Abstract
Hepatic pedicle clamping reduces intraoperative blood loss and the need for transfusion, but its long-term effect on survival and recurrence remains controversial. The aim of this meta-analysis was to evaluate the effect of the Pringle maneuver (PM) on long-term oncological outcomes in patients with primary or metastatic liver malignancies who underwent liver resection. Literature was searched in the Cochrane Central Register of Controlled Trials (CENTRAL), Medline (via PubMed), and Web of Science databases. Survival was measured as the survival rate or as a continuous endpoint. Pooled estimates were represented as odds ratios (ORs) using the Mantel–Haenszel test with a random-effects model. The literature search retrieved 435 studies. One RCT and 18 NRS, including 7480 patients who underwent liver resection with the PM (4309 cases) or without the PM (3171 cases) were included. The PM did not decrease the 1-year overall survival rate (OR 0.86; 95% CI 0.67–1.09; P = 0.22) or the 3- and 5-year overall survival rates. The PM did not decrease the 1-year recurrence-free survival rate (OR 1.06; 95% CI 0.75–1.50; P = 0.75) or the 3- and 5-year recurrence-free survival rates. There is no evidence that the Pringle maneuver has a negative effect on recurrence-free or overall survival rates.
Similar content being viewed by others
Introduction
Liver resection remains the only curative treatment for hepatic malignancies, and can improve long-term survival1. Improvements in surgical techniques, better selection of patients, and improved perioperative care have increased the number of hepatectomies performed worldwide each year1,2. There is growing evidence that excessive blood loss during hepatectomy and the subsequent need for blood transfusions may contribute to a poor outcome for non-cirrhotic and cirrhotic liver resections1,2. Perioperative blood transfusion has been associated with recurrence and poorer long-term survival due to an immune response dysfunction3.
Vascular occlusion techniques have been used by some surgeons during hepatic resection to minimize intraoperative blood loss, especially in large tumors or tumors that are adjacent to major vessels4,5. Pringle described a technique whereby transient hepatic inflow was occluded by clamping the portal triad. Portal clamping in the Pringle maneuver (PM) has been modified several times in form of intermittent portal clamping6,7 and selective portal clamping8. These modifications can control intraoperative blood loss and decrease the need for transfusion. Some surgeons believe that this reduction in the rate of blood transfusions can improve long-term oncological outcomes. On the other hand, some argue that the PM may increase the risk of ischemia–reperfusion injury to the liver, which may impair hepatocyte function4,6,7.
The present systematic review and meta-analysis aimed to evaluate the effect of the PM on long-term oncological outcomes in patients with primary or metastatic liver malignancies who underwent liver resection.
Results
Literature search strategy and included studies
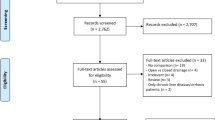
The literature search retrieved 435 studies excluding duplicates. Of these, 416 papers were excluded for various reasons, including redundant information and insufficient data on survival. In the end, 19 articles were included in the current meta-analysis (Fig. 1). During the primary evaluation, included articles were subdivided into three groups regarding their suggestions and conclusion on the effect of PM on oncological outcomes of the patients: in favor of the PM, neutral, and not in favor of the PM (Fig. 2).
Risk of bias assessment for included studies
Of the 19 articles included in this meta-analysis, only one was an RCT. This study included 80 patients (39 cases with PM and 41 cases without PM). The other 18 NRS included 7400 patients (4270 cases with PM and 3130 cases without PM). All studies were published between 2002 and 2020 (Table 1). As shown in Table 2, most studies had moderate bias.
Recurrence-free survival rate
One-year recurrence-free survival rate
One-year RFS rates were reported for 6758 patients from 17 studies (4223 patients were in the PM group and 2744 patients in the non-PM group). The recurrence of malignant hepatic lesions was reported in 1023 cases (24.2%) in the PM group and in 742 cases (27%) in the non-PM group. Meta-analysis indicated that the PM did not decrease 1-year RFS rate (OR 1.06; 95% CI 0.75–1.50; P = 0.75; Fig. 3A) using a random-effects model. There was considerable heterogeneity among the studies (I2 = 84%; P < 0.00001).
Three-year recurrence-free survival rate
Recurrence of malignant lesions during the first 3 years after hepatectomy was reported in 6138 cases from 15 studies. Of these, recurrence was reported in 2037 patients (54.6%) in the PM group and in 1233 patients (51.1%) in the non-PM group. Meta-analysis revealed no significant difference in 3-year RFS rate between the groups (OR 0.99; 95% CI 0.74–1.34; P = 0.97) using the random-effects model (Fig. 3B). The studies that reported 3-year RFS rates were not homogeneous (I2 = 81%; P < 0.00001).
