Abstract
Secondary hyperparathyroidism (sHPT) as a result of chronic kidney disease (CKD) is a common health problem and has been reported to manifest at the sacroiliac joints (SIJ). The aim of this investigation was to systematically assess sacroiliac joint changes in asymptomatic sHPT as detected by high-resolution CT. Included in this IRB-approved retrospective case–control study were 56 patients with asymptomatic sHPT as well as 259 matched controls without SIJ disease. Demographic data were retrieved from electronic patient records. High-resolution computed tomography datasets of all patients were subjected to a structured scoring, including erosions, sclerosis, osteophytes, joint space alterations and intraarticular calcifications. Chi2 tests were used to compare frequencies of lesions. Erosions were significantly more prevalent in patients with sHPT, and were found mainly in the ventral (28.6% vs. 13.9%; p = 0.016) and middle (17.9% vs. 7.7%; p = 0.040) iliac portions of the SIJ. Partial ankylosis was rare in both cohorts (3.6% vs. 5.0%; p > 0.999); complete ankylosis was not observed. Neither extent not prevalence of sclerosis or calcifications differed significantly between groups. Joint lesions reminiscent of sacroiliitis can be found in a substantial portion of asymptomatic patients with secondary hyperparathyroidism. Further investigations into the clinical significance of these findings are warranted.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a global health problem, estimated to affect up to 10% of the general population, with a rising prevalence over the last decades1,2. A frequent complication in patients requiring haemodialysis is the development of osteo-articular disease3, especially secondary hyperparathyroidism (sHPT)4. Parathyroid hormone (PTH) has a catabolic effect on bone metabolism and has been shown to decrease bone-mineral density of cortical bone5; longstanding elevated PTH levels may lead to osteitis fibrosis cystica, a high turnover bone disease presenting with lytic lesions on imaging as a result of replacement of mineralized bone with fibrous tissue6. These findings are most likely the reason why HPT is sometimes considered a differential diagnosis in sacroiliitis imaging7, as subchondral bone resorption may be difficult to distinguish from true erosions on imaging. Additionally, HPT is an important predisposing factor of calcium pyrophosphate dihydrate deposition (CPPD)8, which in turn manifests at the sacroiliac joints in up to 50% of CPPD patients and may cause bilateral erosions, joint space narrowing and sclerosis9,10.
A recent study by Tezcan et al. has investigated MRI features of asymptomatic primary hyperparathyroidism in 49 patients11 and found bone marrow edema in 16.3% of patients, though no significant difference to healthy controls could be detected. In terms of secondary hyperparathyroidism, published evidence of manifestations at the SIJs to date is limited to case reports12, investigations undertaken more than 30 years ago13 or radiographic studies14.
To our knowledge, no systematic investigation on the pattern of arthropathy of the SIJ in sHPT as detected by computed tomography has been undertaken thus far. The aim of this study was to systematically describe the pattern of arthropathy in asymptomatic secondary hyperparathyroidism compared the normal population.
Materials and methods
Ethical approval and patient consent
Prior to data acquisition, approval was attained from the ethics review board of the Charité Universitätsmedizin Berlin (EA1/300/19). Due to the retrospective nature of the investigation the ethics review board waived individual written informed consent. Consent for use of de-identified imaging data in scientific publications was obtained as a routine practice in our institution. The study was conducted in compliance with the Declaration of Helsinki and local legislation and ethical standards.
Patients
Included into the case-group of this retrospective case–control study were patients with known secondary hyperparathyroidism who had received a computed tomography of abdomen and pelvis between March 2016 and March 2019. Clinical data was retrieved from electronic patient records. Patients with known disease of the sacroiliac joints, known rheumatic disease, cutaneous psoriasis, inflammatory bowel disease, uveitis, malignancy of the skeletal system (both primary and metastatic) and fractures of the pelvis were excluded. The patients were matched with subjects from an existing, retrospectively acquired cohort from our institution, who had undergone imaging during the same period and for whom the same clinical information (except parameters of sHPT) were available. Indications for CT examinations in the case group were oncological staging (15/56), infection (10/56), trauma/bleeding (5/56) and other, including evaluation before kidney donation as recipient (27/56). Indications for CT examination in the control group were oncological staging (130/259), infection (83/259), trauma/bleeding (6/259) and other, including evaluation before kidney donation (27/56). Matching was performed in a ration of 4 controls for every case.
