Abstract
Risk factors associated with severity and mortality attributable to COVID-19 have been reported in different cohorts, highlighting the occurrence of acute kidney injury (AKI) in 25% of them. Among other, SARS-CoV-2 targets renal tubular cells and can cause acute renal damage. The aim of the present study was to evaluate the usefulness of urinary parameters in predicting intensive care unit (ICU) admission, mortality and development of AKI in hospitalized patients with COVID-19. Retrospective observational study, in a tertiary care hospital, between March 1st and April 19th, 2020. We recruited adult patients admitted consecutively and positive for SARS-CoV-2. Urinary and serum biomarkers were correlated with clinical outcomes (AKI, ICU admission, hospital discharge and in-hospital mortality) and evaluated using a logistic regression model and ROC curves. A total of 199 COVID-19 hospitalized patients were included. In AKI, the logistic regression model with a highest area under the curve (AUC) was reached by the combination of urine blood and previous chronic kidney disease, with an AUC of 0.676 (95%CI 0.512–0.840; p = 0.023); urine specific weight, sodium and albumin in serum, with an AUC of 0.837 (95% CI 0.766–0.909; p < 0.001) for ICU admission; and age, urine blood and lactate dehydrogenase levels in serum, with an AUC of 0.923 (95%CI 0.866–0.979; p < 0.001) for mortality prediction. For hospitalized patients with COVID-19, renal involvement and early alterations of urinary and serum parameters are useful as prognostic factors of AKI, the need for ICU admission and death.
Similar content being viewed by others
Introduction
Coronavirus disease (COVID-19), produced by the new RNA virus called SARS-CoV-2, was discovered in late 2019 in China1,2. COVID-19 is characterized by a wide spectrum of symptoms, ranging from an asymptomatic state to an acute respiratory distress syndrome in the adult (ARDS), being the acute respiratory tract infection the main affection, possibly progressing to a severe pneumonia3,4,5.
In different retrospective cohorts, several severity and mortality-associated risk factors have been reported6,7,8, with acute kidney injury (AKI) being described in up to 25% of patients6,9. The onset of AKI in individuals with ARDS implies a significant increase in ARDS-associated mortality rates10. Different studies highlight the importance of an elevation of inflammatory mediators as the pathogenic basis for the development of AKI, produced by an endothelial dysfunction, microangiopathy and tubular damage11.
Patients with severe COVID-19 may present with a status of systemic hyperinflammation or cytokine storm12. In addition, SARS-CoV-2 uses the angiotensin-converting enzyme receptor 2 (ACE2) as target for cell invasion, which is also present on the surface of renal tubular cells13. AKI onset has been described on the 9 days after hospital admission due to COVID-19, and associated to factors such as age, infection severity, cardiorenal syndrome and diabetes6,9.
Renal complications are common in individuals with comorbidities that predispose to kidney disease, being difficult to evaluate by means of serum creatinine measurements. To date, some previous studies have reported the usefulness of urine strip biochemical results on the prediction of severity of COVID-19 patients14.
In this line, the aim of this study was to assess the urinary parameters that, in combination with other laboratory tests in serum, demographics variables and comorbidities, could be useful for predicting the development of AKI, requirement of ICU admission and in-hospital mortality, in hospitalized patients with COVID-19.
Methods
Study design and participants
This is a retrospective observational study performed in a tertiary care hospital between March 1st and April 19th, 2020. Patients above 18 years old were consecutively selected through a search on the laboratory information system, including only patients with a positive result on the real-time reverse transcriptase–polymerase chain reaction (RT-PCR) due to a clinical suspicion of COVID-19 and a urine dipstick study requested. Individuals without hospital admission were excluded, alongside with those with hospital admission and a confirmed urinary tract infection (UTI), defined as a positive urine culture.
The prevalence of AKI15 and/or admission to the Intensive Care Unit (ICU) was recorded, and patients were monitored until ICU discharge, hospital discharge or patient’s death. Patients who at the time of retrospective recruitment did not have a final event and remained in ICU were excluded.