Five-year recurrence-free survival rate
A total of 14 studies with 3781 patients in the PM group and 2591 patients in the non-PM group reported 5-year recurrence. As is seen in Fig. 3C, recurrence was reported in 2521 patients (66.67%) in the PM group and in 1862 patients (71.86%) in the non-PM group. The meta-analysis showed that 5-year RFS rate is not significantly different between the two groups (OR 0.82; 95% CI 0.65–1.04; P = 0.1) using the random-effects model (Fig. 3C). The studies that reported 5-year RFS rates were not homogeneous (I2 = 67%; P = 0.0002).
Overall survival rates
One-year overall survival rate
Fifteen studies including 5569 patients reported the 1-year OS rate. Of these, 2776 patients (87.15%) were in the PM group and 2092 patients (87.7%) were in the non-PM group. According to our analysis using the random-effects model, the 1-year OS rate was not significantly different between the PM and non-PM group (OR 0.86; 95% CI 0.67–1.09; P = 0.22) (Fig. 4A). The I2 was 31% with a P value of 0.12.
Three- and five-year overall survival rates
The 3-year OS rate was 64% in the PM group and 63.6% in the non-PM group. The 5-year OS rate was 46.11% in the PM group and 42.9% in the non-PM group (Fig. 4B,C). Meta-analysis indicated that the 3- and 5-year OS rates were not significantly different between the PM and non-PM groups.
Subgroup analysis
The type of malignant tumor (i.e., primary or metastatic) had no significant effect on the 1-year RFS rate in the PM and non-PM groups (primary tumors: OR 1.16; 95% CI 0.86–1.56; P = 0.34; metastatic tumors: OR 0.82; 95% CI 0.43–1.56; P = 0.56), nor did it have an effect on the 3-year RFS rate (primary tumors: OR 0.96; 95% CI 0.68–1.37; P = 0.84; metastatic tumors: OR 1.10; 95% CI 0.60–2.02; P = 0.77) or the 5-year RFS rate (primary tumors: OR 0.84; 95% CI 0.63–1.12; P = 0.24; metastatic tumors: OR 0.77; 95% CI 0.47–1.24; P = 0.28).
The type of malignant tumor had no significant effect on the 1-year OS rate in the PM and non-PM groups (primary tumors: OR 0.87; 95% CI 0.67–1.14; P = 0.31; metastatic tumors: OR 0.74; 95% CI 0.49–1.12; P = 0.15), nor did it have an effect on the 3-year OS rate (primary tumors: OR 1.04; 95% CI 0.85–1.29; P = 0.69; metastatic tumors: OR 1.12; 95% CI 0.72–1.76; P = 0.61) or 5-year OS rate (primary tumors: OR 1.10; 95% CI 0.88–1.38; P = 0.39; metastatic tumors: OR 0.89; 95% CI 0.62–1.28; P = 0.53) (Supplemental Figs. 1 and 2).
Discussion
Intraoperative bleeding is one of the most common and life-threatening complications during liver surgery, and has been associated with increased long-term morbidity and mortality26. In addition, intraoperative hemorrhage increases the rate of blood transfusions, which have a negative impact on long-term postoperative outcomes by reducing the patient’s immune defense26,27. Excessive bleeding and blood transfusion also reduce patient survival26,27. Excessive intraoperative bleeding and vascular occlusion are both associated with an increased risk of postoperative surgical complications and unfavorable clinical outcomes. Therefore, the optimal approach to liver resection is to perform surgery without hepatic vascular occlusion while minimizing blood loss and the need for blood transfusion.
Despite several strategies to reduce intraoperative bleeding, the PM remains the most commonly used technique because it was shown to reduce blood loss with high efficacy in initial randomized trials10,26. However, some studies have not confirmed these initial findings and have even suggested a higher risk of ischemia–reperfusion injury for healthy liver tissue28,29. Furthermore, an increased rate of postoperative complications has been shown in patients who undergo PM during hepatectomies in some studies30. To prevent liver injuries, portal pedicle clamping was modified in the PM to an intermittent approach31. Despite this modification, the overall efficacy of the PM remains controversial32,33. Whether the PM promotes liver injury remains a topic of debate. Furthermore, how the PM affects recurrence and survival in patients with malignant lesions who underwent hepatectomy is not well understood. Although some studies have suggested that prolonged PM increases recurrence1,20, others have demonstrated no effect15,19,34. For instance, Al-Saeedi et al. revealed that a PM of less than 20 min did not increase the recurrence rate after 3 years9. Recent studies showed that the PM has no significant positive impacts on clinical outcomes after minor liver surgeries13,32. However, major liver resections, which have more intraoperative blood loss, probably benefit more from the PM. To address this controversy, we performed a meta-analysis to compare the long-term oncological outcomes of hepatectomy with and without a PM.
