Abstract
Acoustic cardiography can provide simultaneous electrocardiography and acoustic cardiac data to assess the electronic and mechanical heart functions. The aim of this study was to assess whether changes in acoustic cardiographic parameters (ACPs) before and after hemodialysis (HD) are associated with overall and cardiovascular (CV) mortality in HD patients. A total of 162 HD patients was enrolled and ACPs were measured before and after HD, including left ventricular systolic time (LVST), systolic dysfunction index (SDI), third (S3) and fourth (S4) heart sounds, and electromechanical activation time (EMAT). During a follow-up of 2.9 years, 25 deaths occurred with 16 from CV causes. Multivariate analysis showed that high △SDI (per 1; hazard ratio [HR], 2.178; 95% confidence interval [CI], 1.189–3.990), high △EMAT (per 1%; HR, 2.218; 95% CI 1.382–3.559), and low △LVST (per 1 ms; HR, 0.947; 95% CI 0.912–0.984) were independently associated with increased overall mortality. In addition, high △EMAT (per 1%; HR, 2.141; 95% CI 1.117–4.102), and low △LVST (per 1 ms; HR, 0.777; 95% CI 0.637–0.949) were associated with increased CV mortality. In conclusion, the changes in ACPs before and after HD may be a useful clinical marker and stronger prognostic marker of overall and CV mortality than ACPs before HD.
Similar content being viewed by others
Introduction
The incidence and prevalence of end-stage renal disease (ESRD) are increasing worldwide. Cardiovascular (CV) disease is common in patients with ESRD, including coronary artery disease, left ventricular (LV) hypertrophy, heart failure and arrhythmias1,2, and it is also the major cause of morbidity and mortality in dialysis patients1. Cardiac structural and functional abnormalities are also known to increase the risk of CV disease3,4, and therefore identifying these risk factors in these patients as early as possible is crucial. Patients with ESRD have a high prevalence of LV dysfunction, which has been associated with an increased risk of sudden death in ESRD patients5,6,7. Cardiac function is most commonly evaluated using echocardiography, however the accuracy of echocardiography is operator-dependent and time consuming.
Acoustic cardiography can provide simultaneous information on both electrocardiography and acoustic cardiac data to assess the electronic and mechanical functions of the heart8. Acoustic cardiographic parameters (ACPs) include the measurement of heart sounds and systolic time intervals. Previous studies have demonstrated the use of acoustic cardiography to non-invasively detect increased LV filling pressure and LV systolic dysfunction9,10. In addition, it has also been used to detect LV hypertrophy, ischemic heart disease, ventricular fibrillation, heart failure, constrictive pericarditis and sleep apnea8.
Our recent study investigated ACPs in hemodialysis (HD) patients before and after HD, and found that the fourth heart sound (S4) and LV systolic time (LVST) were decreased and electromechanical activation time (EMAT) was increased after HD11. However, whether changes in ACPs before and after HD (△ACPs) can be used as a prognosis marker remains to be determined. Therefore, the aim of this study was to assess whether △ACPs before and after HD are associated with overall and CV mortality in HD patients.
Results
The mean age of the included 162 HD patients was 60.4 ± 10.9 years. The clinical characteristics of the survivor and non-survivor groups are shown in Table 1. Compared to the survivors, the non-survivors had a lower serum albumin level, higher △heart rate, lower △PR interval and lower △LVST.
Determinants of △ACPs
Table 2 shows the determinants of △ACPs using multivariable linear regression analysis. In the multivariable analysis after adjusting for age, sex, diabetes mellitus, hypertension, coronary artery disease, arteriovenous access type, dialysis vintage, systolic and diastolic blood pressures, albumin, log triglyceride, total cholesterol, hemoglobin, total calcium, phosphorous, calcium × phosphorus (CaXP) product, potassium, uric acid, Kt/V, blood flow, and medications including angiotensin converting enzyme inhibitors (ACEIs) and/or angiotensin II receptor blockers (ARBs), β-blocker, statin and aspirin, diabetes, high triglyceride, high hemoglobin, and patients withoutβ-blocker use were significantly associated with high △heart rate. Diabetes was significantly associated with high △QRS Duration. Hypertension, high potassium, and patients without ACEI and/or ARB use were related with low △PR interval. High systolic blood pressure was correlated with low △S3, and low blood flow was associated with △S4. Diabetes and low systolic blood pressure were associated with high △SDI. Low systolic blood pressure, high diastolic blood pressure, high total calcium, high phosphorous, low CaXP product were associated with high △EMAT. Low systolic blood pressure, high diastolic blood pressure, high triglyceride, high total calcium, high phosphorous, low CaXP product were associated with high △EMAT%. Lastly, low systolic blood pressure, high diastolic blood pressure, high triglyceride, high total calcium, and low blood flow were associated with low △LVST. There were no significant variables associated with △QTc and △LVST%.