Clinical and biochemical records
Epidemiologic, demographic, clinical and biochemical variables were analyzed for all individuals.
The recorded data included time from positive RT-PCR to urinalysis, time from urinalysis to clinical endpoints (AKI, ICU admission, discharge or death) and presence of AKI risk factors coded in the medical record (history of heart failure, obesity, diabetes, hypertension and chronic kidney disease (CKD)).
Laboratory confirmation of SARS-CoV-2 infection was defined as a positive result of RT-PCR assay on samples collected on nasal and pharyngeal swabs16. Laboratory data included a complete urinalysis and urine sediment, which were performed on an automatic biochemical and flow cytometer sediment analyzer (Sysmex series UC-3500, UF-5000 and UD-10; Sysmex, Japan; Urine Dipstick MEDITAPE UC-11A and UC-9A), respectively. Approximately 10 mL of midstream urine samples, or collected from urinary catheter in critical patients, were obtained from individuals, and all of them were analyzed within 2 h. To confirm doubtful formed elements in urine, samples were centrifuged 5 min at 1000 × g and checked by optical microscopy by a senior consultant.
Blood was also collected from patients in the Emergency Room or during hospitalization on the same day of urinalysis and onwards. The following tests were performed: complete blood count (Cell-Dyn Sapphire platform, Abbott Diagnostics, US), D-dimer (HemosIL D-Dimer HS, DDU, ACL-TOP 700, Instrumentation Laboratory, US), serum biomarkers: liver enzymes, C-reactive protein (CRP), lactate dehydrogenase (LDH), creatinine phosphokinase (CK), ferritin, urea, albumin, creatinine, potassium, sodium and chloride (Architect ci16200 platform, Abbott Diagnostics, US).
Definitions
For the diagnosis and staging of AKI, the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) definition was used15. Individuals were screened considering the AKI 2012 KDIGO definition as major criterion. For individuals without any previous serum creatinine result, a 7-day follow-up was performed for the establishment of the AKI onset during the episode. Serum creatinine concentration upon admission was considered as baseline.
Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) < 60 ml/min per 1.73 m2 or a urine albumin-creatinine ratio ≥ 30 µg/mg at least 3 months before admission17. Presence of proteinuria and hematuria were defined as more than trace albumin or blood on urine dipstick tests (> 0.3 g/L and > 0.06 mg/dL, respectively). The cut-off values for the other parameters included in the urine dipstick analysis were the following: urobilinogen (> 34.0 µmol/L), glucose (> 2.8 mmol/L), ketones (> 0.93 mmol/L) and bilirubin (> 8.6 µmol/L). Obesity was defined as a body mass index over than 30 kg/m2. AKI recovery was defined as a complete recovery of the kidney function, with a serum creatinine decreasing below threshold or to the baseline.
Statistical analysis
The sample size was calculated for the smallest of the differences described in the bibliography for the three events, the smallest being the need for admission to the ICU with a percentage difference of 16.4 points18 to local data, for a statistical power of 90% and an alpha type error of 0.05, 183 subjects are required in total for the present study. This criterion was used because it was the data we could most reliably approximate, since both deaths were strongly age-dependent and included percentage ranges ranging from values below 1% to age groups with more than 20% mortality. On the other hand, the incidence of AKI in these patients has also been subject to controversy, and the series' ranges vary widely. The sample size calculation and given the retrospective nature of the analysis, we included 61 COVID-19 patients by group, given the study of the three endpoints (mortality, ICU admission and AKI), results in total of 183 patients were sufficient to detect the a priori differences expected from the literature. Regard to group subanalyses, if we consider a subsample group vs. the total sample with a statistical power of 90%, an alpha error of 5% and a beta error of 10%, for an expected increase in incidence of 16.4%, the number of events we need in each subgroup is 3 patients presenting the event death, ICU admission and AKI.
