Abstract
Reading typically involves phonological mediation, especially for transparent orthographies with a regular letter to sound correspondence. In this study we ask whether phonological coding is a necessary part of the reading process by examining prelingually deaf individuals who are skilled readers of Spanish. We conducted two EEG experiments exploiting the pseudohomophone effect, in which nonwords that sound like words elicit phonological encoding during reading. The first, a semantic categorization task with masked priming, resulted in modulation of the N250 by pseudohomophone primes in hearing but not in deaf readers. The second, a lexical decision task, confirmed the pattern: hearing readers had increased errors and an attenuated N400 response for pseudohomophones compared to control pseudowords, whereas deaf readers did not treat pseudohomophones any differently from pseudowords, either behaviourally or in the ERP response. These results offer converging evidence that skilled deaf readers do not rely on phonological coding during visual word recognition. Furthermore, the finding demonstrates that reading can take place in the absence of phonological activation, and we speculate about the alternative mechanisms that allow these deaf individuals to read competently.
Similar content being viewed by others
Introduction
Learning to read is a transformative experience for socio-economic mobility and for our cognitive development1,2. Knowing how to read is closely tied to our knowledge of the language we are reading. The written form is a visual representation of auditory words, and learning to read an alphabetic script typically involves associating letters with sounds. During reading acquisition, phonological processes are the first to develop3,4,5 and lexical access involves phonological mediation6,7,8; reading deficits, such as dyslexia, are associated with poor phonological awareness9. The extent to which phonological representations play a role in reading depends on reading proficiency but also on the transparency of the writing system10,11,12. More proficient readers make greater use of strategies that bypass phonology by recognizing orthographic units or even whole words13,14. Transparent orthographies, in which there is a regular correspondence between graphemes and phonemes, favour phonological coding during word recognition15 and may impact the acquisition of syntactic rules16. In contrast, in opaque orthographies, readers can process printed words by using alternative strategies, such as relying on the visual-orthographic structure. In a language with a transparent orthography, like Spanish, phonological codes are automatically accessed during reading17,18. In this study, we ask whether phonological processing is required for word identification in a transparent orthography. To do this, we examine a population that reads without access to the phonological forms of words, namely, deaf individuals.
A variety of factors, including visuo-perceptual processing and reading experience, impact reading outcomes19 but in the case of deaf readers, their lack of audition severely limits their access to phonology. Consequently, the connection between the written word and the sound-based form that it represents has led various authors and educators to highlight the importance of phonological coding and awareness for reading skills in this population20,21,22. Many studies that look at the role of phonology in deaf readers do so through meta-phonological tasks and/or phonological awareness tasks that require explicit phonological judgments, such as rhyme detection23,24,25,26,27,28,29. Since our aim is to determine whether phonology is automatically activated in word reading, we wish to avoid task-driven effects that might activate phonological representations: we focus on implicit reading tasks that do not involve making decisions about the phonological form of the word.
Despite the evidence of phonological encoding from studies with explicit tasks, phonology seems to be much less important for deaf readers9,30. Reading ability in English of primary school children has been linked to language ability but not phonemic awareness31. A major study of over 200 secondary school deaf readers (aged between 11 and 16 years old) of four different languages (with transparent and opaque orthographies) found that syntactic rather than phonological knowledge characterized skilled readers from those less skilled16. In a meta-analysis of 57 studies looking at reading in deaf individuals, phonological awareness predicted only 11% of the variance in reading ability; in contrast, overall language ability (either spoken or signed) predicted up to 35%32. Further support comes from specific studies that have found no behavioural evidence of phonological encoding in deaf readers33. Skilled deaf readers of French activated visual, orthographic and semantic codes during reading, but not phonological codes34. In an eye tracking study of the phonological and orthographic preview benefit in the parafovea in readers of English, both hearing and deaf readers benefited from orthographic coding in parafoveal vision but only the hearing group benefited from phonological coding35.
Much of the previous work on deaf readers34,35,36 has been conducted in languages with opaque orthographies, particularly English. Since orthographic depth can modulate the role of phonological coding during reading4,10,11,12, the absence of phonological coding in deaf readers of languages like English31 or Hebrew16 may be due to the opaque orthography. With a transparent orthography, the properties of the orthographic representation lead us to expect the engagement of phonological encoding: a study of deaf readers of Spanish found evidence of phonological coding37. Critically, the deaf readers in that study had a broad range of reading abilities. In this study we focus on the minority of deaf readers who have good reading skills. For those deaf individuals who read a transparent orthography well, what role does phonology play?
The present study investigates the involvement of phonological processes during visual word recognition in deaf skilled readers of a transparent language (Spanish) using tasks that do not explicitly require phonological analysis (a semantic categorization task and a lexical decision task) while measuring behavioural responses and electrophysiological activity (EEG), and to compare the group with hearing readers. EEG provides sufficient temporal precision to capture the time course of sub-lexical processes (i.e., early and late effects). An important feature of this study is that deaf participants are skilled readers with reading levels comparable to hearing readers of the same age. We carried out two experiments, each looking at different aspects of the word recognition process: the first focuses on sub-lexical processing during word recognition to look for evidence of phonological activation before lexical access takes place; the second experiment examines lexical analysis and the possible role of phonological coding during lexical access. We exploit the pseudohomophone effect, based on pseudowords that sound like real words, one of the strongest indicators of phonological processing in visual word recognition38,39,40,41. Our goal is to discover whether these proficient deaf readers activate phonological representations and to what extent they differ from hearing readers. More broadly, we inquire how central phonology is for proficient reading in a language with a transparent orthography.