Risk of overall mortality
The median follow-up period was 2.9 (range 2.4–3.4) years for all patients, during which 25 patients died (15.4%), including CV death (n = 16), malignancy (n = 3), infectious disease (n = 3), and others (n = 3). Table 3 shows the relationships between ACPs before HD and △ACPs to overall mortality. In the multivariable analysis after adjustment, high △heart rate (per 1 beat/min; hazard ratio [HR], 1.148; 95% confidence interval [CI], 1.053–1.253; p = 0.002), low △PR interval (per 1 ms; HR, 0.930; 95% CI 0.888–0.975; p = 0.002), high △SDI (per 1; HR, 2.178; 95% CI 1.189–3.990; p = 0.012), high EMAT (per 1%; HR, 1.508; 95% CI 1.056–2.154; p = 0.024), high △EMAT (per 1%; HR, 2.218; 95% CI 1.382–3.559; p = 0.001), low LVST (per 1 ms; HR, 0.9679; 95% CI 0.946–0.994; p = 0.014), and low △LVST (per 1 ms; HR, 0.947; 95% CI 0.912–0.984; p = 0.005) were independently associated with increased overall mortality.
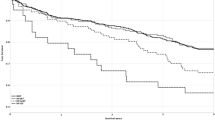
The adjusted Cox regression survival curves [adjusted for age, sex, diabetes mellitus, hypertension, coronary artery disease, arteriovenous access type, dialysis vintage, systolic and diastolic blood pressures, albumin, log triglyceride, total cholesterol, hemoglobin, total calcium, phosphorous, CaXP product, potassium, uric acid, Kt/V, blood flow, and medications including ACEIs and/or ARBs, β-blocker, statin and aspirin] for overall survival in the patients according to the 75th percentile of △EMAT (1%) are shown in Fig. 1. Patients with △EMAT ≥ 1% had a worse overall survival than those with △EMAT < 1% (HR, 9.554; 95% CI 1.560–58.518; p = 0.015).
The adjusted Cox regression survival curves for overall survival in the patients according to the 75th percentile of △LVST (9 ms) are shown in Fig. 2. The patients with △LVST < 9 ms had a worse overall survival than those with △LVST ≥ 9 ms (HR, 0.018; 95% CI 0.001–0.455; p = 0.015).
Risk of CV mortality
There were 16 documented CV deaths during follow-up, including heart failure (n = 7), myocardial infarction (n = 3), ventricular fibrillation (n = 4) and hemorrhagic stroke (n = 2). Table 4 lists the predictors for CV mortality after adjusting for demographic, clinical, and biochemical factors, and ACPs before HD and △ACPs. Multivariable analysis revealed that low △PR interval (per 1 ms; HR, 0.910; 95% CI 0.846–0.980; p = 0.012), high △EMAT (per 1%; HR, 2.141; 95% CI 1.117–4.102; p = 0.022), low LVST (per 1 ms; HR, 0.837; 95% CI 0.730–0.960; p = 0.011), and low △LVST (per 1 ms; HR, 0.777; 95% CI 0.637–0.949; p = 0.013) were significantly associated with increased CV mortality.
Discussion
To the best of our knowledge, this is the first study to report associations between changes in ACP before and after HD with overall and CV mortality. Our results showed that high △heart rate, low △PR interval, high △SDI, high EMAT, high △EMAT, low △LVST, and low △LVST were significantly associated with increased overall mortality. In addition, low △PR interval, high △EMAT, low LVST and low △LVST were significantly associated with increased CV mortality.
