Abstract
Porous hollow fibres made of polyvinylidene fluoride were employed as membrane contactor for carbon dioxide (CO2) absorption in a gas–liquid mode with methyldiethanolamine (MDEA) based nanofluid absorbent. Both theoretical and experimental works were carried out in which a mechanistic model was developed that considers the mass transfer of components in all subdomains of the contactor module. Also, the model considers convectional mass transfer in shell and tube subdomains with the chemical reaction as well as Grazing and Brownian motion of nanoparticles effects. The predicted outputs of the developed model and simulations showed that the dispersion of CNT nanoparticles to MDEA-based solvent improves CO2 capture percentage compared to the pure solvent. In addition, the efficiency of CO2 capture for MDEA-based nanofluid was increased with rising MDEA content, liquid flow rate and membrane porosity. On the other hand, the enhancement of gas velocity and the membrane tortuosity led to reduced CO2 capture efficiency in the module. Moreover, it was revealed that the CNT nanoparticles effect on CO2 removal is higher in the presence of lower MDEA concentration (5%) in the solvent. The model was validated by comparing with the experimental data, and great agreement was obtained.
Similar content being viewed by others
Introduction
Fossil fuel consumption has been increased over the last 150 years to meet its demand for power generation. However, rising fuel consumption has caused considerable emissions of greenhouse gases especially carbon dioxide (CO2), which is one of the current major issues in the world dealing with environmental impact. It can lead to climate change, and a gradual increase in globe temperature and consequently has a negative impact on the world population, environmental health and economic development1. Therefore, development of effective and environmental-friendly technology to store or capture CO2 has great significance from the environmental point of view. Various CO2 capture procedures, including physical and chemical absorption2, solid adsorption3, cryogenic4 and membrane technology5 have been utilized to treat gas streams containing CO2 and other pollutant gases.
Chemical absorption in aqueous amine solvents is the most common approach for CO2 capture at industrial scale because of its advantages such as high efficiency and mature process6. Methyldiethanolamine (MDEA) is a tertiary amine and cost-effective absorbent which is widely used for CO2 removal owing to its acceptable CO2 absorption capacity, carbamate formation, low corrosion rate, and lower heat regeneration7. There is direct contact between amine solutions and gas phase in conventional systems, causing some difficulties, including weeping, foaming, entrainment, flooding, and excessive loading. Furthermore, high energy consumption and their complexity are their main drawbacks. Membrane technology can be utilized as a promising method for the separation of CO2 and other gaseous pollutants from various gas mixtures to overcome the drawbacks of traditional separation techniques8.
Dispersion of nanoparticles (NPs) into either organic or aqueous solvents has been proposed as one of the useful ways to enhance CO2 absorption7,9. It has been revealed that CO2 removal enhances by 4.5% and 5.6% when there is Al2O3 and SiO2 NPs in methanol solvent at 20 °C, respectively9. The mass transfer coefficient for CO2 absorption would be improved when NPs are added to the absorbent solution. The interphase boundary layer mixing because of the presence of NP and Grazing effect phenomena occurs when NPs are properly dispersed in the solvent media10,11,12,13. NPs dispersion increases the fluid turbulence in the gas–liquid boundary layer and subsequently improves CO2 mass transfer rate14. Grazing effect means a strong affinity of CO2 molecules into dispersed NPs and then reduction of gas concentration in the solvent, which can increase driving force between liquid and gas phases15. Brownian motion of NPs in the solvent results in inducing micro-convections in the liquid which would improve the mass transfer performance of the employed process16.
In terms of theoretical evaluation, mechanistic transport phenomena modeling of hollow fibre membrane contactor have been widely investigated to understand the effect of operating conditions, membrane specification, solvent type, and etc.17,18,19. Indeed, model-based process development approach has been employed for process intensification and improvement of separation efficiency. However, in the previous developed models, Grazing and Brownian motion of NPs effects which happen in the presence of NPs were not considered in detail.
A DEAB-based nanofluid (NF) for carbon dioxide removal was studied in a hollow fibre membrane contactor, confirming the dispersion of carbon nanotube (CNT) and SiO2 NPs considerably increase the removal rate. The effect of adding a number of NPs to water on the CO2 absorption was investigated by Peyravi et al.20 and it was found that solvent velocity and NP content had significant influences on CO2 removal from the gas phase. As it can be seen, using NPs in solvent for improvement of CO2 absorption in Hollow-Fiber Membrane Contactors (HFMCs) is increasing. Therefore, it will be highly important to investigate these systems to obtain a detailed understanding of the process. Computational fluid dynamics (CFD) is recognized as a sophisticated computational technique for understanding transport phenomena in different processes, and has attracted much attention.