Results
Experiment 1: semantic categorization task
In Experiment 1 we tested the role of phonological processing with a go/no-go semantic categorization task (identifying animals) with masked priming. In the critical, no-go trials, primes were pseudowords and pseudohomophones of the target word. Masked priming has revealed early phonological effects in visual word recognition: the N250 component, with a broad scalp distribution, reflects sub-lexical processing that involves associating orthographic and phonological representations, and takes place before lexical access has occurred42. Compared with control pseudoword primes, pseudohomophone primes modulate the N250 component43 and we expect this effect for the hearing readers. If deaf readers activate phonological codes, we expect a comparable modulation; conversely, if they do not activate phonological codes the response should be similar for both types of prime (pseudohomophones and control pseudowords).
Behavioural
In the semantic categorization task all participants showed high levels of accuracy, pressing the button response when the animal names were presented as target (range 80–100%, mean 99.0%, SD 3.43). When the animal names were presented as prime (for just 50 ms), the response rate was close to zero (mean 1.38%, SD 2.53) indicating that the primes were successfully masked. There were only few false alarms in the experimental trials (pseudohomophone primes: mean 2.56%, SD 2.68; pseudoword primes: mean 2.0%, SD 1.98) suggesting that participants paid attention to the content of the stimuli.
ERPs
As planned, we analysed the EEG signal of no-go trials in an early time window to examine the effect of Group (hearing, deaf), PrimeType (pseudohomophone, pseudoword) and topographic factors (see Methods) on the N250 component. Additionally, visual inspection of the grand average waveform revealed a divergence between prime types later on (see Fig. 1); analysis of a 500–700 ms window yielded a trend for a main effect of PrimeType that did not reach significance (see Supplementary Materials S1 for details).
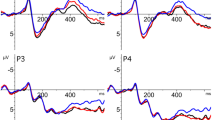
ERP results for Experiment 1 (semantic categorization task). Top: grand average ERP waveforms for deaf (left) and hearing (right) groups. Midline electrodes (Fz, Cz, Pz) are displayed. ERPs in response to the target words are compared for pseudohomophone and pseudoword primes. Bottom: N250 topographical maps created by subtracting the average voltage of pseudoword prime condition from the average voltage of pseudohomophone prime condition. PH Pseudohomophones, PW Pseudowords.
230–270 ms
There was a significant interaction Group × PrimeType (F(1,38) = 4.10, p = 0.049, ŋ2 = 0.097; see Fig. 1) suggesting that in the hearing group targets preceded by pseudohomophones elicited a greater negativity as compared to targets preceded by pseudowords (t(19) = 2.55, p = 0.039). The two conditions did not differ in the deaf group (t(19) = 0.79, p = 0.439). No other significant effect was found (Group: F(1,38) = 0.14, p = 0.712, ŋ2 = 0.004; PrimeType: F(1,38) = 0.89, p = 0.351, ŋ2 = 0.023).
Experiment 2: Lexical decision task
Experiment 2 tested phonological processing in a lexical decision task with words and two types of nonwords: pseudohomophones and control pseudowords. Behaviourally, the pseudohomophone effect shows up as slower reaction times and/or more errors for pseudohomophones compared to control pseudowords40. In electrophysiological measures, the N400, a component with a typically centro-parietal distribution, has been associated with semantic incongruence and semantic processing in written word recognition44. Reading nonwords compared with words elicits a more negative amplitude around 400ms45. However, phonology can modulate the ERP response46: nonwords that sound like real words—pseudohomophones—attenuate the N400 amplitude38,47,48. The reduced N400 effect for pseudohomophones suggests that the phonological representation facilitates semantic integration in visual word recognition49. We expect to replicate the results from previous experiments in the hearing group: a pseudohomophone effect in the behavioural and ERP responses. If deaf skilled readers use phonological encoding during lexical access, we expect similar results for this group also. Conversely, if deaf readers are not sensitive to phonological codes, we expect their responses to pseudohomophones not to differ from those of pseudowords, with similar error rates and reaction times, and a similar N400 amplitude.
Behavioural measures
Error rates
The deaf group was more accurate than the hearing group (F1(1, 38) = 10.05, p = 0.003; F2(1, 237) = 30.37, p < 0.001, ŋ2 = 0.209; see Table 1 and Fig. 2). The effect of WordType (word, pseudohomophone, pseudoword) was also significant (F1(2, 76) = 21.70, p < 0.001; F2(2, 237) = 16.00, p < 0.001, ŋ2 = 0.363), indicating that participants made the highest number of errors with pseudohomophones as compared to the other two types of stimuli (words: t(117) = 4.46, p < 0.001; pseudowords: t(117) = 4.36, p < 0.001). The interaction Group × WordType (F1(2, 76) = 15.24, p < 0.001; F2(2, 237) = 24.22, p < 0.001, ŋ2 = 0.286) showed that this difference was present in the hearing group (pseudohomophones vs. words: t(114) = 6.57, p < 0.001; pseudohomophones vs. pseudowords: t(114) = 6.50, p < 0.001), but not in the deaf group (pseudohomophones vs. words: t(114) = 0.62, p = 0.950; pseudohomophones vs. pseudowords: t(114) = 0.53, p = 0.950).
