comprehensive characterization and optimization of anti-LRRK2 (leucine-rich repeat kinase 2) monoclonal antibodies. Biochem. J. 453, 101–113 (2013).
Mandemakers, W., Snellinx, A., O’Neill, M. J. & de Strooper, B. LRRK2 expression is enriched in the striosomal compartment of mouse striatum. Neurobiol. Dis. 48, 582–593 (2012).
Henry, A. G. et al. Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum. Mol. Genet. 24, 6013–6028 (2015).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2016).
Andreone, B. J. et al. Alzheimer’s-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat. Neurosci. 23, 927–938 (2020).
Bonet-Ponce, L. et al. LRRK2 mediates tubulation and vesicle sorting from lysosomes. Sci. Adv. 6, eabb2454 (2020).
Eguchi, T. et al. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc. Natl. Acad. Sci. 115, E9115–E9124 (2018).
Cook, D. A. et al. LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Parkinsons Dis. 3, 11 (2017).
Gardet, A. et al. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J. Immunol. 185, 5577–5585 (2010).
Thévenet, J., Pescini Gobert, R., Hooft van Huijsduijnen, R., Wiessner, C. & Sagot, Y. J. Regulation of LRRK2 expression points to a functional role in human monocyte maturation. PLoS ONE 6, e21519 (2011).
Hui, K. Y. et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med. 10, eaai7795 (2018).
Melachroinou, K. et al. Elevated in vitro kinase activity in peripheral blood mononuclear cells of leucine-rich repeat kinase 2 G2019S carriers: A novel enzyme-linked immunosorbent assay-based method. Mov. Disord. 35, 2095–2100 (2020).
Padmanabhan, S. et al. An assessment of LRRK2 serine 935 phosphorylation in human peripheral blood mononuclear cells in idiopathic Parkinson’s disease and G2019S LRRK2 cohorts. J. Parkinsons Dis. 10, 623–629 (2020).
Heckman, M. G. et al. Population-specific frequencies for LRRK2 susceptibility variants in the Genetic Epidemiology of Parkinson’s Disease (GEO-PD) Consortium. Mov. Disord. 28, 1740–1744 (2013).
Ouled Amar Bencheikh, B. et al. LRRK2 protective haplotype and full sequencing study in REM sleep behavior disorder. Parkinson. Relat. Disord. 52, 98–101 (2018).
Tan, E. K. et al. Multiple LRRK2 variants modulate risk of Parkinson disease: A Chinese multicenter study. Hum Mutat 31, 561–568 (2010).
Nixon-Abell, J. et al. Protective LRRK2 R1398H variant enhances GTPase and Wnt signaling activity. Front. Mol. Neurosci. 9, 18 (2016).
Burbulla, L. F. et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357, 1255–1261 (2017).
Miklossy, J. et al. LRRK2 expression in normal and pathologic human brain and in human cell lines. J. Neuropathol. Exp. Neurol. 65, 953–963 (2006).
Volpicelli-Daley, L. A. et al. G2019S-LRRK2 expression augments α-synuclein sequestration into inclusions in neurons. J. Neurosci. 36, 7415–7427 (2016).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Langston, R. G. et al. Association of a common genetic variant with Parkinson’s disease is propagated through microglia. bioRxiv https://doi.org/10.1101/2021.01.15.426824 (2021).
Schilder, B. M. & Raj, T. Fine-mapping of Parkinson’s disease susceptibility loci identifies putative causal variants. bioRxiv https://doi.org/10.1101/2020.10.22.340158 (2020).
Moehle, M. S. et al. LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 32, 1602–1611 (2012).
Panagiotakopoulou, V. et al. Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat. Commun. 11, 5163 (2020).
Kluss, J. H. et al. Detection of endogenous S1292 LRRK2 autophosphorylation in mouse tissue as a readout for kinase activity. npj Parkinson’s Dis. 4, 13 (2018).