In the current work, a mechanistic model and simulation were developed to investigate NPs presence in an amine solvent (MDEA) on the CO2 absorption in a HFMC. Changes in CNT concentration and operation conditions on the CO2 mass transfer rate were studied in terms of carbon dioxide recovery from the gas stream. A numerical method based on finite element discretization scheme was implemented to solve the nonlinear system’s equations. The modeling outputs were compared with measured results to evaluate validity of the developed mechanistic method.
Experimental
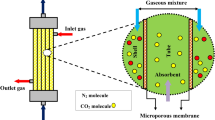
The polyvinylidene fluoride (PVDF) fibres were made using the thermally induced phase inversion technique. Then, the prepared PVDF fibres were packed in perspex glass as a shell of the module. It was supplied by Sign Trade. It should be pointed out that the blockage of the fibres was checked before packing into the module. Then, the top and bottom sides of the contactor were sealed by resin. The CNTs nanoparticles were provided by Cheap Tubes Ins. Company (USA). In CO2 absorption experiments, the gas phase flow rate was adjusted using a flow controller, whereas the liquid flow rate was set via a Masterflex L/S Digital Pump (Cole Parmer). At the exit of gas stream, a CO2 Analyzer (CAI-600 Seri, Gas Analyzers, USA) was employed to find the CO2 content in the gas stream. The gas-phase passed through the shell-side of HFMC, and the MDEA based NF flew through the tube section in a counter-current configuration. The volumetric flow rate of the CO2 and N2 mixture was measured using mass flow controller. The NPs were stable in the solution without adding any surfactant. The diagram of the used experimental system for membrane CO2 absorption was provided in Fig. 1. Experimental conditions are given in Table 1.
To prepare the solvent for the gas absorption experiments, solution of MDEA was initially prepared at 3 different percentages of 5, 10, and 20 wt%. Then, 0.5 wt% of CNT particles was added to each sample and sonicated by the high-intensity ultrasonic liquid processor for 60 min to stabilize it. The mixture was then ready to be utilized in the experiments as solvent where no surfactant was required to stabilize the suspension21,22,23 . Table 2 lists properites of the PVDF hollow fiber membrane used in the absorption process in this study.
Model development
Figure 2 illustrates a schematic demonstration of CO2 absorption process into MDEA-based NF using a microporous/hydrophobic (non-wetted) PVDF contactor module. To simplify the model development, one fibre is considered, which is then divided into three subdomains: i.e. tube subdomain (NF), polymeric membrane, and shell subdomain (N2 and CO2 mixture). As can be observed from Fig. 2, MDEA-based NF flows in the tube subdomain, whereas the gas mixture fed to the shell subdomain in the opposite direction. The mechanistic model is built axis-symmetrically (two dimensional) because of the non-existence of angular gradient6. Here, we used Happel’s formula to estimate the shell side radius (Fig. 2) around the fibre24:
where φ denotes the void fraction. The membrane specification was provided in Table 3. The following assumptions are used for process modeling:
-
Steady-state and isothermal fluid flow.
-
Gas velocity profile is fully developed.
-
Ideal gas behavior was considered.
-
Laminar flow for liquid phase in the system.
-
Gas mixture only fills the fiber pores at all operating conditions.
Shell side's equations
The mathematical formula that describes the transport of CO2 molecules from the gas phase (N2 and CO2 mixture) to MDEA-based NF is the continuity equation, which can be expressed as25,26:
where C, R and N refer to the CO2 concentration, reaction rate, and mass flux, respectively. The mass transfer flux is calculated using6,26:
where D is the diffusivity and Vz is the velocity. The steady-state mass transfer equation in the shell side is then derived as27:
Velocity field is computed using27:
where p is the pressure in the shell compartment of contactor. The density (ρ) and viscosity (µ) of the fluid is assumed to be constant. The symbol \({ }V_{z - shell} { }\) denotes the velocity in the axial coordinate. Also, the following equation is used for non-compressible fluid27:
Membrane’s equations
The main equation for transport of CO2 through the fiber pores considering gas filled pores can be expressed as follows27,28:
Here, the fibers are considered to be filled with the gas phase due to the hydrophobic polymer used as the membrane and controlling the operational conditions6.