RTs
The deaf group was faster than the hearing group (F1(1, 38) = 10.39, p = 0.003; F2(1, 237) = 424.59, p < 0.001, ŋ2 = 0.215; see Table 1 and Fig. 2). A main effect of WordType (F1(2, 76) = 93.22, p < 0.001; F2(2, 237) = 90.53, p < 0.001, ŋ2 = 0.710) indicated that responses to the words were faster as compared to the two types of nonwords (pseudohomophones: t(117) = 4.08, p < 0.001; pseudowords: t(117) = 3.34, p = 0.002). Importantly, this effect interacted with Group (F1(2, 76) = 6.57, p = 0.014; F2(2, 237) = 8.03, p = 0.023, ŋ2 = 0.147), suggesting that the difference between pseudohomophones and words was statistically more evident in the hearing group as compared to the deaf group (hearing: t(114) = 3.99, p < 0.001; deaf: t(114) = 2.42, p = 0.024), while the difference between pseudowords and words was similarly present in both groups (hearing: t(114) = 2.63, p = 0.018; deaf: t(114) = 2.62, p = 0.018). These behavioural results were confirmed when by-item and by-participant variability were accounted for by mixed linear models analyses (see Supplementary Materials S2).
ERPs
300–500 ms
There was a main effect of WordType (F(2, 76) = 18.31, p < 0.001, ŋ2 = 0.325, see Fig. 3), suggesting that the greatest negativity was elicited by the pseudowords, which were followed by the pseudohomophones and the words (pseudowords vs. pseudohomophones: t(39) = 2.88, p = 0.007; pseudohomophones vs. words: t(39) = 3.44, p = 0.003; pseudowords vs. words: t(39) = 6.29, p < 0.001). The interactions between WordType and the topographical factors (Type × Anteriority: F(4, 152) = 9.89, p < 0.001, ŋ2 = 0.206; WordType × Laterality: F(4, 152) = 3.62, p = 0.010, ŋ2 = 0.087; WordType × Anteriority × Laterality: F(8, 304) = 2.10, p = 0.057, ŋ2 = 0.052) indicated that these differences were more evident over central right electrodes (all ts > 2.60), which is compatible with the N400 distribution. In addition, the factor WordType interacted with Group (Group x WordType: F(2, 76) = 3.47, p = 0.036, ŋ2 = 0.084; Group × WordType × Anteriority: F(4, 152) = 3.10, p = 0.037, ŋ2 = 0.075), indicating different N400 effects for deaf and hearing participants. While in the hearing group only pseudowords elicited a greater N400 as compared to words and pseudohomophones (pseudowords vs. words: t(19) = 3.93, p = 0.003; pseudowords vs. pseudohomophones: t(19) = 3.96, p = 0.003; pseudohomophones vs. words: t(19) = 0.84, p = 0.413), in the deaf group both pseudowords and pseudohomophones elicited a greater N400 as compared to words (pseudowords vs. words: t(19) = 5.09, p < 0.001; pseudohomophones vs. words: t(19) = 4.12, p = 0.003; pseudowords vs. pseudohomophones: t(19) = 1.09, p = 0.413). These differences were more evident over central and posterior sites (hearing: all ts > 3.00; deaf: all ts > 4.00). Similar ERP results were obtained using the same amount of items for each level of the factor WordType (see Supplementary Materials S3).
ERP results for Experiment 2 (lexical decision task). Top: grand average ERP waveforms for deaf (left) and hearing (right) groups. Midline electrodes (Fz, Cz, Pz) are displayed. Bottom: N400 topographical maps for 300–500 ms created by subtracting the average voltage of words from the average voltage of pseudowords, and the average voltage of words from the average voltage of pseudohomophones. PW Pseudowords, PH Pseudohomophones, W Words.
Discussion
The pseudohomophone effect in hearing readers provides clear evidence of phonological activation in word reading. In experiment 1, pseudohomophone primes modulated hearing readers’ N250 response, indicating that phonological representations participate in sub-lexical processing. In experiment 2, hearing readers made significantly more errors in classifying pseudohomophones as nonwords compared to control pseudowords, and their ERP response also showed a reduced N400 effect for pseudohomophones compared to control pseudowords, replicating previous studies that showed that phonological information interferes in the process of lexical decision38,39,41.
In contrast to the hearing readers, the skilled deaf readers in this study showed no difficulty in classifying pseudohomophones as nonwords: they showed no difference in error rates (or reaction times) or in the N400 response for pseudohomophones and control pseudowords. These deaf readers treated pseudohomophones as pseudowords; the fact that they sound like real words created no interference. We take this as evidence that these skilled deaf readers do not need to activate phonological coding during word reading. Even so, phonological coding might occur but not be apparent (because another, more effective mechanism achieves the word recognition process independently). Two pieces of evidence suggest that this is not the case. Firstly, in the semantic categorization task, pseudohomophones did not modulate deaf readers’ N250, suggesting that even at the sub-lexical level phonological coding is not activated. Secondly, in the lexical decision task, deaf readers responded significantly faster than hearing readers across all conditions. Previous studies have reported faster reaction times in word reading tasks for deaf compared to hearing readers20,50,51. Deaf individuals show enhanced reactivity in simple visual detection tasks52 and this may explain part of this advantage. An additional contributing factor for the discrepancy between deaf and hearing readers in word detection tasks may be precisely that phonological competitors are not activated for deaf readers, making word recognition faster51,53. This proposal fits with the general trend for faster, more proficient reading to rely less on phonological encoding and more on orthographic chunking13,54.