The PM, regardless of whether it is complete or intermittent, was shown to be an independent risk factor for cancer recurrence in one study13. However, other studies have reported no negative impact of the PM on patient survival and disease recurrence17,18. In a recent randomized-controlled trial, the intermittent PM did not affect disease-free survival after hepatectomy, but did improve the OS rate10. The positive effect of the intermittent PM was particularly promising in patients with hepatic disorders such as cirrhosis10. In the present analysis, we observed no significant differences in 1-, 3-, and 5-year overall and recurrence-free survival between the PM and non-PM groups. Furthermore, subgroup analysis revealed no significant effects of tumor type (i.e., primary or metastatic) on 1-, 3-, and 5-year survival between the PM and non-PM groups. This is in accordance with previous findings from large patient cohorts and clinical trials.
The PM was shown to be a risk factor for disease recurrence in several studies. It has been hypothesized that ischemia during portal pedicle clamping causes microvascular damage by breaking adhesions between tumor cells and endothelial cells35. The hepatic ischemia-perfusion cycle might increase the expression of E-selectin, which plays a crucial role in cancer cell metastasis36,37. However, we found no significant increase in disease recurrence following hepaectomy with the PM, indicating that the PM is not associated with disease recurrence after hepatectomy.
During reperfusion, liver parenchymal cells are thought to be injured by cytokines and radical oxygen species, which are produced by active Kupffer cells38. However, a meta-analysis reported no significant patient benefits of hemihepatic vascular occlusion over complete hepatic vascular occlusion, despite a lower rate of liver injury39. This suggests that significant hepatic injury is not caused by the PM, and that the potential benefits outweigh the potential disadvantages. In addition, of enrolled studies in this meta-analysis, four studies (2335 cases) reported the number of patients with steatosis, and no significant difference was observed in means of fatty liver distribution among patients with and without PM a. However, included studies failed to provide more detailed data on clinical or oncological impacts of liver texture characteristics (e.g. macrovesicular or microvesicular liver steatosis, or liver fibrosis) on outcomes of the pringle maneuver, which prohibited us from carrying out subgroup analyses.
A study by Fagenson et al. reported that patients undergoing minor liver resection and cases with metastatic disease had a worse outcome when PM was performed40. This finding is in similar line with our previously published report. Our results showed that PM is useful in patients who underwent extended liver resection, but this surgical maneuver may not be beneficial in minor hepatectomies9. It can be derived that PM is associated with encouraging early perioperative outcomes without worsening the long-term survival among well-selected patients. On this basis, it cannot be denied that the selection of patients undergoing PM plays a principal role in increasing of safety and efficacy of PM.
There are some limitations to the present study. The main weakness is the variability in PM techniques, underlying liver disease, tumor stage status, and preoperative liver function between the included studies. Due to lack of subgroup results regarding the underlying liver disease, especially liver cirrhosis, it was not possible to assess the impact of PM in cirrhotic patients. In addition, although several studies have compared the PM with non-PM techniques, the number of RCTs is low, and most studies have a retrospective design, which can have a selection bias because PM enable surgeons to perform more aggressive hepatectomy in patients with more advance tumors with worse prognosis. We have added to study from the same center in our meta-analysis6,13; the first study was performed between January 2007 and December 201013 and the second study was performed between January 2010 and December 20126. These two studies may include overlapping patients in 2010 which can create some bias in present meta-analysis.
In conclusion, the present study shows that the PM is a suitable surgical technique for managing intraoperative bleeding during liver resection, and does not increase tumor recurrence and long-term mortality. We believe that the PM is a useful and acceptable aopproach to major or extended liver resection. However, further studies in large patient cohorts and randomized trials are needed to comprehensively evaluate the advantages and disadvantages of this procedure.
Methods
This systematic review and meta-analysis was reported according the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines41.
Eligibility criteria
The research question was formulated according to the PICOS strategy.