Descriptive statistics included frequency analysis (percentages) for categorical variables and means and standard deviation or medians and interquartile ranges amplitude (IQR = p75-p25) for continuous variables. Sample normality was assessed through the Kolmogorov–Smirnov’s test and comparisons were determined by the Student’s t or Mann–Whitney’s U tests for continuous variables, as appropriate, and by the use of the Chi squared test or Fisher’s exact test for categorical variables, after verifying the variances using the Levene´s test. A binary and multiple logistic regression was performed, and stepwise regression with forward selection method (best Fisher’s test sequence) was used for predicting variables. Odds ratios (ORs) were calculated for each significant variable and receiver-operating characteristics (ROC) curve analyses were performed with the different cut-off values, selecting those with the best Chi squared value, of the significant parameters of the logistic regression model for the discrimination of COVID-19 clinical endpoints. The differences were considered statistically significant when the alpha type error was less than 0.05. SPSS v.24 software was used for data analysis (IBM Corporation, US).
Ethics approval and consent to participate
The Local Ethics Committee (Comitè d’Ètica de la Investigació de les Illes Balears) approved the study (IB4258/20PI) and all individuals or their legal representatives gave their informed consent. Because of Spanish laws, the research team cannot share the full database used for the current paper. Moreover, because COVID-19 admitted patients to our hospital was limited, data could contains potentially identifying or sensitive patient information. However, other researchers who meet the criteria for access to confidential data, may request to gain access to the minimal data set underlying the results under request at the Ethics Committee (contact via https://www.caib.es/sites/comiteetic/es/portada44578/?campa=yes), (e-mail address: ceic_ib@caib.es).
Human investigations
We certify that all protocols and methods are carried out in accordance with relevant guidelines and regulations.
Results
COVID-19 screening population
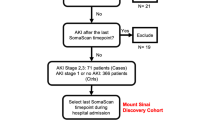
During the study period, a total of 713 patients with a RT-PCR analysis for SARS-CoV-2 were included, of which 240 (33.7%) had a positive result (Fig. 1).
A total of 199 (82.9%) individuals with a positive result required hospital admission. Clinical data and biochemical parameters of COVID-19 hospitalized patients are compared in Table 1, has been described with differentiated genders, as gender is an independent variable associated with the risk of presenting the three endpoints described. Median time from a positive RT-PCR result to urinalysis was 0.32 days (IQR: 2.16).
Clinical endpoints for hospitalized patients with COVID-19
Intensive care unit admission
Twenty-four patients needed ICU admission, which represents 12.1% of the number of hospitalized patients. Clinical variables and biochemical results for critical and non-critical patients are shown in Table 2. The ROC curve analyses performed on the different significant clinical variables and biomarkers revealed that the highest area under the curve (AUC) was reached by a combination of urine specific weight, sodium and albumin in serum, with an AUC of 0.837 (95% CI 0.766–0.909; p < 0.001). Different cut-off values of specific weight, sodium and albumin in serum were tested in order to evaluate the OR for all of them. Figure 2 summarizes those cut-off values for the prediction for this endpoint and the OR and ROC curve obtained thereof. Median time from urinalysis result to ICU admission was 2.17 days (IQR: 2.91).
Acute kidney injury
Fifteen patients developed AKI during the clinical episode, which represents 7.5% of the number of hospitalized patients. Clinical data and biochemical results for AKI and non-AKI patients are shown in Table 3. The ROC curve analyses performed on the different clinical variables and biomarkers revealed that the highest AUC was reached by a model including the presence of blood in urine and CKD, with an AUC of 0.676 (95% CI 0.512–0.840; p = 0.023). Different cut-off values of urine blood were tested in order to evaluate their ORs. Figure 3 summarizes cut-off values of urine blood for AKI prediction. Median time from urinalysis result to AKI development was 3.84 days (IQR: 6.09) and the specific features of these patients are summarized in Table 4.