These results provide converging behavioural and electrophysiological evidence that these skilled deaf readers read words without activating phonology, as has already been shown behaviourally for other languages with transparent orthographies, such as German or Turkish19. This finding casts doubt on the assumption that deaf individuals need to access phonology in reading tasks to be competent readers21. Although some studies show a correlation between phonology and reading skills in deaf individuals24,26,55,56, our finding adds neuroimaging evidence to the body of studies that question the importance of phonological coding for proficient reading in the prelingually deaf16,19,20,30,31,32,34,35,57,58,59. Central to this issue is the type of deaf reader: this study has focused on highly skilled deaf readers, that is, individuals who have overcome the condition of deafness to acquire competent reading skills. Other deaf readers may have different processing mechanisms; a previous study with deaf readers of Spanish who were not highly skilled did find evidence of phonological encoding37.
This leads to an important caveat for our finding in terms of what it tells us about deaf readers: our electrophysiological data support the claim that highly skilled deaf readers do not need to—and in fact do not—automatically activate phonological encoding. We have examined the end state of the process of acquiring reading but make no claims about the process of learning to read. For hearing learning readers, phonology seems to play an important role14. The evidence for phonological coding in deaf children learning to read is mixed60,61 and more work is required to understand what phonology does and does not contribute to reading for different deaf individuals, but growing evidence suggests that the difference may lie at the sentence or discourse level rather than the lexical level19. This caveat limits the implications of this study for educational practice. Explicit training of phonological awareness may not help deaf individuals to achieve high reading proficiency22,24,31. Deaf readers do not need phonology during automatic stages of reading processing, suggesting that these readers avail of alternative strategies. One possibility is a visual, purely orthographic representation, supported by neuroimaging evidence that deaf readers have finely tuned orthographic representations but weak phonological sensitivity in the reading network62; another is mediation of the written word through the sub-lexical units of sign language9,63. Future studies should investigate how these processes support skilled deaf readers in acquiring a high level of literacy and how these mechanisms could be exploited and fostered to improve reading skills in deaf students.
To conclude, our findings tell us something important about reading in general. Contrary to previous claims17,18, phonological mediation is not necessarily an automatic part of reading a language with a transparent orthography. Skilled deaf readers in the current study did not activate phonological codes, showing that phonological access is not necessary for competent reading in Spanish. Even though phonological knowledge may facilitate learning to read a transparent orthography, the present results demonstrate that this does not mean that phonology is required during skilled reading.
Methods
Experiment 1: semantic categorization task
Participants
Twenty adult severely (70–90 dB) to profoundly (> 90 dB) deaf adults (14 females; age range 23–45 years old; mean 33; SD 7) participated in the study. All participants were born deaf or lost their hearing before the age of 3 (i.e., prelingual deafness); none had cochlear implants. They learned Spanish Sign Language before the age of 10 and used it as the main language for communication. Most of them learned to read at an early age, at school, except two individuals who learned to read after the age of 16. Twenty hearing Spanish readers (10 females; age range = 20–42 years old; mean 29; SD 6) were included as a control group. All deaf participants and hearing controls were skilled readers in Spanish (i.e., with performances above the 75th centile of the ECL-2 Test64) and the two groups did not differ in non-verbal intelligence, Spanish reading comprehension, and Spanish vocabulary size (see Table 2). Before taking part in the experiment all participants signed an informed consent form that was approved by the BCBL Ethics Committee. The experiment was performed following the BCBL Ethics Committee’s guidelines and regulations.
Materials
The experimental targets were 80 Spanish words (4–6 letters long; mean number of letters 5.29; mean log word frequency 3.96, range 2.61–4.65) selected from the EsPal database65. The targets were preceded by nonword primes that could be: (1) pseudohomophones (40 items), which were created by replacing one letter of the target word by another letter that corresponded to the same phoneme (e.g., the pseudohomophone nobio created from the base word novio [boyfriend]), and (2) pseudowords (40 items), in which one letter of the target was replaced by another letter that corresponded to a different sound (e.g., the pseudoword notio created from the same target word). Two lists were constructed such that each target word used to generate the nonwords was presented once in each list, either as a pseudohomophone or as a pseudoword.
In addition, we selected an additional 20 Spanish words (4–6 letters long) corresponding to animal names (mean log word frequency 3.49, range 2.26–4.50; mean number of letters: 4.50), each of which was paired with a Spanish word with the same number of letters (mean log word frequency 3.16, range 2.51–3.50; mean number of letters 4.50). Each word pair was not semantically related and was presented twice, once with the animal name as target and once with the animal name as prime. This was done to control for prime visibility.
Participants were randomly assigned to one of the two lists. In total, each participant completed 120 trials: 80 experimental trials with nonword-prime/word-target pairs (40 pseudohomophone primes and 40 pseudoword primes), 20 trials with word-prime/animal-target pairs, and 20 trials with animal-prime/word-target pairs.
Procedure
The experiment was run in a silent and dimly lit room using Presentation software (version 0.70). Each trial began with a fixation cross for 500 ms, followed by a forward mask consisting of a row of hash marks (#####) for 500 ms (the number of hash marks matched the length of the prime). Then, a prime appeared in lowercase (25-pt. Courier New) and remained on the screen for 50 ms. The prime was followed immediately by the target stimulus, also in lowercase. Stimuli subtended a visual angle of 1.9 to 2.9 degrees depending on the number of letters. The target remained on the screen for 1500 ms or until the participant’s response (see Fig. 4). Participants were instructed to press a button on the keyboard (space bar) only when they saw an animal name, and to respond as quickly and accurately as possible. The order of presentation of the stimuli was randomized for each participant.