-
Population: all adult patients who underwent liver resection
-
Intervention: PM during liver resection
-
Comparators: no PM
-
Outcome: overall or recurrence-free survival rates
-
Study design: all study types methodological designs, including human subjects, except case series with less than ten patients, narrative or systematic reviews, letters, conference abstracts, and study protocols.
Duplicate publications or overlapping cohorts were excluded.
Search strategy
According to Goossen et al.42 the following databases were searched.
-
1.
Cochrane Central Register of Controlled Trials (CENTRAL)
-
2.
Medline (via PubMed)
-
3.
Web of Science
Databases were last searched for relevant publications in May 2020. The references of each included study were also searched for additional relevant articles. The combination of search terms is presented in Supplemental Text 1.
Study selection
Two investigators (SS and AH) independently screened all papers identified by the search strategy and selected eligible studies based on the PICOS criteria. Two authors (SAHS and AR) then reviewed and evaluated the full-text of eligible articles and extracted the data. Discrepancies were settled by a discussion with a third author (EK).
Outcomes and data items
Recurrence-free survival rate
The recurrence-free survival (RFS) rate was defined as the number of the patients who survived without signs of recurrence after primary liver resection. We measured the RFS after 1, 3, and 5 years.
Overall survival rate
The overall survival (OS) rate was defined as the number of patients who survived after liver resection, regardless of disease recurrence. We measured the OS at 1, 3, and 5 years.
Quality assessment
The Cochrane risk-of-bias tool was used to assess the quality of randomized-controlled trials (RCT) and the ROBINS-I tool was used to assess the quality of non-randomized studies (NRS)43,44. The Cochrane risk-of-bias tool evaluated several items, including bias arising from the randomization process, bias arising from the timing of identification and recruitment of individual participants in relation to the timing of randomization, bias due to deviations from intended interventions, bias due to missing outcome data, and bias in the selection of the reported result. The overall risk of bias was low if the study was judged to be at low risk of bias for all domains. There were some concerns of bias if some concern of bias was detected in at least one domain. The risk of bias was high if the study was judged to be at high risk of bias in at least one domain or if some concerns of bias were detected in multiple domains.
Statistical analysis
Statistical analyses were performed by RevMan version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). Pooled results were analyzed using the Mantel–Haenszel method. Results were presented as odds ratios (OR) or as survival rates with 95% confidence intervals (CI). Because of clinical heterogeneity between studies, a random‐effects model was used. A P value < 0.05 for the Q-test or a I2 index more than 75% indicated statistical heterogeneity among studies. An I2 index between 50 and 75% indicated moderate statistical heterogeneity.
References
Liu, S. et al. Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J. Surg. Oncol. 114, 112–118. https://doi.org/10.1002/jso.24271 (2016).
Zhen, Z. J., Lau, W. Y., Wang, F. J. & Lai, E. C. Laparoscopic liver resection for hepatocellular carcinoma in the left liver: Pringle maneuver versus tourniquet method. World J. Surg. 34, 314–319. https://doi.org/10.1007/s00268-009-0320-z (2010).
Koch, M. et al. Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann. Surg. 241, 199–205. https://doi.org/10.1097/01.sla.0000151795.15068.27 (2005).
Famularo, S. et al. Does the Pringle maneuver affect survival and recurrence following surgical resection for hepatocellular carcinoma? A western series of 441 patients. J. Surg. Oncol. 117, 198–206 (2018).
Huntington, J. T., Royall, N. A. & Schmidt, C. R. Minimizing blood loss during hepatectomy: A literature review. J. Surg. Oncol. 109, 81–88. https://doi.org/10.1002/jso.23455 (2014).
Hao, S., Chen, S., Yang, X. & Wan, C. Adverse impact of intermittent portal clamping on long-term postoperative outcomes in hepatocellular carcinoma. Ann. R Coll. Surg. Engl. 99, 22–27. https://doi.org/10.1308/rcsann.2016.0183 (2017).
Liu, L. et al. Influence of hepatic artery occlusion on tumor growth and metastatic potential in a human orthotopic hepatoma nude mouse model: Relevance of epithelial-mesenchymal transition. Cancer Sci. 101, 120–128. https://doi.org/10.1111/j.1349-7006.2009.01363.x (2010).
Si-Yuan, F. U. et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am. J. Surg. 201, 62–69. https://doi.org/10.1016/j.amjsurg.2009.09.029 (2011).
Al-Saeedi, M. et al. Pringle maneuver in extended liver resection: A propensity score analysis. Sci. Rep. 10, 8847. https://doi.org/10.1038/s41598-020-64596-y (2020).