Diffusivity of CO2 in the membrane pores is obtained as6,28:
where tortuosity factor (\(\uptau\)) is calculated using the fiber porosity (ε)29:
Tube side's equations
Dispersion of CNT NPs will enhance the mass transfer rate in the system due to the synergistic effects. To consider Brownian and Grazing effects into developed mathematical model and simulation, several possible mechanisms were proposed in the literature for gas absorption in NFs. The diffusion coefficient for the MDEA-based nanofluid can be expressed as30,31:
The modified diffusion coefficient is written as follows30,31 :
The symbol \(\varphi\) denotes NP volume fraction in the liquid solvent. Sc (Schmidt) and Re (Reynolds) dimensionless numbers can be determined as follows32:
where K, dp, and Db,f are Boltzmann constant, NP diameter (8 nm), and carbon dioxide diffusion coefficient (1.45 × 10–9 m2/s), respectively.
To involve the Grazing effect in the developed model and its effect on CO2 mass transfer in the membrane contactor, the MDEA-based solvent phase was considered as two distinct solid and liquid and phases. Therefore, three mass transfer equations were derived and used in the developed model. Continuity equation of CO2 in the solid phase is derived as22,33:
where CS denotes CO2 concentration at the solid–liquid interface and q refers to the amount of CO2 adsorbed by the CNT NPs, which is determined with Langmuir isotherm33:
where qm (29.45 mol/kg) refers to the maximum adsorption by CNT NPs, kd (0.00049 m3/mol) is Langmuir constant. In Eq. 15, the symbol \(\alpha_{p}\) is the specific surface area of NP and kp is the mass-transfer coefficient between solid CNT nanoparticles and MDEA solvent, which is estimated as33:
The effective density of NF is written as33:
In addition, the mass transfer equation for CO2 in the MDEA-based nanofluid (steady-state) and MDEA solvent is expressed as follows27,33:
Velocity distribution in the tube is determined using27:
where \({\overline{\text{V}}}\) is the average velocity. MDEA structure, as well as the reaction rate of CO2 with MDEA are listed in Table 4. The reaction constant and reaction rate units are (m3 mol−1 s−1) and (mol1 m−3 s−1).
Table 5 provides the boundary conditions of mass and momentum transfer equation for the tube, membrane, and shell subdomains of the contactor.
Numerical solution
For solving the governing equations of MDEA-based solvent and gas phase, a finite element method is employed via COMSOL Multiphysics 5.4. The adaptive meshing and error control were employed, and PARDISO solver as one of the effective solvers for simulating membrane systems was employed to minimize the calculations errors35,36. It was found that the numerical solution time for solving the governing mass and momentum equations was about 3 min.
Results and discussion
Model validation
The mathematical model’s findings were verified by comparing with experimental data in terms of CO2 removal percentage. The CO2 absorption in the contactor module for different solvents containing MDEA reactant and MDEA-based nanofluid are presented in Figs. 3 and 4. There has been a great agreement between experimental data and modeling values for both membrane contactors with and without CNT nanoparticles. As observed, increasing solvent and MDEA-based nanofluid flow rate enhances the removal of carbon dioxide37. The liquid without NPs and MDEA-based nanofluid velocity can change the convection mass flux and consequently improves the overall mass transfer of carbon dioxide from the gas phase to the solvent phase. Figures 3 and 4 also indicated that MDEA-based nanofluid flow rate shows a considerable impact on the removal rate. In the system without NPs, increasing solvent flow rate from 10 to 40 ml/min led to the enhancement of carbon dioxide from 38.46 to 46.17%, 45.71 to 51.12%, and 53.82 to 57.56% for 5, 10, and 20% MDEA concentrations, respectively.
For the membrane contactor with 0.5 wt% CNT NPs, it is observed increase in CO2 removal from 41.28 to 51.49%, 47.11 to 55.00%, and 54.71 to 60.35% when MDEA-based nanofluid is increased from 10 to 40 ml/min for three different MDEA concentrations. Dispersion of 0.5 wt% CNT NPs into base fluid increases CO2 removal by 5.32, 3.88, 2.70% in the presence of 5, 10, and 20% MDEA when liquid flow rate is 40 ml/min. The effect of CNT NPs on CO2 removal is higher when there is lower MDEA concentration in the liquid phase.