Mortality
Twenty patients died during hospital admission, which represents 10.1% of the number of hospitalized patients. Clinical data and biochemical results for survivors and non-survivors are shown in Table 5. The ROC curve analyses performed on the different clinical variables and biomarkers revealed that the highest AUC was reached by a model including age, presence of blood in urine and LDH levels in serum, with an AUC of 0.923 (95% CI 0.866–0.979; p < 0.001). Different cut-off values for age, urine blood and LDH levels in serum were tested in order to evaluate their ORs. Figure 4 summarizes the cut-off values of these variables for mortality prediction. Median time from urinalysis result to death was 8.04 days (IQR: 19.02).
Laboratory findings
In the study of serum and urine biomarkers in hospitalized patients, we observed interesting results in urine pH values, presence of blood and protein in the urine dipstick of those patients who develop AKI, as well as urate and chloride in blood. Regarding the need for admission to the ICU, a higher urine specific weight, ketones, glucose and protein were found in a greater percentage in individuals that presented a critical evolution than in those that didn’t, also highlighting a higher presence of pathological casts in the urinary sediments of these patients. In serum, increased concentrations of D-dimer, creatinine, LDH and CRP were observed, as well as a decrease in the albumin and sodium. Concerning mortality, the differential value of the urine dipstick with respect to the presence of blood and protein in a greater proportion in non-surviving patients was again noteworthy, as well as increased levels of D-dimer, LDH, CK and CRP, again observing a decrease in the level of serum albumin.
Discussion
In this study, for patients with COVID-19, urine blood > 0.03 mg/dL and urine specific weight > 1.026 were shown to be associated with a high incidence of AKI, ICU admission and mortality. In addition, we found that patients with adverse clinical outcomes were predominantly older men with an age above 65 years, chronic kidney disease, lower levels of sodium and albumin in serum and an LDH activity > 400 U/L upon hospital admission, which correlated with the severity of the in-hospital episode compared to those hospitalized patients without these initial findings.
Biochemical parameters in urine can be used for UTI diagnosis, monitoring of kidney diseases and the follow-up of treatment effects, due to the easy sample collection, test automation and cost-effectiveness19. Despite that, only few studies reported on the correlation between urine biochemical parameters and the prediction of a critical evolution of COVID-19. Liu et al.14 found significant differences in the proportion of positive blood and protein in the urine dipstick between patients with COVID-19 and healthy controls. In addition, infected patients presented higher pH values and a decrease in urine specific weight. Regarding the severity of the COVID-19, the groups with the highest proportion of positive glucose and protein results were patients with the greatest severity; up to 60% of critical patients were positive for glucose in the urine dipstick and 50% for protein. These findings are consistent with those seen in our study regarding the group of patients admitted to the ICU versus those who did not, reporting a significant increase in the percentage of patients with positive values in urine dipstick glucose, protein, ketones and urine specific weight.
In a retrospective study, Pei G, et al.18 described that 75.4% of COVID-19 patients had renal involvement upon admission, 65.8% of which with proteinuria and 41.7% with hematuria. In their study as a whole, the presence of AKI was 4.7%, with a higher incidence during hospital stay than upon admission (86.4% vs 13.6%). Critically ill patients showed a high incidence of proteinuria and hematuria (85.7% and 69.6%, respectively), and also of AKI (42.9%). Mortality is proportional to the stage of AKI, reaching 90.9% in stage 3. However, overall mortality amounted 8.7% of admitted patients, and up to 52.8% of the critical patients. These results are consistent with those found in our study where the presence of proteinuria upon hospital admission was 62.8%, with a presence of 83.3% in the critical patient. The overall prevalence of AKI was 14.2%, and 26.7% of such patients required ICU admission. Mortality was higher among patients with AKI (33.3% vs. 8.2%), and the presence of blood in urine was a good predictor of in-hospital mortality in these patients.
Conversely, there are studies which question the relationship of CKD with the development of AKI and highlight that, in patients without CKD, the presence of COVID-19 does not increase the development of AKI, although this is a complication with a high mortality20. Our results confirm the presence of CKD as a predictor of the development of AKI in COVID-19 patients, with a risk 5 times higher than in COVID-19 patients without CKD. Moreover, the presence of AKI is related to a greater need for ICU admission and higher mortality in our population. These findings support the idea that the development of AKI in the COVID-19 patient is promoted by the patient's comorbidities, pharmacological treatments and the presence of a systemic inflammatory state as a consequence of viremia, and that this leads to a worse in-hospital evolution21,22. This observation is further supported by some case series reporting 19% of AKI in the population requiring ICU admission23.