EEG recording and analysis
The electroencephalogram (EEG) was recorded with a 32-channel BrainAmp system (Brain Products GmbH) at a 500 Hz sampling rate. Scalp voltages were collected from 27 Ag/AgCl electrodes placed in an EasyCap recording cap (Fp1, Fp2, F7, F8, F3, F4, FC5, FC6, FC1, FC2, T7, T8, C3, C4, CP5, CP6, CP1, CP2, P3, P4, P7, P8, O1, O2, Fz, Cz, Pz). Two external electrodes were placed on the mastoids, with the right one as the online reference. An additional electrode at FCz served as ground, and four electrodes (two above and below the right eye, and two on the ocular canthi) recorded the electro-oculogram (EOG). Impedance was kept below 5KΩ for mastoids and scalp electrodes, and below 10KΩ for EOG electrodes. The EEG signal was re-referenced offline to the average activity of the two mastoids. A notch filter and a bandpass filter were applied (50 Hz, 0.01–30 Hz, 12 dB/octave). Ocular artifacts were corrected using Independent Component Analysis (ICA): the EEG of each subject was decomposed into independent components and those that explained the highest variance of EOG channels were identified and visually inspected before being removed from the original signal. Only trials corresponding to the experimental items were included in the analysis: all animal go trials and experimental trials with incorrect go-responses were excluded. For the remaining trials, EEG epochs of 1200 ms time-locked to the target presentation onset were obtained (including 200 ms pre-stimulus baseline). Residual artifacts exceeding ± 100 µV in amplitude were removed. Overall, 8.56% of trials were rejected, with no differences across experimental conditions (F(1,78) = 0.17, p = 0.68). The remaining clean epochs were averaged across conditions, with a baseline correction between – 200 and 0 ms.
We analysed the data using R66. A repeated-measure analysis of variance (ANOVA) was applied to the N250 time window, which was defined based on visual inspection (230–270 ms). An additional late time window was added to the analysis after visually inspecting the ERP grand averages (500–700 ms). The average activity of three contiguous electrodes was calculated resulting in nine clusters: Left Anterior (F3, F7, FC5), Left Central (C3, T7, CP5), Left Posterior (P3, P7, O1), Medial Anterior (Fp1, Fp2, Fz), Medial Central (FC1, FC2, Cz), Medial Posterior (CP1, CP2, Pz), Right Anterior (F4, F8, FC6), Right Central (C4, T8, CP6), Right Posterior (P4, P8, O2). To probe the scalp distribution of any effects, these clusters were included in the statistical analyses as different levels of two topographical factors: Anteriority (Anterior, Central, Posterior) and Laterality (Left, Middle, Right). Each ANOVA included the within-subject factor of PrimeType (Pseudohomophone, Pseudoword), the between-subject factor of Group (Deaf, Hearing), and the two topographical factors (Anteriority and Laterality). The Greenhouse–Geisser procedure was applied when the sphericity assumption was violated. Effects of topographical factors will be reported only when they interact with the experimental factors. Two-tailed t-tests were conducted as follow-up analyses and corrected for multiple comparisons67.
Experiment 2: lexical decision task
Participants
The participants were the same as those in Experiment 1.
Materials
For word trials, we selected 80 Spanish words between four and six letters long (mean number of letters 5; average log frequency 3.57, range 3.01–3.87) from the EsPal database65. For nonword trials, 80 Spanish base words between four and six letters long (mean number of letters 5.29) with a similar frequency to the first set were also selected (mean log word frequency 3.96, range 2.61–4.65). These words were then used to create two types of nonwords: (1) pseudohomophones by replacing one letter by another letter that corresponded to the same phoneme (e.g., the pseudohomophone javón created from the base word jabón [soap]), and (2) pseudowords in which one letter was replaced by another letter that corresponded to a different sound (e.g., the pseudoword jacón created from the same base word). Two lists were constructed such that each base word used to generate the nonwords was presented once in each list, either as a pseudohomophone or as a pseudoword. Participants were randomly assigned to the two lists. In total, each participant completed 160 trials: 80 trials with words and 80 trials with nonwords (40 pseudohomophones and 40 pseudowords).
Procedure
The experiment was run in a silent and dimly lit room using Presentation software (version 0.70). Each trial began with a fixation cross at the centre of the screen for 500 ms, followed by a blank screen for 200 ms. Then, a stimulus (word or nonword) was presented in lowercase font (45-pt. Courier New, subtending a visual angle of 3.2 to 5.0 degrees) for 1500 ms or until the participant’s response (see Fig. 5). Participants were instructed to press one of two buttons on the keyboard (‘M’ and ‘Z’) to indicate whether the letter string was a word or a nonword. They were asked to respond as quickly and accurately as possible. The order of presentation of the stimuli was randomized for each participant and the two response buttons were counterbalanced for word and nonword responses. Participants completed eight practice trials before starting the experiment.
EEG recording and analysis
The EEG recording and pre-processing were the same as in Experiment 1. ERPs time-locked to the onset of the stimulus presentation were obtained only for the trials followed by a correct behavioural response. Overall, the average number of rejected ERP trials was 11.17%. Statistical analyses were conducted on both behavioural and ERP data.