Gas flow rate effect on CO2 absorption
Based on the literature, one of the main advantages of the membrane technology is simple scale-up, since it is not difficult to find the surface area compared to conventional absorption processes. The enhancement of the gas flow rate can positively impact CO2 flux by reducing the mass transfer resistance. On the other hand, the residence time of the gas in the contactor is reduced with a rising gas velocity which results in the reduction of CO2 absorption efficiency. Figure 5 illustrates the influence of gas mixture flow rate on the carbon dioxide absorption at three different MDEA concentrations, including 5, 10, and 20 wt% As it was expected, increasing gas mixture flow rates from 10 to 50 ml/min reduces CO2 capture by 31%, 35%, and 40% in the presence of 0.5 wt.% CNT NPs and 5%, 10%, and 20% MDEA. Furthermore, increasing MDEA concentration increases CO2 removal. Given that the reaction kinetics of MDEA with carbon dioxide is of elementary type (Table 3), it depends directly on MDEA concentration.
CO2 diffusive and convective flux distribution in the gas on the shell subdomain of the contactor is illustrated for three different gas mixture flow rates, including 5, 10, 15 ml/min in Fig. 6. As it can be seen, the contribution of convective flux along the membrane contactor is significant in comparison with axial diffusive flux and the convective flux is maximum in the centre of the shell side where the gas velocity is maximum. This could be due to the fact that the velocity is predominant which can result in high convective flux38. Figure 6 illustrates that both diffusive and convective fluxes are reduced along the fiber length because of reducing the driving force in the z-direction. In addition, the convective flux is about 7 times higher than diffusive flux at higher gas flow rates.
CO2 concentration distribution in the contactor
Concentration distribution of CO2 in 3 subdomains of the contactor at three different MDEA concentrations is indicated in Fig. 7. The concentration profile of CO2 is presented in r-direction. It is observed that concentration reduction of carbon dioxide in the microporous membrane subdomain, as well as the MDEA-based nanofluid, is significant, while there is not any significant change of the concentration profile in the shell side which gas stream flows. This phenomenon is due to the amount of diffusion coefficient in the gas phase, which is 10 and 104 times higher than membrane and tube subdomains of membrane contactor, respectively. Moreover, it is indicated in Fig. 7 that CO2 concentration reduction in the membrane subdomain is higher when MDEA concentration is high. There is a sharp reduction in CO2 concentration near the membrane surface for all three different MDEA concentrations. As it can be seen, the change in CO2 concentration in the membrane subdomain is considerable, so it will be useful to investigate membrane specifications such as porosity and tortuosity on the CO2 absorption to enhance the separation efficiency.
Effect of microporous membrane porosity and tortuosity
The effect of microporous membrane porosity on the CO2 removal using MDEA-based nanofluid is illustrated in Fig. 8. The fibre tortuosity was calculated using Eq. (10) for each amount of porosity. It was seen that the enhancement of the CO2 removal from gas stream can happen with increasing the membrane porosity. This is because of increasing diffusivity factor in the fibre pores with increasing porosity and consequently decrement of the mass transfer resistance for the transport of CO2 from gas to the solvent phase39. Furthermore, the slope of CO2 removal is sharper when the porosity is between 0.2 and 0.5, after that, the slope was decreased. It can be said that the used membrane (porosity = 0.4585) in this study is suitable for CO2 absorption using MDEA-based nanofluid. It should also be paid attention that with increasing porosity, the stability of membrane decreases, therefore, the optimum porosity should be selected based on operating condition. Moreover, the amount of tortuosity decreases with increasing the fibre porosity. It means that the removal percentage is decreased by enhancing the membrane tortuosity factor. Based on Eq. (9), the diffusion coefficient in the membrane subdomain is becoming less with increasing tortuosity factor and subsequently, the mass transfer resistance for CO2 diffusion through the microporous membrane would be increased40,41.