Lee, K. F. et al. Impact of intermittent pringle maneuver on long-term survival after hepatectomy for hepatocellular carcinoma: Result from two combined randomized controlled trials. World J. Surg. 43, 3101–3109. https://doi.org/10.1007/s00268-019-05130-8 (2019).
Jiang, J. H. et al. Comparison of hepatectomy with or without hepatic inflow occlusion in patients with hepatocellular carcinoma: A single-center experience. Minerva Med. 108, 324–333. https://doi.org/10.23736/S0026-4806.17.04788-7 (2017).
Xu, W. et al. Continuous Pringle maneuver does not affect outcomes of patients with hepatocellular carcinoma after curative resection. Asia Pac. J. Clin. Oncol. 13, e321–e330 (2017).
Hao, S., Chen, S., Yang, X. & Wan, C. Impact of intermittent portal clamping on the early recurrence of hepatocellular carcinoma after surgery. Surg. Today 46, 1290–1295. https://doi.org/10.1007/s00595-016-1316-6 (2016).
Tsang, M. E. et al. The impact of portal pedicle clamping on survival from colorectal liver metastases in the contemporary era of liver resection: A matched cohort study. HPB (Oxford) 17, 796–803. https://doi.org/10.1111/hpb.12458 (2015).
Huang, J. W. et al. Intermittent hepatic inflow occlusion during partial hepatectomy for hepatocellular carcinoma does not shorten overall survival or increase the likelihood of tumor recurrence. Medicine https://doi.org/10.1097/MD.0000000000000288 (2014).
Weiss, M. J. et al. Hepatic pedicle clamping during hepatic resection for colorectal liver metastases: No impact on survival or hepatic recurrence. Ann. Surg. Oncol. 20, 285–294. https://doi.org/10.1245/s10434-012-2583-0 (2013).
Xia, F. et al. Does hepatic ischemia–reperfusion injury induced by hepatic pedicle clamping affect survival after partial hepatectomy for hepatocellular carcinoma?. World J. Surg. 37, 192–201 (2013).
De Carlis, L. et al. Colorectal liver metastases: Hepatic pedicle clamping during hepatectomy reduces the incidence of tumor recurrence in selected patients. Case-matched analysis. Eur. J. Surg. Oncol. 39, 726–733. https://doi.org/10.1016/j.ejso.2013.03.015 (2013).
Ferrero, A. et al. Does Pringle maneuver affect survival in patients with colorectal liver metastases?. World J. Surg. 34, 2418–2425 (2010).
Nijkamp, M. W. et al. Prolonged portal triad clamping during liver surgery for colorectal. Liver metastases is associated with decreased time to hepatic tumour recurrence. Ejso 36, 182–188. https://doi.org/10.1016/j.ejso.2009.10.016 (2010).
Giuliante, F. et al. Does hepatic pedicle clamping affect disease-free survival following liver resection for colorectal metastases?. Ann. Surg. 252, 1020–1026 (2010).
Wang, C. C. et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann. Surg. Oncol. 16, 1832–1842. https://doi.org/10.1245/s10434-009-0448-y (2009).
Wong, K. et al. Intermittent Pringle manoeuvre is not associated with adverse long-term prognosis after resection for colorectal liver metastases. Br. J. Surg. 95, 985–989 (2008).
Tanaka, K. et al. Clinical features of hepatocellular carcinoma developing extrahepatic recurrences after curative resection. World J. Surg. 32, 1738–1747. https://doi.org/10.1007/s00268-008-9613-x (2008).
Buell, J. F. et al. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann. Surg. 236, 658–666. https://doi.org/10.1097/00000658-200211000-00017 (2002).
Alkozai, E. M., Lisman, T. & Porte, R. J. Bleeding in liver surgery: Prevention and treatment. Clin. Liver Dis. 13, 145–154. https://doi.org/10.1016/j.cld.2008.09.012 (2009).
Cata, J. P., Wang, H., Gottumukkala, V., Reuben, J. & Sessler, D. I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br. J. Anaesth. 110, 690–701. https://doi.org/10.1093/bja/aet068 (2013).
van Wagensveld, B. A. et al. Continuous or intermittent vascular clamping during hemihepatectomy in pigs: hyaluronic acid kinetics in the assessment of early microvascular liver damage. Eur. J. Surg. 166, 255–261. https://doi.org/10.1080/110241500750009375 (2000).