The first important finding of this study is that high △EMAT before and after HD was associated with increased overall and CV mortality. EMAT is defined as the amount of time required for the left ventricle to generate sufficient force to close the mitral valve, and therefore reflects the velocity of force generation during systole12. Previous studies have shown a strong association between EMAT and impaired LV contractility, and that EMAT increases steadily with age in patients with heart failure13,14. EMAT has also been shown to be a useful prognostic marker of CV outcomes. In a study of hospitalized patients with congestive heart failure, Zhang et al. found that elevated EMAT measured at admission was an independent risk factor for major adverse CV events15. In patients with acute heart failure syndrome, Chao et al. reported that EMAT could predict CV outcomes independently of LV ejection fraction, LV diastolic function, and serum N-terminal pro-brain natriuretic peptide16. In addition, Sung et al. reported that a high %EMAT or S3 strength may indicate an increased risk of de-compensation and mortality in patients with heart failure, and that intensifying diuretic and/or vasodilator therapy may alleviate potential adverse events17. But why did the EMAT increase after HD in our study? The potential mechanism may be insufficient coronary perfusion and cause resulting heart ischemia and impaired heart systolic function. Furthermore, there may be a stenosis lesion on coronary artery, so the coronary perfusion is susceptible to volume depletion (decrease in effective circulating volume) by ultrafiltration. In patients with elevated EMAT after HD, this may contribute to higher cardiovascular risk. In the present study, △EMAT was a stronger predictor of adverse outcomes than EMAT before HD. Therefore, dynamic changes in EMAT before and after HD may reflect impaired LV contractility more accurately than resting APCs before HD.
The second important finding of this study is that low △LVST before and after HD was associated with increased overall and CV mortality. In 1970, Garrard et al. reported a significant correlation between LVST and angiographically determined LV ejection fraction and left ventricular end-diastolic volume18. Patients with LV systolic dysfunction often have a shorter LVST as more time is required to generate sufficient force to open the aortic valve and keep it open in situations with impaired ventricular contraction19. Zuber et al. also showed that patients with an LV ejection fraction < 50% had a higher prevalence of S3, lower mean LVST, and higher mean EMAT and EMAT/LVST. The authors concluded that these parameters can improve detection of LV systolic dysfunction20. In addition, in patients with intermediate brain natriuretic peptide levels (100 to 500 pg/mL), Shapiro et al. showed that EMAT/LVST could detect LV dysfunction with a high specificity (95%) and moderate sensitivity (55%) and improve the detection of LV dysfunction21. In the present study, we found that low △LVST was associated with increased overall and CV mortality, and that this was a stronger predictor of adverse outcomes than LVST before HD.
Another important finding of this study is that high △SDI before and after HD was correlated with increased overall mortality. In patients with heart failure, previous studies have shown that SDI can discriminate patients with severe LV systolic dysfunction (ejection fraction [EF] ≤ 35%) from those with moderate LV systolic dysfunction (35% < EF < 50%) with an SDI > 522. Dillier et al. reported that both SDI (≥ 5) and S3 (4) score were strong independent predictors of mortality and could identify high-risk patients requiring more aggressive monitoring and therapy23. In the present study, we found that high △SDI was associated with higher overall mortality, and that it was a stronger predictor of adverse outcomes than SDI before HD.
In this study, the patients with an increased heart rate after HD had higher overall mortality. The reason for an increase in heart rate after HD is not fully understood, but it may be related to progressive volume depletion by ultrafiltration, sympatho-activation and changes in electrolyte levels24,25. The autonomic system controls heart rate and rhythm via a balance between parasympathetic and sympathetic systems26. A previous study reported that HD patients are characterized by vagal withdrawal and sympathetic overactivity, and that this was linked to a poor CV prognosis27. In addition, Barnas et al. investigated hemodynamic patterns during dialysis hypotension, and demonstrated that tachycardia induced by ultrafiltration is part of the spectrum of normal CV autonomic activation. The authors concluded that tachycardia develops by increasing baroreflex-mediated sympathetic activity24. Rogovoy et al. investigated sudden cardiac death in ESRD patients using continuous electrocardiography monitoring, and found that sudden increases in heart rate during or after dialysis preceded non-sustained ventricular tachycardia events26. In addition, Severi et al. reported a significant increase (11%) in heart rate following changes in calcium, potassium and pH, without significant changes in autonomic activity indices25.