Conclusion
A robust and reliable mechanistic model and simulation methodology was implemented to study the effects of CNT nanoparticles dispersion into MDEA-based solvent on the performance of the hollow fibre membrane contactor in terms of CO2 removal. In the current study, Brownian as well as Grazing mechanisms were taken into account as the main mechanisms of mass-transfer improvement in the separation system. There was a great agreement between the simulation results and experimental data reported in the literature. The CO2 removal increased from 41.28 to 51.49%, 47.11 to 55.00%, and 54.71 to 60.35% with the enhancement of MDEA concentration from 5 to 20% in the range of 10–40 ml/min MDEA-based nanofluid flow rate. The carbon dioxide absorption was increased by 14% with increasing porosity from 0.2 to 0.7, while it was decreased from 52.93% to 22.58% when the membrane tortuosity increased from 1 to 25. In addition, the main mass transfer resistance was in the tube subdomain where MDEA-based fluid flows.
References
Seo, S., Lages, B. & Kim, M. Catalytic CO2 absorption in an amine solvent using nickel nanoparticles for post-combustion carbon capture. J. CO2 Util. 36, 244–252. https://doi.org/10.1016/j.jcou.2019.11.011 (2020).
Dave, A., Dave, M., Huang, Y., Rezvani, S. & Hewitt, N. Process design for CO2 absorption from syngas using physical solvent DMEPEG. Int. J. Greenh. Gas Control 49, 436–448. https://doi.org/10.1016/j.ijggc.2016.03.015 (2016).
Ho, M. T., Allinson, G. W. & Wiley, D. E. Reducing the cost of CO2 capture from flue gases using pressure swing adsorption. Ind. Eng. Chem. Res. 47, 4883–4890. https://doi.org/10.1021/ie070831e (2008).
Mehrpooya, M., Esfilar, R. & Moosavian, S. M. A. Introducing a novel air separation process based on cold energy recovery of LNG integrated with coal gasification, transcritical carbon dioxide power cycle and cryogenic CO2 capture. J. Clean. Prod. 142, 1749–1764. https://doi.org/10.1016/j.jclepro.2016.11.112 (2017).
Chen, G. et al. Mass transfer performance and correlation for CO2 absorption into aqueous 3-diethylaminopropylamine solution in a hollow fiber membrane contactor. Chem. Eng. Process. Process Intensif. 152, 107932. https://doi.org/10.1016/j.cep.2020.107932 (2020).
Shirazian, S., Taghvaie Nakhjiri, A., Heydarinasab, A. & Ghadiri, M. Theoretical investigations on the effect of absorbent type on carbon dioxide capture in hollow-fiber membrane contactors. PLoS ONE 15, e0236367 (2020).
Rahmatmand, B., Keshavarz, P. & Ayatollahi, S. Study of absorption enhancement of CO2 by SiO2, Al2O3, CNT, and Fe3O4 nanoparticles in water and amine solutions. J. Chem. Eng. Data 61, 1378–1387. https://doi.org/10.1021/acs.jced.5b00442 (2016).
Gabelman, A. & Hwang, S.-T. Hollow fiber membrane contactors. J. Membr. Sci. 159, 61–106. https://doi.org/10.1016/S0376-7388(99)00040-X (1999).
Lee, J. W., Jung, J.-Y., Lee, S.-G. & Kang, Y. T. CO2 bubble absorption enhancement in methanol-based nanofluids. Int. J. Refrig. 34, 1727–1733. https://doi.org/10.1016/j.ijrefrig.2011.08.002 (2011).
Pishnamazi, M. et al. ANFIS grid partition framework with difference between two sigmoidal membership functions structure for validation of nanofluid flow. Sci. Rep. 10, 15395. https://doi.org/10.1038/s41598-020-72182-5 (2020).
Nguyen, Q., Taghvaie Nakhjiri, A., Rezakazemi, M. & Shirazian, S. Thermal and flow visualization of a square heat source in a nanofluid material with a cubic-interpolated pseudo-particle. ACS Omega 5, 17658–17663. https://doi.org/10.1021/acsomega.0c02173 (2020).
Rezakazemi, M., Darabi, M., Soroush, E. & Mesbah, M. CO2 absorption enhancement by water-based nanofluids of CNT and SiO2 using hollow-fiber membrane contactor. Sep. Purif. Technol. 210, 920–926. https://doi.org/10.1016/j.seppur.2018.09.005 (2019).
Hajilary, N. & Rezakazemi, M. CFD modeling of CO2 capture by water-based nanofluids using hollow fiber membrane contactor. Int. J. Greenh. Gas Control 77, 88–95. https://doi.org/10.1016/j.ijggc.2018.08.002 (2018).