We analysed the data using R66 and the lme4 package68. For the behavioural data, we calculated error rates and reaction times (RTs). Trials with incorrect responses (5.94%) were excluded from RT analysis. RTs that were more than 2.5 SDs away from the mean of each condition and each participant were also excluded from the analysis of the response latencies (2.28%). A by-subject repeated-measures ANOVA was conducted on the error rates and the RTs including Group (Deaf, Hearing) as a between-subject factor, and WordType (Word, Pseudohomophone, Pseudoword) as a within subject factor. Similar ANOVAs were conducted by item, including Group as a within-item factor and WordType as a between-item factor.
For the EEG data, a repeated-measures ANOVA was conducted on the average ERP amplitude within the N400 time window (300–500 ms). This analysis included Group (Deaf, Hearing) as a between-subject factor and WordType (Word, Pseudohomophone, Pseudoword) as a within-subject factor. As in Experiment 1, two topographical factors were also added (Anteriority, Laterality). The Greenhouse–Geisser procedure was applied when the sphericity assumption was violated. Effects of topographical factors will be reported only when they interact with the experimental factors. In both behavioural and EEG analyses, two-tailed t-tests were conducted as follow-up analyses and corrected for multiple comparisons67.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Carreiras, M. et al. An anatomical signature for literacy. Nature 461, 983–986 (2009).
Dehaene, S., Cohen, L., Morais, J. & Kolinsky, R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci. 16, 234–244 (2015).
Doctor, E. A. & Coltheart, M. Children’s use of phonological encoding when reading for meaning. Mem. Cognit. 8, 195–209 (1980).
Frith, U. Beneath the surface of developmental dyslexia. In Surface Dyslexia: Neuropsychological and Cognitive Studies of Phonological Reading (eds Patterson, K. E. et al.) 301–330 (Taylor & Francis, Milton Park, 2017). https://doi.org/10.4324/9781315108346.
Reitsma, P. Sound priming in beginning readers. Child Dev. 55, 406–423 (1984).
Coltheart, M., Rastle, K., Perry, C., Langdon, R. & Ziegler, J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol. Rev. 108, 204–256 (2001).
Frost, R. Toward a strong phonological theory of visual word recognition: true issues and false trails. Psychol. Bull. 123, 71–99 (1998).
Van Orden, G. C., Johnston, J. C. & Hale, B. L. Word identification in reading proceeds from spelling to sound to meaning. J. Exp. Psychol. Learn. Mem. Cogn. 14, 371–386 (1988).
Clark, M. D. et al. The importance of early sign language acquisition for deaf readers. Read. Writ. Q. 32, 127–151 (2016).
Ehri, L. C. Sources of difficulty in learning to spell and read. Adv. Dev. Behav. Pediatr. 7, 121–195 (1986).
Harm, M. W. & Seidenberg, M. S. Computing the meanings of words in reading: cooperative division of labor between visual and phonological processes. Psychol. Rev. 111, 662–720 (2004).
Share, D. L. Phonological recoding and self-teaching: sine qua non of reading acquisition. Cognition 55, 151–218 (1995).
Joseph, H. S. S. L., Liversedge, S. P., Blythe, H. I., White, S. J. & Rayner, K. Word length and landing position effects during reading in children and adults. Vision Res. 49, 2078–2086 (2009).
Tiffin-Richards, S. P. & Schroeder, S. Word length and frequency effects on children’s eye movements during silent reading. Vision Res. 113, 33–43 (2015).
Katz, L. & Frost, R. Orthography, phonology, morphology, and meaning: an overview. Adv Psychol 94, 1–8 (1992).
Miller, P. et al. Factors distinguishing skilled and less skilled deaf readers: evidence from four orthographies. J. Deaf Stud. Deaf Educ. 17, 439–462 (2012).
Carreiras, M., Perea, M., Vergara, M. & Pollatsek, A. The time course of orthography and phonology: ERP correlates of masked priming effects in Spanish. Psychophysiology 46, 1113–1122 (2009).
Pollatsek, A., Perea, M. & Carreiras, M. Does conal prime canal more than cinal? Masked phonological priming effects in Spanish with the lexical decision task. Mem. Cognit. 33, 557–565 (2005).
Kargin, T. et al. Differences in word processing skills of deaf and hearing individuals reading in different orthographies. J. Dev. Phys. Disabil. 24, 65–83 (2012).
Hanson, V. L. & Fowler, C. A. Phonological coding in word reading: evidence from hearing and deaf readers. Mem. Cognit. 15, 199–207 (1987).
Perfetti, C. A. & Sandak, R. Reading optimally builds on spoken language: implications for deaf readers. J. Deaf Stud. Deaf Educ. 5, 32–50 (2000).
Nielsen, D. C. & Luetke-Stahlman, B. Phonological awareness: one key to the reading proficiency of deaf children. Am. Ann. Deaf 147, 11–19 (2002).
Aparicio, M., Gounot, D., Demont, E. & Metz-Lutz, M.-N. Phonological processing in relation to reading: an fMRI study in deaf readers. Neuroimage 35, 1303–1316 (2007).
Campbell, R. & Wright, H. Deafness, spelling and rhyme: how spelling supports written word and picture rhyming skills in deaf subjects. Q. J. Exp. Psychol. 40, 771–788 (1988).
Colin, C., Zuinen, T., Bayard, C. & Leybaert, J. Phonological processing of rhyme in spoken language and location in sign language by deaf and hearing participants: a neurophysiological study. Clin. Neurophysiol. 43, 151–160 (2013).