Imaging technique
For all CT scans a special reconstruction of the pelvic skeleton was available, yielding images in a similar quality as in dedicated SIJ imaging. All image datasets were scored by one radiological resident with 7 years of experience in MSK imaging (KZ), blinded for all clinical data. Images were read using dedicated software (Horos v3.3.6, The Horos Project, public license) in random order and predominantly in oblique-coronal and axial orientation, using bone-window settings.
Scoring system
Expanding on previous work of our research group15, a scoring system was used that divides the sacroiliac joints into 12 joint regions on each side. In each region, erosions and sclerosis were assessed in a categorical fashion, laid out in Table 1.
Additionally, joint space alterations (including pseudo-widening and ankylosis) and intraarticular calcifications (both per side) as well as osteophytes (ventral and dorsal, separately for each side) were scored. Prior to scoring, an atlas was assembled from example patients not included in this analysis, which was used as a reference during scoring. A teaching session on 15 test cases (not included in the study) was carried out with a consultant radiologist with expertise in MSK radiology (TD) before commencement of the scoring process. In order to calculate inter- and intra-reader reliability, a random sample of 50 study patients was scored by both junior (KZ) and senior reader (TD)—in case of the junior reader a second time.
Statistical analysis
Statistical analyses were carried out using SPSS Version 25 (IBM Corporation, New York, USA). Patients were matched with controls using the dedicated propensity score matching tool; age, gender and weight were chosen as covariables, with a defined tolerance of 0.01. Scoring results were summarised as sum scores for each structural lesion separately; on the patient level, positivity for erosions was defined as a sum score ≥ 2. Frequencies of structural lesions were compared using Chi2 tests. Sum scores of structural lesions were compared using Mann–Whitney-U tests. Intraclass-correlation coefficients were calculated for inter- and intra-reader reliability between readers, using a two-way mixed model ICC(3,2)16 on sum scores for each lesion type. A significance level of p < 0.05 was assumed for all tests.
Results
Patients’ characteristics
A total of 315 patients (56 sHPT, 259 controls) were included in this investigation; a summary of patient flow and clinical characteristics is provided as Fig. 1. As per study design, mean age and gender distribution did not differ between groups.
Patient flow. sHPT secondary hyperparathyroidism, GFR glomerular filtration rate, SD standard deviation, iPTH intact parathyroid hormone, ADPKD autosomal dominant polycystic kidney disease, GN glomerulonephritis. Distribution of clinical characteristics was compared between sHPT and controls with appropriate statistical tests (t-test, Chi2 test)—no significant differences were detected.
Frequency and distribution of structural lesions
A complete table of frequencies of structural lesions as well as mean sum scores is provided as Table 2.
Erosions were observed in significantly more sHPT patients than their matched controls (11/56 vs. 10/259; p < 0.001). The difference was most pronounced in the iliac joint portions with 28.6% vs. 13.9% ventrally (p = 0.001), 17.9% vs. 7.7% in the middle (p = 0.040) and 10.7% vs. 3.5% dorsally (p = 0.033). Neither sclerosis nor joint space alterations including partial ankylosis differed in frequency and extent between sHPT patients controls; there was no instance of complete ankylosis in either cohort of this investigation. Intraarticular calcifications were seen in 26.8% of sHPT patients and 17.4% of controls—the difference was not statistically significant (p = 0.132). Osteophytes were more prevalent in controls than sHPT patients with 51.7% vs. 32.1% (p = 0.008) in the dorsal aspect of the joint. Imaging examples from the patient cohort are supplied as Fig. 2.