Kim, Y. I. et al. Successful intermittent application of the Pringle maneuver for 30 min during human hepatectomy: A clinical randomized study with use of a protease inhibitor. Hepatogastroenterology 54, 2055–2060 (2007).
Xiaobin, F., Zipei, L., Shuguo, Z., Jiahong, D. & Xiaowu, L. The Pringle manoeuvre should be avoided in hepatectomy for cancer patients due to its side effects on tumor recurrence and worse prognosis. Med. Hypotheses 72, 398–401 (2009).
Nomi, T. et al. Modified Pringle maneuver for laparoscopic liver resection. Ann. Surg. Oncol. 22, 852. https://doi.org/10.1245/s10434-014-4088-5 (2015).
Lordan, J. T., Worthington, T. R., Quiney, N., Fawcett, W. J. & Karanjia, N. D. Operative mortality, blood loss and the use of Pringle manoeuvres in 526 consecutive liver resections. Ann. R. Coll. Surg. Engl. 91, 578–582. https://doi.org/10.1308/003588409x432473 (2009).
Sanjay, P., Ong, I., Bartlett, A., Powell, J. J. & Wigmore, S. J. Meta-analysis of intermittent P ringle manoeuvre versus no P ringle manoeuvre in elective liver surgery. ANZ J. Surg. 83, 719–723 (2013).
Ariizumi, S. et al. Surgical shunt closure via the lumen of an intrahepatic portal aneurysm. Dig. Surg. 23, 259–261. https://doi.org/10.1159/000096157 (2006).
Yamamoto, M. & Asanuma, K. Portal vein clamp and subsequent blood reflow enhance liver metastasis of colon ACL-15 cells administered intrasplenically in F344/DU rats. Shinshu Med. J. 59, 249–257 (2011).
Uotani, H. et al. Induction of E-selectin after partial hepatectomy promotes metastases to liver in mice. J. Surg. Res. 96, 197–203. https://doi.org/10.1006/jsre.2001.6095 (2001).
Antoine, M., Tag, C. G., Gressner, A. M., Hellerbrand, C. & Kiefer, P. Expression of E-selectin ligand-1 (CFR/ESL-1) on hepatic stellate cells: Implications for leukocyte extravasation and liver metastasis. Oncol. Rep. 21, 357–362 (2009).
Bhogal, R. H., Curbishley, S. M., Weston, C. J., Adams, D. H. & Afford, S. C. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 16, 1303–1313. https://doi.org/10.1002/lt.22157 (2010).
Wang, H. Q., Yang, J. Y. & Yan, L. N. Hemihepatic versus total hepatic inflow occlusion during hepatectomy: A systematic review and meta-analysis. World J. Gastroenterol. 17, 3158–3164. https://doi.org/10.3748/wjg.v17.i26.3158 (2011).
Fagenson, A. M., Gleeson, E. M., Nabi, F., Lau, K. N. & Pitt, H. A. When does a Pringle Maneuver cause harm? HPB https://doi.org/10.1016/j.hpb.2020.07.014 (2020).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med 6, 1-6. https://doi.org/10.1371/journal.pmed.1000097 (2009).
Goossen, K. et al. Optimal literature search for systematic reviews in surgery. Langenbecks Arch. Surg. 403, 119–129. https://doi.org/10.1007/s00423-017-1646-x (2018).
Sterne, J. A. et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. https://doi.org/10.1136/bmj.i4919 (2016).
Sterne, J. A. C. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. https://doi.org/10.1136/bmj.l4898 (2019).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
E.K., S.S., S.A.H.A.S., A.R., A.H., O.G. designed the study, collected and analyzed data, and wrote the manuscript. M.A., N.R., and C.R. contributed knowledge and revised the manuscript. H.O., P.P. and A.M. co-designed the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khajeh, E., Shafiei, S., Al-Saegh, S.AH. et al. Meta-analysis of the effect of the pringle maneuver on long-term oncological outcomes following liver resection. Sci Rep 11, 3279 (2021). https://doi.org/10.1038/s41598-021-82291-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82291-4
This article is cited by
-
Effect of intermittent Pringle maneuver on perioperative outcomes and long-term survival following liver resection in patients with hepatocellular carcinoma: a meta-analysis and systemic review
World Journal of Surgical Oncology (2023)
-
Statin use is associated with the reduction in hepatocellular carcinoma recurrence after liver surgery
BMC Cancer (2022)
-
Laparoscopic central hepatectomy using a parenchymal-first approach: how we do it
Surgical Endoscopy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.