Studies on the association between PR interval and mortality have reported inconsistent results. Magnani et al. conducted a prospective cohort study with 2722 older participants (age 70–79 years), and multivariable-adjusted analyses showed that every SD increase (29 ms) in baseline PR interval was associated with 13% increases in the 10-year risk of both incident atrial fibrillation and heart failure. However, they did not find an association between PR interval and all-cause mortality28. Skampardoni et al. published a review of patients with chronic kidney disease or dialysis, and showed varying results of the association between PR interval prolongation and CV outcomes and overall mortality29. Moreover, Flueckiger et al. reported an association between prolonged PR interval and increased mortality30, while Kestenbaum et al. reported that a longer PR interval could not independently predict incident CV events or mortality31. In addition, Silva et al. reported that prolonged PR interval could predict bradyarrhythmias32. Regarding the difference between PR intervals before and after HD, Badarau et al. reported that an increased PR interval after HD was associated with lower rates of CV events and mortality33. During dialysis, changes in electrolyte levels, fluid shift and autonomic response may influence the PR interval29. In the present study, the patients who had low △PR interval before and after HD were associated with increased overall and CV mortality. Therefore, further studies are needed to investigate the significance and prognostic value of decreased PR interval after dialysis.
There are several limitations to this study. First, because relatively few patients died, the statistical power of the study was reduced. Further studies with a larger sample size should be performed to verify our findings. In addition, the quality of acoustic cardiography data is influenced by exogenous and endogenous noises. Finally, the ACPs were measured only before and after HD, and repeated measurements may be necessary.
In conclusion, we demonstrated that high △SDI, high △EMAT and low △LVST were independently associated with increased overall mortality. In addition, high △EMAT and low △LVST were significantly associated with increased CV mortality. Changes in ACP before and after HD (△ACP) may be a useful clinical marker and stronger prognostic marker of overall and CV mortality than ACPs before HD. Acoustic cardiography is a non-invasive cost-effective technique to screen dialysis patients who are at high risk of adverse clinical outcomes.
Materials and methods
Ethics statement
Written informed consent was obtained from each participant in accordance with institutional requirements and the principles of the Declaration of Helsinki. Moreover, the current study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20160071).
Study patients and design
This study was conducted at the dialysis unit of a regional hospital in southern Taiwan from October 2016 to April 2018, and enrolled 162 patients (86 [53.1%] males, 76 females [46.9%]) receiving HD treatment three times a week. Each HD session lasted for 3.5 to 4 h. The dialyzer blood flow rates ranged from 250 to 300 mL/min with a dialysate flow rate of 500 mL/min. The study protocol was approved by the Institutional Review Board of Kaohsiung Medical University Hospital, and informed consent was obtained from all of the enrolled patients.
Demographic, medical, and laboratory data collection
Age, sex, smoking status (ever vs. never) and comorbid conditions of the patients were obtained from medical records and interviews. After overnight fasting, blood samples were obtained and biomarkers including triglycerides, hemoglobin, albumin, fasting glucose, uric acid, and total cholesterol, calcium, phosphorus, and potassium were measured. The efficiency of dialysis was determined by Kt/V according to the Daugirdas method34. All blood samples were obtained within 1 month of enrollment into the study.
Acoustic cardiography
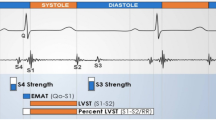
Acoustic cardiographic examinations were performed in the supine position 30 min before and 30 min after a mid-weekly HD session. Acoustic cardiographic data including heart sounds and related systolic time intervals were analyzed using an AUDICOR system (Inovise Medical). The following ACPs were measured8,12,22:
-
1.
EMAT and %EMAT: the time from Q wave onset to the mitral component of the first heart sound (S1). EMAT was defined as the time required for the left ventricle to generate sufficient force to close the mitral valve.
-
2.
LVST: the time from S1 to the second heart sound (S2).
-
3.
The third heart sound (S3): the probability that S3 existed. A single value between 0 and 10 was reported, and the presence of S3 was defined as a value ≥ 5.
-
4.
S4: as with S3, a single value between 0 and 10 was reported, and the presence of S4 was defined as a value ≥ 5.
-
5.
Systolic dysfunction index (SDI): SDI was derived from the nonlinear transformation of [(S3 score ÷ 10) × QRS duration × QR interval × %EMAT] and mapped onto a scale of 0 to 10.