Kars, R. L., Best, R. J. & Drinkenburg, A. A. H. The sorption of propane in slurries of active carbon in water. Chem. Eng. J. 17, 201–210. https://doi.org/10.1016/0300-9467(79)80104-5 (1979).
Saidi, M. CO2 absorption intensification using novel DEAB amine-based nanofluids of CNT and SiO2 in membrane contactor. Chem. Eng. Process. Process Intensif. 149, 107848. https://doi.org/10.1016/j.cep.2020.107848 (2020).
Krishnamurthy, S., Bhattacharya, P., Phelan, P. E. & Prasher, R. S. Enhanced mass transport in nanofluids. Nano Lett. 6, 419–423. https://doi.org/10.1021/nl0522532 (2006).
Cao, F., Gao, H., Ling, H., Huang, Y. & Liang, Z. Theoretical modeling of the mass transfer performance of CO2 absorption into DEAB solution in hollow fiber membrane contactor. J. Membr. Sci. 593, 117439. https://doi.org/10.1016/j.memsci.2019.117439 (2020).
Rosli, A., Shoparwe, N. F., Ahmad, A. L., Low, S. C. & Lim, J. K. Dynamic modelling and experimental validation of CO2 removal using hydrophobic membrane contactor with different types of absorbent. Sep. Purif. Technol. 219, 230–240. https://doi.org/10.1016/j.seppur.2019.03.030 (2019).
Sohaib, Q., Muhammad, A., Younas, M. & Rezakazemi, M. Modeling pre-combustion CO2 capture with tubular membrane contactor using ionic liquids at elevated temperatures. Sep. Purif. Technol. 241, 116677. https://doi.org/10.1016/j.seppur.2020.116677 (2020).
Peyravi, A., Keshavarz, P. & Mowla, D. Experimental investigation on the absorption enhancement of CO2 by various nanofluids in hollow fiber membrane contactors. Energy Fuels 29, 8135–8142. https://doi.org/10.1021/acs.energyfuels.5b01956 (2015).
Jiang, J., Zhao, B., Zhuo, Y. & Wang, S. Experimental study of CO2 absorption in aqueous MEA and MDEA solutions enhanced by nanoparticles. Int. J. Greenh. Gas Control 29, 135–141. https://doi.org/10.1016/j.ijggc.2014.08.004 (2014).
Golkhar, A., Keshavarz, P. & Mowla, D. Investigation of CO2 removal by silica and CNT nanofluids in microporous hollow fiber membrane contactors. J. Membr. Sci. 433, 17–24. https://doi.org/10.1016/j.memsci.2013.01.022 (2013).
Rahimi, K., Riahi, S. & Abbasi, M. Effect of host fluid and hydrophilicity of multi-walled carbon nanotubes on stability and CO2 absorption of amine-based and water-based nanofluids. J. Environ. Chem. Eng. 8, 103580. https://doi.org/10.1016/j.jece.2019.103580 (2020).
Happel, J. Viscous flow relative to arrays of cylinders. AIChE 5, 174–177 (1959).
Poling, B. E., Prausnitz, J. M. & O’Connell, J. P. The Properties of Gases and Liquids 5th edn. (McGraw-Hill, New York, 2004).
Faiz, R. & Al-Marzouqi, M. Insights on natural gas purification: Simultaneous absorption of CO2 and H2S using membrane contactors. Sep. Purif. Technol. 76, 351–361. https://doi.org/10.1016/j.seppur.2010.11.005 (2011).
Bird, R. B., Stewart, W. E. & Lightfoot, E. N. Transport Phenomena, vol. 16 2nd edn. (Wiely, Hoboken, 2002).
Faiz, R. & Al-Marzouqi, M. Mathematical modeling for the simultaneous absorption of CO2 and H2S using MEA in hollow fiber membrane contactors. J. Membr. Sci. 342, 269–278 (2009).
Srisurichan, S., Jiraratananon, R. & Fane, A. Mass transfer mechanisms and transport resistances in direct contact membrane distillation process. J. Membr. Sci. 277, 186–194 (2006).
Nagy, E., Feczkó, T. & Koroknai, B. Enhancement of oxygen mass transfer rate in the presence of nanosized particles. Chem. Eng. Sci. 62, 7391–7398 (2007).