The prediction of in-hospital mortality based on urinary parameters has already been evaluated by Bonetti G. et al.24 for COVID-19 patients attended at the Emergency Room. Urinary sediment was found to be a useful prognostic tool, as a higher percentage of in hospitalized non-survivors presented hyaline-granular casts and renal tubular cells, alongside with elevated serum creatinine and urea concentrations. In our study, no significant differences were seen in the presence of renal tubular cells between survivors and non-survivors, although assessed by another analytical technology25. Nevertheless, we highlighted the presence of a higher percentage of patients with pathological casts and higher serum creatinine values requiring ICU admission, which confirms the usefulness and convenience of an initial assessment of urine parameters (urine dipstick and sediment) and renal function.
Necropsy studies performed in non-survivors admitted to the ICU26 confirm that SARS-CoV-2 infection directly affects renal tissue and triggers the development of AKI, which is further exacerbated by systemic hypoxia, coagulation abnormalities and iatrogenic rhabdomyolysis. These observations would be reinforced by the fact that our study shows a higher incidence of AKI in ICU patients (16.7%), who also present an increase in D-Dimer, LDH and CRP levels, adding the finding of a higher CK activity in non-survivors. Other studies agree on the remark of a decreased serum albumin level upon hospital admission found in our study, as an independent factor for the development of stage 3 AKI and a fatal outcome27, adding value to early predictors of AKI by monitoring renal changes28. Our results shown in Table 4 confirm the differences between the different KDIGO stages of AKI, with a higher proportion of stage 3 patients with ICU admission, need for orotracheal intubation, renal replacement treatment, more days to renal function recovery and a high rate of mortality than those in stage 1 and 2.
In this regard, recent studies have described low serum albumin levels in young COVID-19 patients, along with elevated D-Dimer, LDH and the presence of proteinuria and obesity as predictors of critical disease progression29. Our findings are coincidental, since we reported a higher proportion of obese patients who develop AKI and require ICU admission. In addition, the presence of proteinuria, elevated D-dimer, LDH, CRP and decreased serum albumin are observed in those patients who are critical and non-survivors. Some authors have reported that the development of AKI during hospital episode combined with heart failure (HF) is related with high mortality rate30. In our study, HF does not appear to be associated with a worse evolution of the development of AKI. Altogether, our findings suggest that renal involvement with AKI presentation and the presence of urinary parameter alterations may add to respiratory involvement as prognostic factors of severity in COVID-1931.
The observed in-hospital mortality of 10.1% and the predictive model, including an age greater than 65 years, are in concordance with the findings reported in a recent meta-analysis, placing our data in an intermediate term between the Chinese cohorts with low mortality and the Spanish and US cohorts with in-hospital mortality rates of around 20%32. An influence of gender on adverse events has also been described33, the male gender presenting with a higher proportion of AKI, ICU admissions and mortality in our study. However, variables included in other studies34 such as lymphocyte and platelet counts and serum ferritin, were not included in our logistic regression models. We found that elevated LDH values above 400 U/L, together with an age above 65 years (more if it is greater than 85 years) and hematuria upon hospital admission have predictive capacity on the in-hospital mortality of COVID-19 patients. These findings are consistent with other Spanish and North American series35,36,37.
To the best of our knowledge, this study adds evidence to those who have included the prognostic role of urinary markers and their association with the three endpoints (AKI, need for ICU admission and in-hospital mortality) in small populations with COVID-19 individuals38,39,40.