Definition of overall and CV mortality
Cases of CV and overall mortality were verified from medical records by two cardiologists, with disagreements being resolved by a third cardiologist. Patients were followed until death or September 2020. Patients who did not die were censored at the end of follow-up.
Statistical analysis
Data are expressed as percentages, mean ± standard deviation for ACPs, mean ± standard error of mean for △ACP, or median (25th–75th percentile) for triglycerides. The patients were divided into two groups as survivors and non-survivors. Between-group differences in categorical variables were assessed using the chi-square test. Approximately normally distributed continuous variables were analyzed using the independent t-test, and continuous variables with skewed distribution were analyzed using the Mann–Whitney U test. △ACP was defined as the difference between the ACP measurement before and after HD. Multivariable linear regression analysis was used to identify the determinants for △ACP. Multivariable Cox proportional hazard analysis was used to identify associations between an ACP before HD and △ACP and overall and CV mortality. Survival curves for overall and CV mortality were derived using Cox-regression analysis. A p value < 0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS 19.0 for Windows (SPSS Inc. Chicago, USA).
References
Cozzolino, M. et al. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 33, 28–34 (2018).
Cheung, A. K. et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 65, 2380–2389 (2004).
Zoccali, C. et al. Left ventricular systolic function monitoring in asymptomatic dialysis patients: a prospective cohort study. J. Am. Soc. Nephrol. 17, 1460–1465 (2006).
Rodriguez-Iturbe, B. & Correa-Rotter, R. Cardiovascular risk factors and prevention of cardiovascular disease in patients with chronic renal disease. Expert Opin. Pharmacother. 11, 2687–2698 (2010).
Zoccali, C. et al. Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J. Am. Soc. Nephrol. 15, 1029–1037 (2004).
Zoccali, C. Left ventricular systolic dysfunction: a sudden killer in end-stage renal disease patients. Hypertension 56, 187–188 (2010).
Wang, A. Y. et al. Sudden cardiac death in end-stage renal disease patients: a 5-year prospective analysis. Hypertension 56, 210–216 (2010).
Wen, Y. N., Lee, A. P., Fang, F., Jin, C. N. & Yu, C. M. Beyond auscultation: acoustic cardiography in clinical practice. Int. J. Cardiol. 172, 548–560 (2014).
Zuber, M. et al. Acoustic cardiography augments prolonged QRS duration for detecting left ventricular dysfunction. Ann. Noninvasive Electrocardiol. 12, 316–328 (2007).
Collins, S. P. et al. Bedside prediction of increased filling pressure using acoustic electrocardiography. Am. J. Emerg. Med. 27, 397–408 (2009).
Tsai, H. J. et al. Investigation of acoustic cardiographic parameters before and after hemodialysis. Dis. Mark. 2019, 5270159 (2019).
Roos, M. et al. Noninvasive detection of left ventricular systolic dysfunction by acoustic cardiography in cardiac failure patients. J. Card. Fail. 14, 310–319 (2008).
Efstratiadis, S. & Michaels, A. D. Computerized acoustic cardiographic electromechanical activation time correlates with invasive and echocardiographic parameters of left ventricular contractility. J. Card. Fail. 14, 577–582 (2008).
Dillier, R., Zuber, M., Arand, P., Erne, S. & Erne, P. Assessment of systolic and diastolic function in heart failure using ambulatory monitoring with acoustic cardiography. Ann. Med. 43, 403–411 (2011).
Zhang, J., Liu, W. X. & Lyu, S. Z. Predictive value of electromechanical activation time for in-hospital major cardiac adverse events in heart failure patients. Cardiovasc. Ther. 2020, 4532596 (2020).
Chao, T. F. et al. Electromechanical activation time in the prediction of discharge outcomes in patients hospitalized with acute heart failure syndrome. Intern. Med. 49, 2031–2037 (2010).
Sung, S. H. et al. Effect of acoustic cardiography-guided management on 1-year outcomes in patients with acute heart failure. J. Card. Fail. 26, 142–150 (2020).
Garrard, C. L. Jr., Weissler, A. M. & Dodge, H. T. The relationship of alterations in systolic time intervals to ejection fraction in patients with cardiac disease. Circulation 42, 455–462 (1970).
Roos, M., Toggweiler, S., Zuber, M., Jamshidi, P. & Erne, P. Acoustic cardiographic parameters and their relationship to invasive hemodynamic measurements in patients with left ventricular systolic dysfunction. Congest. Heart Fail. 12(Suppl 1), 19–24 (2006).