Bahmanyar, A., Khoobi, N., Moharrer, M. M. A. & Bahmanyar, H. Mass transfer from nanofluid drops in a pulsed liquid–liquid extraction column. Chem. Eng. Res. Des. 92, 2313–2323 (2014).
Prasher, R., Bhattacharya, P. & Phelan, P. E. Thermal conductivity of nanoscale colloidal solutions (nanofluids). Phys. Rev. Lett. 94, 025901 (2005).
Lu, S., Xing, M., Sun, Y. & Dong, X. Experimental and theoretical studies of CO2 absorption enhancement by nano-Al2O3 and carbon nanotube particles. Chin. J. Chem. Eng. 21, 983–990. https://doi.org/10.1016/S1004-9541(13)60550-9 (2013).
Haimour, N., Bidarian, A. & Sandall, O. C. Kinetics of the reaction between carbon dioxide and methyldiethanolamine. Chem. Eng. Sci. 42, 1393–1398. https://doi.org/10.1016/0009-2509(87)85011-X (1987).
Pishnamazi, M. et al. Molecular investigation into the effect of carbon nanotubes interaction with CO2 in molecular separation using microporous polymeric membranes. Sci. Rep. 10, 13285. https://doi.org/10.1038/s41598-020-70279-5 (2020).
Razavi, S. M. R., Rezakazemi, M., Albadarin, A. B. & Shirazian, S. Simulation of CO2 absorption by solution of ammonium ionic liquid in hollow-fiber contactors. Chem. Eng. Process. Process Intensif. 108, 27–34. https://doi.org/10.1016/j.cep.2016.07.001 (2016).
Rezakazemi, M. et al. CFD simulation of natural gas sweetening in a gas–liquid hollow-fiber membrane contactor. Chem. Eng. J. 168, 1217–1226. https://doi.org/10.1016/j.cej.2011.02.019 (2011).
Qatezadeh Deriss, A., Langari, S. & Taherian, M. Computational fluid dynamics modeling of ibuprofen removal using a hollow fiber membrane contactor. Environ. Prog. Sustain. Energy 40, e13490. https://doi.org/10.1002/ep.13490 (2020).
Zhang, W. et al. Effect of porosity on mass transfer of gas absorption in a hollow fiber membrane contactor. J. Membr. Sci. 470, 399–410. https://doi.org/10.1016/j.memsci.2014.06.059 (2014).
Sohaib, Q. et al. Rigorous non-isothermal modeling approach for mass and energy transport during CO2 absorption into aqueous solution of amino acid ionic liquids in hollow fiber membrane contactors. Sep. Purif. Technol. 254, 117644. https://doi.org/10.1016/j.seppur.2020.117644 (2021).
Younas, M. et al. Post-combustion CO2 capture with sweep gas in thin film composite (TFC) hollow fiber membrane (HFM) contactor. J. CO2 Util. 40, 101266. https://doi.org/10.1016/j.jcou.2020.101266 (2020).
Acknowledgements
Saeed Shirazian gratefully acknowledges the supports by the Government of the Russian Federation (Act 211, contract 02.A03.21.0011) and the Ministry of Science and Higher Education of the Russian Federation (Grant FENU-2020-0019).
Author information
Authors and Affiliations
Contributions
Y.C.: Conceptualization, Analysis. Z.U.R.: Experiments, Analysis, Measurements. N.G.: Supervision, Revision, Experiments, Analysis, Resources. M.A-M.: Supervision, Data analysis, Methodology. N.A.: Measurements, Experiments, Validation. A.T.N.: Writing-draft, Data curing, Software. M.G: Simulations, Validation, Resources. M.R.: Conceptualization, Modeling, Software, Writing-review. A.M.: Supervision, Project administration. M.P.: Writing-draft, Analysis, Validation. S.S.: Supervision, Funding acquisition, Revision, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, Y., Rehman, Z.U., Ghasem, N. et al. Intensification of CO2 absorption using MDEA-based nanofluid in a hollow fibre membrane contactor. Sci Rep 11, 2649 (2021). https://doi.org/10.1038/s41598-021-82304-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82304-2
This article is cited by
-
Performance enhancement of hollow fiber membrane contactors for CO2 absorption using MEA-based functionalized nanofluids
Microfluidics and Nanofluidics (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.