Dyer, A., MacSweeney, M., Szczerbinski, M., Green, L. & Campbell, R. Predictors of reading delay in deaf adolescents: the relative contributions of rapid automatized naming speed and phonological awareness and decoding. J. Deaf Stud. Deaf Educ. 8, 215–229 (2003).
MacSweeney, M., Goswami, U. & Neville, H. The neurobiology of rhyme judgment by deaf and hearing adults: an ERP study. J. Cogn. Neurosci. 25, 1037–1048 (2013).
Transler, C., Leybaert, J. & Gombert, J. Do deaf children use phonological syllables as reading units?. J. Deaf Stud. Deaf Educ. 4, 124–143 (1999).
Waters, G. & Doehring, D. G. The nature and role of phonological information in reading acquisition: Insights from congenitally deaf children who communicate orally BT—Reading and its development: component skills approach. In Reading and its development: component skills approach (eds Carr, T. & Levy, B. A.) 220–232 (Academic Press, Cambridge, 1990).
Miller, P. & Clark, M. D. Phonemic awareness is not necessary to become a skilled deaf reader. J. Dev. Phys. Disabil. 23, 459–476 (2011).
Izzo, A. phonemic awareness and reading ability: an investigation with young readers who are deaf. Am. Ann. Deaf 147, 18–28 (2002).
Mayberry, R. I., del Giudice, A. A. & Lieberman, A. M. Reading achievement in relation to phonological coding and awareness in deaf readers: a meta-analysis. J. Deaf Stud. Deaf Educ. 16, 164–188 (2011).
Fariña, N., Duñabeitia, J. A. & Carreiras, M. Phonological and orthographic coding in deaf skilled readers. Cognition 168, 27–33 (2017).
Bélanger, N. N., Baum, S. R. & Mayberry, R. I. Reading difficulties in adult deaf readers of French: phonological codes, not guilty!. Sci. Stud. Read. 16, 263–285 (2012).
Bélanger, N. N., Mayberry, R. I. & Rayner, K. Orthographic and phonological preview benefits: parafoveal processing in skilled and less-skilled deaf readers. Q. J. Exp. Psychol. 66, 2237–2252 (2013).
Emmorey, K., Weisberg, J., McCullough, S. & Petrich, J. A. F. Mapping the reading circuitry for skilled deaf readers: an fMRI study of semantic and phonological processing. Brain Lang. 126, 169–180 (2013).
Gutierrez-Sigut, E., Vergara-Martínez, M. & Perea, M. Early use of phonological codes in deaf readers: an ERP study. Neuropsychologia 106, 261–279 (2017).
Briesemeister, B. B. et al. The pseudohomophone effect: evidence for an orthography–phonology-conflict. Neurosci. Lett. 455, 124–128 (2009).
Ferrand, L. & Grainger, J. Effects of orthography are independent of phonology in masked form priming. Q. J. Exp. Psychol. 47, 365–382 (1994).
Rubenstein, H., Lewis, S. S. & Rubenstein, M. A. Evidence for phonemic recoding in visual word recognition. J. Verbal Learn. Verbal Behav. 10, 645–657 (1971).
Ziegler, J. C., Jacobs, A. M. & Klüppel, D. Pseudohomophone effects in lexical decision: still a challenge for current word recognition models. J. Exp. Psychol. Hum. Percept. Perform. 27, 547–559 (2001).
Holcomb, P. J. & Grainger, J. On the time course of visual word recognition: an event-related potential investigation using masked repetition priming. J. Cogn. Neurosci. 18, 1631–1643 (2006).
Grainger, J., Kiyonaga, K. & Holcomb, P. J. The time course of orthographic and phonological code activation. Psychol. Sci. 17, 1021–1026 (2006).
Kutas, M. & Federmeier, K. D. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP). Annu. Rev. Psychol. 62, 621–647 (2011).
Holcomb, P. J. Semantic priming and stimulus degradation: Implications for the role of the N400 in language processing. Psychophysiology 30, 47–61 (2007).
Kramer, A. F. & Donchin, E. Brain potentials as indices of orthographic and phonological interaction during word matching. J. Exp. Psychol. Learn. Mem. Cogn. 13, 76–86 (1987).
Holcomb, P. J. & Neville, H. J. Auditory and visual semantic priming in lexical decision: a comparison using event-related brain potentials. Lang. Cogn. Process. 5, 281–312 (1990).
Vergara-Martínez, M., Perea, M., Gómez, P. & Swaab, T. Y. ERP correlates of letter identity and letter position are modulated by lexical frequency. Brain Lang. 125, 11–27 (2013).
Newman, R. L. & Connolly, J. F. Determining the role of phonology in silent reading using event-related brain potentials. Cogn. Brain Res. 21, 94–105 (2004).
Brown, P. M. & Brewer, L. C. Cognitive processes of deaf and hearing skilled and less skilled readers. J. Deaf Stud. Deaf Educ. 1, 263–270 (1996).
Morford, J. P., Occhino-Kehoe, C., Piñar, P., Wilkinson, E. & Kroll, J. F. The time course of cross-language activation in deaf ASL–English bilinguals. Biling. Lang. Cogn. 20, 337–350 (2017).
Pavani, F. & Bottari, D. Visual abilities in individuals with profound deafness. In The Neural Bases of Multisensory Processes (eds Murray, M. M. & Wallace, M. T.) 423–448 (CRC Press/Taylor & Francis, Boca Raton, 2011). https://doi.org/10.1201/b11092-28.