Zuber, M., Kipfer, P. & Attenhofer Jost, C. Systolic dysfunction: correlation of acoustic cardiography with Doppler echocardiography. Congest. Heart Fail. 12(Suppl 1), 14–18 (2006).
Shapiro, M. et al. Diagnostic characteristics of combining phonocardiographic third heart sound and systolic time intervals for the prediction of left ventricular dysfunction. J. Card. Fail. 13, 18–24 (2007).
Wang, S. et al. Rapid bedside identification of high-risk population in heart failure with reduced ejection fraction by acoustic cardiography. Int. J. Cardiol. 168, 1881–1886 (2013).
Wang, S. et al. Prognostic value of acoustic cardiography in patients with chronic heart failure. Int. J. Cardiol. 219, 121–126 (2016).
Barnas, M. G., Boer, W. H. & Koomans, H. A. Hemodynamic patterns and spectral analysis of heart rate variability during dialysis hypotension. J. Am. Soc. Nephrol. 10, 2577–2584 (1999).
Severi, S., Cavalcanti, S., Mancini, E. & Santoro, A. Heart rate response to hemodialysis-induced changes in potassium and calcium levels. J. Nephrol. 14, 488–496 (2001).
Rogovoy, N. M. et al. Hemodialysis procedure-associated autonomic imbalance and cardiac arrhythmias: insights from continuous 14-day ECG monitoring. J. Am. Heart Assoc. 8, e013748 (2019).
Chan, C. T. et al. Determinants of cardiac autonomic dysfunction in ESRD. Clin. J. Am. Soc. Nephrol. 5, 1821–1827 (2010).
Magnani, J. W. et al. Electrocardiographic PR interval and adverse outcomes in older adults: the Health, Aging, and Body Composition study. Circ. Arrhythm. Electrophysiol. 6, 84–90 (2013).
Skampardoni, S., Poulikakos, D., Malik, M., Green, D. & Kalra, P. A. The potential of electrocardiography for cardiac risk prediction in chronic and end-stage kidney disease. Nephrol. Dial. Transplant. 34, 1089–1098 (2019).
Flueckiger, P., Pastan, S., Goyal, A., McClellan, W. W. & Patzer, R. E. Associations of ECG interval prolongations with mortality among ESRD patients evaluated for renal transplantation. Ann. Transplant. 19, 257–268 (2014).
Kestenbaum, B. et al. Kidney function, electrocardiographic findings, and cardiovascular events among older adults. Clin. J. Am. Soc. Nephrol. 2, 501–508 (2007).
Silva, R. T. et al. Predictors of arrhythmic events detected by implantable loop recorders in renal transplant candidates. Arq. Bras. Cardiol. 105, 493–502 (2015).
Badarau, S. et al. Electrocardiogram abnormalities and heart rate variability in predicting mortality and cardiovascular events among hemodialyzed patients. Int. Urol. Nephrol. 47, 1703–1708 (2015).
Daugirdas, J. T. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J. Am. Soc. Nephrol. 4, 1205–1213 (1993).
Acknowledgement
This work was granted by Kaohsiung Municipal Siaogang Hospital (kmhk-109-001).
Author information
Authors and Affiliations
Contributions
Conceptualization, T.-L.C. and S.-C.C.; Data curation, T.-L.C., Y.-H.L., J.-C.H., P.-Y.W., and S.-C.C.; Formal analysis, Y.-H.L., J.-C.H., P.Y.W., and S.-C.C.; Investigation, Y.-H.L., J.-C.H., P.-Y.W., and S.-C.C.; Methodology, T.-L.C., Y.-H.L., J.-C.H. and S.-C.C.; Resources, J.-C.H., P.-Y.W., and S.-C.C.; Software, J.-C.H., P.-Y.W., and S.-C.C.; Supervision, S.-C.C. and J.-M.C.; Writing—original draft, T.-L.C.; Writing—review and editing, S.-C.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, TL., Liu, YH., Huang, JC. et al. Changes in acoustic cardiographic parameters before and after hemodialysis are associated with overall and cardiovascular mortality in hemodialysis patients. Sci Rep 11, 1559 (2021). https://doi.org/10.1038/s41598-021-81286-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81286-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.