Reynolds, A., Doggett, E. A., Riddle, S. M., Lebakken, C. S. & Nichols, R. J. LRRK2 kinase activity and biology are not uniformly predicted by its autophosphorylation and cellular phosphorylation site status. Front. Mol. Neurosci. 7, 54–54 (2014).
West, A. B. et al. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 16, 223–232 (2007).
Fan, Y. et al. Interrogating Parkinson’s disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochem. J. 475, 23–44 (2018).
Ross, O. A. et al. Association of LRRK2 exonic variants with susceptibility to Parkinson’s disease: A case–control study. Lancet Neurol. 10, 898–908 (2011).
Herzig, M. C. et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum. Mol. Genet. 20, 4209–4223 (2011).
Zhao, J., Molitor, T. P., Langston, J. W. & Nichols, R. J. LRRK2 dephosphorylation increases its ubiquitination. Biochem. J. 469, 107–120 (2015).
Ito, G. et al. Phos-tag analysis of Rab10 phosphorylation by LRRK2: A powerful assay for assessing kinase function and inhibitors. Biochem. J. 473, 2671–2685 (2016).
Pandya, H. et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 20, 753–759 (2017).
Eidson, L. N. et al. Candidate inflammatory biomarkers display unique relationships with alpha-synuclein and correlate with measures of disease severity in subjects with Parkinson’s disease. J. Neuroinflamm. 14, 164 (2017).
Acknowledgements
We would like to thank Paul Davies and James Hastie at University of Dundee Medical Research Council Protein Phosphorylation Unit for providing phosphorylated Rab10 protein. We would also like to thank Dario Alessi at University of Dundee for generously sharing with us the RAB8A and RAB10 KO A549 cells. The biospecimens from healthy subjects and PD patients from MJFF were kindly provided by the LRRK2 Biobanking Initiative site at the Columbia University Medical Center under the direction of Dr. Roy Alcalay. The LRRK2 Biobanking Initiative is coordinated and funded by The Michael J. Fox Foundation for Parkinson’s Research. We thank Janet Hurt, Dominique Jacquemet-Engelhart, Rinkal Chaudhary, and Anthony Lucas for their contributions to the PBMC isolation study contributing to the N551K R1398H findings. We also thank Joseph Lewcock, Anthony Estrada, Gilbert Di Paolo, Thomas Sandmann, and Steve Lianoglou for helpful feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
X.W., E.N., M.M., V.B., S.V.A., M.M., C.L., H.N., H.S., A.A., L.P., N.M., S.H.R. and A.G.H. designed experiments. X.W., E.N., R.M., M.M., V.B., S.V.A., M.M., C.L., H.N., H.S. and N.M. performed and analyzed experiments. X.W., S.V.A., S.H.R. and A.G.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
XW, EN, MM, VB, SVA, MM, CL, RM, HN, HS, AA, LP, NM, SHR and AGH are paid employees of Denali Therapeutics Inc. Denali has filed patent applications related to the subject matter of this paper. Biogen also had the opportunity to review this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Negrou, E., Maloney, M.T. et al. Understanding LRRK2 kinase activity in preclinical models and human subjects through quantitative analysis of LRRK2 and pT73 Rab10. Sci Rep 11, 12900 (2021). https://doi.org/10.1038/s41598-021-91943-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91943-4
This article is cited by
-
Single molecule array measures of LRRK2 kinase activity in serum link Parkinson’s disease severity to peripheral inflammation
Molecular Neurodegeneration (2024)
-
A potential patient stratification biomarker for Parkinson´s disease based on LRRK2 kinase-mediated centrosomal alterations in peripheral blood-derived cells
npj Parkinson's Disease (2024)
-
Comprehensive genetic screening of early-onset dementia patients in an Austrian cohort-suggesting new disease-contributing genes
Human Genomics (2023)
-
Elevated urine BMP phospholipids in LRRK2 and VPS35 mutation carriers with and without Parkinson’s disease
npj Parkinson's Disease (2023)
-
Parkinson-causing mutations in LRRK2 impair the physiological tetramerization of endogenous α-synuclein in human neurons
npj Parkinson's Disease (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.