Meade, G., Grainger, J., Midgley, K. J., Holcomb, P. J. & Emmorey, K. ERP Effects of masked orthographic neighbour priming in deaf readers. Lang. Cogn. Neurosci. 34, 1016–1026 (2019).
Grainger, J. & Ziegler, J. C. A dual-route approach to orthographic processing. Front. Psychol. 2, 54 (2011).
Colin, S., Magnan, A., Ecalle, J. & Leybaert, J. Relation between deaf children’s phonological skills in kindergarten and word recognition performance in first grade. J. Child Psychol. Psychiatry 48, 139–146 (2007).
Luetke-Stahlman, B. & Nielsen, D. C. The contribution of phonological awareness and receptive and expressive english to the reading ability of deaf students with varying degrees of exposure to accurate english. J. Deaf Stud. Deaf Educ. 8, 464–484 (2003).
Meade, G., Grainger, J., Midgley, K. J., Holcomb, P. J. & Emmorey, K. An ERP investigation of orthographic precision in deaf and hearing readers. Neuropsychologia 146, 107542 (2020).
Kyle, F. E. & Harris, M. Concurrent correlates and predictors of reading and spelling achievement in deaf and hearing school children. J. Deaf Stud. Deaf Educ. 11, 273–288 (2006).
Leybaert, J. & Alegria, J. Is word processing involuntary in deaf children?. Br. J. Dev. Psychol. 11, 1–29 (1993).
Ormel, E., Hermans, D., Knoors, H., Hendriks, A. & Verhoeven, L. Phonological activation during visual word recognition in deaf and hearing children. J. Speech Lang. Hear. Res. 53, 801–820 (2010).
Transler, C. & Reitsma, P. Phonological coding in reading of deaf children: pseudohomophone effects in lexical decision. Br. J. Dev. Psychol. 23, 525–542 (2005).
Glezer, L. S. et al. Orthographic and phonological selectivity across the reading system in deaf skilled readers. Neuropsychologia 117, 500–512 (2018).
Petitto, L. A. et al. Visual sign phonology: insights into human reading and language from a natural soundless phonology. Wiley Interdiscip. Rev. Cogn. Sci. 7, 366–381 (2016).
De la Cruz, M. V. ECL-2. Evaluación de la comprensión lectora. (Editorial TEA, 1999).
Duchon, A., Perea, M., Sebastián-Gallés, N., Martí, A. & Carreiras, M. EsPal: one-stop shopping for Spanish word properties. Behav. Res. Methods 45, 1246–1258 (2013).
R Core Development Team. R: A language and environment for statistical computing. Vienna, Austria (2019).
Hochberg, Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75, 800–802 (1988).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. https://doi.org/10.18637/jss.v067.i01 (2015).
Raven, J., Raven, J. C. & Court, J. H. Manual for Raven’s progressive Matrices and Vocabulary Scales Section 3s (Oxford Psychologists Press, Oxford, 1998).
Izura, C., Cuetos, F. & Brysbaert, M. Lextale-esp: Un test para la rápida y eficaz evaluación del tamaño del vocabulario en español. Psicológica 35, 49–67 (2014).
Lemhöfer, K. & Broersma, M. Introducing LexTALE: a quick and valid lexical test for advanced learners of english. Behav. Res. Methods 44, 325–343 (2012).
de Bruin, A., Carreiras, M. & Duñabeitia, J. A. The BEST Dataset of Language Proficiency. Front. Psychol. 8, 522 (2017).
Acknowledgements
The authors are grateful for assistance from CNSE (the Spanish National Body of Deaf People), various Deaf People’s Associations throughout Spain and two universities (Pompeu Fabra University in Barcelona and University of La Laguna in Tenerife), which provided facilities to run the experiments. The authors thank the study participants, without whom this work would not have been possible. The authors acknowledge financial support from the Basque Government through the BERC 2018-2021 program, the Spanish Ministry of Science and Innovation (Agencia Estatal de Investigación, AEI), through the Severo Ochoa Programme for Centres/Units of Excellence in R&D (SEV‐2015‐490); and Grants (PSI-2016-76435-P to B.C.; ACELERA RED2018-102615-T, SAPIENTIA-CM H2019/HUM-5705 and PGC2018-097145-B-I00 to J.A.D.; and RTI2018-093547-B-I00 to M.C.); and fellowships (Juan de la Cierva Fellowships FJCI-2017-31806 to B.C. and IJCI-2016-27702 to S.C., and predoctoral fellowship BES-2013-064140 to N.F. from the Spanish Ministry of Science and Innovation (AEI); and H2020-MSCA-IF-2018-837228 to S.C. from the European Commission).
Author information
Authors and Affiliations
Contributions
M.C., J.A.D., N.F. and B.C. conceived and designed the experiment; N.F. performed the experiments; S.C. analysed the data; M.C., J.A.D., B.C. and S.C. interpreted the data; B.C., S.C. and N.F. contributed to writing the manuscript: M.C. and J.A.D. provided critical revisions of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Costello, B., Caffarra, S., Fariña, N. et al. Reading without phonology: ERP evidence from skilled deaf readers of Spanish. Sci Rep 11, 5202 (2021). https://doi.org/10.1038/s41598-021-84490-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84490-5
This article is cited by
-
Electrophysiological signatures of spelling sensitivity development from primary school age to adulthood
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.