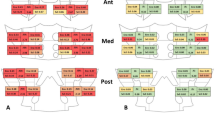
Example of joint alterations in sHPT. Axially reconstructed high-resolution CT images. (A) Healthy control patient with normal SIJ. (B) Patient with secondary hyperparathyroidism: note the subchondral bone resorption mimicking erosions (black arrowheads) as well as the sclerotic rim around the resorption zone (white arrowheads). (B) Patient with secondary hyperparathyroidism: note the irregular, pseudo-widened left joint space (black arrowheads) compared to the regular joint space on the right.
Inter-and intra-reader agreement
Interreader reliability, interpreted according to Koo et al.16, was moderate for erosions (0.57; 95% CI 0.25–0.76; p = 0.002), good for sclerosis (0.78; 95% CI 0.64–0.87; p < 0.001), moderate for joint space alterations (0.74; 95% CI 0.53–0.85; p < 0.001), good for osteophytes (0.83; 95% CI 0.71–0.91; p < 0.001) and good for calcifications (0.85; 95% CI 0.74–0.91; p < 0.001). Intrareader reliability was good for erosions (0.77; 95% CI 0.59–0.87; p < 0.001), good for sclerosis (0.88; 95% CI 0.78–0.93; p < 0.001), good for joint space alterations (0.82; 95% CI 0.68–0.90; p < 0.001), good for osteophytes (0.87; 95% CI 0.77–0.93; p < 0.001) and good for calcifications (0.86; 95% CI 0.76–0.92; p < 0.001).
Discussion
To our knowledge the data presented here is the first systematic exploration of structural lesions of the sacroiliac joints of patients with asymptomatic sHPT. We found a significantly higher prevalence of erosions in sHPT patients, while ankylosis and sclerosis were equally rare in both groups. These findings carry significance in the context of sacroiliitis imaging, where erosions are considered a specific finding of inflammatory joint disease.
Our findings somewhat contradict those of Tezcan et al.11, who examined inflammatory MRI lesions in primary hyperparathyroidism and did not find significant differences between HPT patients and controls. The most likely explanation for this incongruence is that standard SIJ-MRI imaging has known deficits in the depiction of small structural lesions17, such as erosions. Osteophytes, which are considered degenerative rather than inflammatory joint lesions were significantly less prevalent in the sHPT group. This is surprising, as osteoarthritis is considered a common finding in patients with advanced CKD3, and patients were matched with regards to predisposing factors for mechanical SIJ disease such as obesity and parity in women18. A possible explanation may be a comparably larger proportion of immobile patients in the sHPT group. Another unexpected finding is the similar prevalence of joint space calcifications in both groups—as hyperparathyroidism is a known risk factor for developing CPPD8, a comparably higher percentage of sHPT patients with articular calcifications would have been expected. In our opinion, these findings support the hypothesis, that SIJ changes in sHPT are most likely manifestations of osteitis fibrosis cystica rather than sacroiliac CPPD.
Due to the retrospective nature of the investigation, data on back pain in sHPT patients could only be captured from electronic patients records, so that we cannot exclude the possibility that some of the patients may in fact have symptomatic joint disease, somewhat limiting the validity of our results. The results are limited further by the small size of the patient group—larger, prospective cohorts with more detailed information on severity and duration of the secondary hyperparathyroidism, including information on vitamin D, calcium and alkaline phosphatase are needed to illicit the factors that contribute to the development of the lesions described in this analysis.
In conclusion, secondary hyperparathyroidism can mimic sacroiliitis on SIJ CT in a substantial portion of patients and should be considered when assessing joints for inflammatory changes. Further investigations into the clinical significance of these lesions for affected patients are warranted.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Liyanage, T. et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385, 1975–1982. https://doi.org/10.1016/S0140-6736(14)61601-9 (2015).
Mills, K. T. et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 88, 950–957. https://doi.org/10.1038/ki.2015.230 (2015).
Akasbi, N. et al. Rheumatic complications of long term treatment with hemodialysis. Rheumatol. Int. 32, 1161–1163. https://doi.org/10.1007/s00296-010-1756-z (2012).