This study has several limitations. First, during the COVID-19 outbreak, urine dipstick and sediment studies were scarcely requested, probably due to a low evidence in COVID-19 patients since only a few studies have attempted to reveal the prognostic role of urinary markers and their association with the renal injury in early pandemic times. These facts which might represent a selection bias in our retrospective study, especially with regard to the higher proportion of men included. Second, we did not detect SARS-CoV-2 in urine samples and no data was obtained from other biomarkers, biopsy studies or functional testing. Third, the prediction models presented need to be validated in prospective cohorts with control groups, especially since this is a small sample of AKI patients.
Conclusions
This study shows the gender differences of the possible predictive variables in the hospital admission of COVID-19 patients. Three predictive models were included for AKI, need for ICU admission and mortality in which the independent variables derived from the early analysis of urine, together with an age above 65 years old, the presence of CKD and levels of serum markers such as albumin, sodium and LDH may represent a useful tool in the management of patients requiring hospital admission for a SARS-CoV-2 infection.
Abbreviations
- AKI:
-
Acute kidney injury
- ACE2:
-
Angiotensin-converting enzyme receptor 2
- AUC:
-
Area under the curve
- CKD:
-
Chronic kidney disease
- COVID-19:
-
Coronavirus disease
- CRP:
-
C-reactive protein
- CK:
-
Creatinine phosphokinase
- eGFR:
-
Estimated glomerular filtration rate
- HF:
-
Heart failure
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile ranges amplitude
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- LDH:
-
Lactate dehydrogenase
- ROC:
-
Receiver-operating characteristics
- ARDS:
-
Respiratory distress syndrome in the adult
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- UTI:
-
Urinary tract infection
References
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020).
World Health Organization. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail. Published February 11 (2020).
Gorbalenya, A. E. et al. Severe acute respiratory syndrome-related coronavirus: the species and its viruses—a statement of the Coronavirus Study Group. Nat. Microbiol. 5, 536–544 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Li, Q. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020).
Xiong, T.-Y., Redwood, S., Prendergast, B. & Chen, M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 41, 1798–1800 (2020).
Du, Y. et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 201, 1372–1379 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centred, retrospective, observational study. Lancet Respir. Med. 8, 475–481 (2020).
Wilson, J. G. & Calfee, C. S. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit. Care 24, 102 (2020).
Joannidis, M. et al. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 46, 654–672 (2019).
McGonagle, D., Sharif, K., O’Regan, A. & Bridgewood, C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 19, 102537 (2020).
Letko, M., Marzi, A. & Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 5, 562–569 (2020).
Liu, R. et al. The value of urine biochemical parameters in the in the prediction of the severity of coronavirus disease 2019. Clin. Chem. Lab. Med. 58, 1121–1124 (2020).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 120, c179–c184 (2012).
WHO. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance V 1.2. WHO/2019-nCoV/Clinical/2020.4. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. (2020).
Disease, K. Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 3, 1–150 (2013).
Pei, G. et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J. Am. Soc. Nephrol. 31, 1157–1165 (2020).
Erdman, P., Anderson, B., Zacko, J. C., Taylo, K. & Donaldson, K. The accuracy of the Sysmex UF-1000i in urine bacterial detection compared with the standard urine analysis and culture. Arch. Pathol. Lab. Med. 141, 1540–1543 (2017).
Wang, L. et al. Coronavirus disease 19 infection does not result in Acute Kidney Injury: An analysis of 116 hospitalized patients from Wuhan, China. Am. J. Nephrol. 51, 343–348 (2020).
Fanelli, V. et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care. 24, 155 (2020).
Wai-Shun Chan, V. et al. A systematic review on COVID-19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J. Urol. https://doi.org/10.1007/s00345-020-03246-4 (2020).
Ng, J. J., Luo, Y., Phua, K. & Choong, A. Acute kidney injury in hospitalized patients with coronavirus disease 2019 (COVID-2019): a meta-analysis. J. Infect. 81, 647–679 (2020).
Bonetti, G. et al. Urinalysis parameters for predicting severity in coronavirus disease 2019 (COVID-19). Clin. Chem. Lab. Med. 58, e163–e165 (2020).
Oyaert, M., Speeckaert, M., Boelens, J. & Delanghe, R. Renal tubular epitelial cells add value in the diagnosis of upper urinary tract pathology. Clin. Chem. Lab. Med. 58, 597–604 (2020).
Su, H. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 98, 219–227 (2020).
Lim, J. H. et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J. Clin. Med. 9, 1718 (2020).
Gabarre, P. et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 46, 1339–1348 (2020).
Deng, M. et al. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity (Silver Spring) 28, 1815–1825 (2020).
Pelayo, J. et al. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with COVID-19 in a US inner city hospital system. Cardiorenal. Med. 10, 223–231 (2020).
Yang, X. et al. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care. 24, 356 (2020).
Bonanad, C. et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J. Am. Med. Dir. Assoc. 21, 915–918 (2020).
Gebhard, C., Regitz-Zagrosek, V., Neuhauser, H. K., Morgan, R. & Klein, S. L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 11, 29 (2020).
Henry, B. M., Santos de Oliveira, M. H., Benoit, S., Plebani, M. & Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 58, 1021–1028 (2020).
Martos, F. et al. Comorbidity and prognostic factors on admission in a COVID-19 cohort of a general hospital. Rev. Clin. Esp. https://doi.org/10.1016/j.rce.2020.05.017 (2020).
Ayanian, S., Reyes, J., Lynn, L. & Teufel, K. The association between biomarkers and clinical outcomes in novel coronavirus pneumonia in a US cohort. Biomark. Med. 14, 1091–1097 (2020).
Gavin, W. et al. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am. J. Infect. Control. S0196–6553(20), 30689–30691. https://doi.org/10.1016/j.ajic.2020.07.005 (2020).
Ouyang, L., Gong, Y., Zhu, Y. & Gong, J. Association of acute kidney injury with the severity and mortality of SARS-CoV-2 infection: a meta-analysis. Am. J. Emerg. Med. https://doi.org/10.1016/j.ajem.2020.08.089 (2020).
Zahid, U. et al. Acute kidney injury in COVID-19 patients: an inner city hospital experience and policy implications. Am. J. Nephrol. https://doi.org/10.1159/000511160 (2020).
Nadin, M. K. et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-00356-5 (2020).
Funding
The authors declare that they have received funding from the Health Research Institute of the Balearic Islands (IdISBa) to publish this work [Grant number: LIBERI Project LIB21/11].
Author information
Authors and Affiliations
Contributions
D.M.G., D.R.C., C.G.C. and A.G.R. conceived and designed the study. D.M.G., D.R.C., P.A.C., J.A.P., R.A.M. and M.A.B.V. supervised the data collection and managed the data, including quality control. D.R.C. and D.M.G. provided statistical advice on study design and analysed the data; D.M.G., I.L.L., A.A.F. and A.G.R. chaired the data oversight committee. D.M.G., D.R.C., J.M.B. and L.G.G.R. drafted the manuscript, and all authors contributed substantially to its revision. D.M.G., D.R.C., J.M.B. and A.G.R. take responsibility for the paper as a whole. All authors listed on of the manuscript have seen and approved the submission of this version and take full responsibility for the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morell-Garcia, D., Ramos-Chavarino, D., Bauça, J.M. et al. Urine biomarkers for the prediction of mortality in COVID-19 hospitalized patients. Sci Rep 11, 11134 (2021). https://doi.org/10.1038/s41598-021-90610-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90610-y
This article is cited by
-
Machine learning algorithms for predicting COVID-19 mortality in Ethiopia
BMC Public Health (2024)
-
Machine learning-based mortality prediction models for smoker COVID-19 patients
BMC Medical Informatics and Decision Making (2023)
-
A metabolic readout of the urine metabolome of COVID-19 patients
Metabolomics (2023)
-
COVID-19 in Iran: clinical presentations and outcomes in three different surges of COVID-19 infection
Virology Journal (2022)
-
Using dipstick urinalysis to predict development of acute kidney injury in patients with COVID-19
BMC Nephrology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.