Naveh-Many, T. & Volovelsky, O. Parathyroid cell proliferation in secondary hyperparathyroidism of chronic kidney disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21124332 (2020).
Silverberg, S. J. et al. Skeletal disease in primary hyperparathyroidism. J. Bone Miner. Res. 4, 283–291. https://doi.org/10.1002/jbmr.5650040302 (1989).
Yuen, N. K., Ananthakrishnan, S. & Campbell, M. J. Hyperparathyroidism of renal disease. Perm J. 20, 15–127. https://doi.org/10.7812/TPP/15-127 (2016).
Tuite, M. J. Sacroiliac joint imaging. Semin. Musculoskelet. Radiol. 12, 72–82. https://doi.org/10.1055/s-2008-1067939 (2008).
Zhang, W. et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: Terminology and diagnosis. Ann. Rheum. Dis. 70, 563–570. https://doi.org/10.1136/ard.2010.139105 (2011).
Resnick, D. Diagnosis of Bone and Joint Disorders. (Saunders, 2002).
Littlejohn, G. O., Baron, M. & Urowitz, M. B. Sacroiliac joint abnormalities in calcium pyrophosphate crystal deposition disease. Rheumatol. Int. 1, 195–198. https://doi.org/10.1007/BF00541176 (1982).
Tezcan, M. E. et al. Evaluation of acute and chronic MRI features of sacroiliitis in asymptomatic primary hyperparathyroid patients. Clin. Rheumatol. 35, 2777–2782. https://doi.org/10.1007/s10067-016-3172-6 (2016).
Peces, R., Gil, F., Gonzalez, F. & Ablanedo, P. Multiple brown tumors in a female hemodialyzed patient with severe secondary hyperparathyroidism. Nefrologia 22, 79–82 (2002).
Hooge, W. A. & Li, D. CT of sacroiliac joints in secondary hyperparathyroidism. J. Can. Assoc. Radiol. 32, 42–44 (1981).
Shih, C., Chen, K. H., Chang, C. Y., Jim, Y. F. & Chang, T. Articular manifestations of renal osteodystrophy. Zhonghua Yi Xue Za Zhi (Taipei) 52, 372–377 (1993).
Diekhoff, T. et al. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: Results from the SIMACT study. Ann. Rheum. Dis. 76, 1502–1508. https://doi.org/10.1136/annrheumdis-2016-210640 (2017).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163. https://doi.org/10.1016/j.jcm.2016.02.012 (2016).
Diekhoff, T. et al. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: Results from the SIMACT study. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2016-210640 (2017).
Poddubnyy, D. et al. Clinical and imaging characteristics of osteitis condensans ilii as compared with axial spondyloarthritis. Rheumatology (Oxford) https://doi.org/10.1093/rheumatology/keaa175 (2020).
Acknowledgements
The authors thank the Berlin Institute of Health for providing essential infrastructure for data collection. They also thank Mr Robert Röhle, MSc for valuable input on statistical analysis. All authors declare to have no conflict of interest.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization, V.K. and K.Z.; methodology, V.K., L.L. and K.Z.; validation, T.D., D.P., L.L. and K.-G. H.; formal analysis, K.Z., V.K., D.P. and L.L.; investigation, V.K., T.D., L.L., D.P., K.-G.H. and K.Z.; resources, K.-G. H..; data curation, V.K., L.L. and K.Z.; writing—original draft preparation, V.K.; writing—review and editing, V.K., T.D., L.L., D.P., K.-G.H. and K.Z..; visualization, K.Z., V.K. and T.D.; supervision and project administration, K.Z.. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kreutzinger, V., Diekhoff, T., Liefeldt, L. et al. Asymptomatic secondary hyperparathyroidism can mimic sacroiliitis on computed tomography. Sci Rep 11, 4323 (2021). https://doi.org/10.1038/s41598-021-83989-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83989-1
This article is cited by
-
Chirurgie des Hyperparathyreoidismus
Die Chirurgie (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.