Abstract
Multicentre, retrospective cohort study with multivariable Cox proportional-hazards modelling and survival-time inverse-probability-weighting, evaluating the impact of different treatments on survival of proven COVID-19 patients admitted to two Hospitals in the province of Piacenza, Italy. Use of tocilizumab and of high doses of low molecular weight heparin, but not of antivirals (either alone or in combination), azithromycin, and any corticosteroid, was independently associated with lower mortality. Our results support further clinical evaluation of high doses of low molecular weight heparin and tocilizumab as COVID-19 therapeutics.
Similar content being viewed by others
Introduction
Italy was struck by the first epidemic wave of coronavirus disease 2019 (COVID-19) in early 20201. Overall, about 44 000 excess deaths occurred in this 3-month period2, largely concentrated in Northern Italy. At that time, virtually no evidence was available on the optimal management of COVID-19. In this study, we aim to evaluate the impact of the medical interventions on survival in hospitalized COVID-19 patients in the province of Piacenza, Italy.
Methods
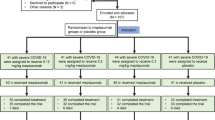
A multicentre, retrospective cohort study was performed among patients hospitalized for COVID-19 from February 21st (date of the first reported COVID-19 case) to May 15th, 2020, at two Hospitals in the province of Piacenza: Guglielmo da Saliceto and Castel San Giovanni. Data for consecutive patients were extracted from electronic medical files, cross-checked, and collated in an anonymized database. Data were censored on June 30th, 2020. Adult (18 years and older) confirmed COVID-19 cases with SARS-CoV-2 reverse transcriptase real-time polymerase chain reaction test on nasal/pharyngeal swab3 were included. Part of the cohort was described previously4. The study was approved by the local Ethics Committee (Area Vasta Emilia Nord), which waived the requirement for informed consent. Continuous data were presented as median and interquartile range (IQR), categorical data as counts and proportions. Low molecular weight heparin was defined as high-dose (HD-LMWH) when given at therapeutic posology (i.e. enoxaparin 6000 international units daily or more) according to drug package inserts. Prescription of treatment (including the choice of the drug and its posology) was heterogeneous in terms of indication as little evidence and no specific recommendations on their use were available. Missing data were handled with multiple imputation using chained equations with 10 imputed datasets. The proportion of missing observations ranged between 0 and 9%. Multivariable Cox proportional-hazards models were used to assess the association of treatment variables with survival, controlling for potential confounders chosen according to univariate results and a priori plausibility. Two models were used: (1) including a variable coding for treatment with any antiviral drug, and (2) including variables coding for the use of hydroxychloroquine, protease inhibitors, and their combination. Variables were included into the model if associated with the outcome at p-value < 0.20 in univariate and if they fulfilled the proportional hazards assumption. Hazard ratios (HR) were reported with 95%-confidence intervals (CI). A sensitivity analysis was performed excluding patients who died at hospital admission or in the two following days. We used survival-time inverse-probability-weighting (IPW) to assess the effect of treatment variables significantly associated with survival in the Cox model. Treatment and censoring models included age, number of comorbidities, symptoms (cough and diarrhoea), respiratory support, and blood tests (total white blood cells and platelets) at hospital admission. Two-sided α < 0.05 was considered statistically significant. Statistical analysis was performed using Stata software version 15.0 (StataCorp).
Compliance with existing regulations
All methods described in the manuscript were carried out in accordance with relevant guidelines and regulations.
Results
Overall, 623 patients with a COVID-19 diagnosis were assessed for study participation: out of them, 600 were confirmed COVID-19 cases and were included in the study. Baseline characteristics are provided in Supplementary Tables. At hospital admission, 75% patients required oxygen therapy, 10% required high-flow oxygen therapy or non-invasive ventilation, and 3% required invasive ventilation. Most patients (88%) received antiviral treatment and 71% received at least two antivirals, mostly hydroxychloroquine (79%) and darunavir/cobicistat (54%). In addition, 66% of patients received azithromycin, 44% a corticosteroid, and 5% tocilizumab. Prophylaxis with LMWH was administered to 72% of patients, while 26% received HD-LMWH. As of June 30th, 2020, 355 patients (60%) were discharged, 199 (33%) died, and 46 (7%) were still hospitalized. Full results are reported in Supplementary Tables. Table 1 reports the results of the multivariable Cox proportional-hazards model. Overall, antiviral treatment with any drug, alone or in combination, or treatment with specific drugs (hydroxychloroquine or a protease inhibitor) was not independently associated with increased survival. Similar results were found for treatment with any corticosteroid or azithromycin. Conversely, two treatment variables were associated with increased survival: use of HD-LMWH (HR 0.56; 95% CI 0.39–0.81) and of tocilizumab (HR 0.13; 95% CI 0.02–0.92). These results were confirmed in the sensitivity analysis excluding patients who died early after hospital admission (N = 31; data not shown). According to IPW, the estimated average treatment effect in a population receiving HD-LMWH was an 86% increase (95% CI 27–145%) in survival time relative to a population not receiving it. Conversely, the effect of tocilizumab was not statistically significant (relative average treatment effect: − 1%, 95% CI − 14 to 12%).
Conclusions
In our study, the administration of antivirals (alone or in combination), azithromycin, and corticosteroids did not lead to increased survival in a cohort of COVID-19 patients. Conversely, the use of HD-LMWH and tocilizumab was independently associated with lower mortality. Our results confirm the lack of mortality reduction in hospitalized COVID-19 patients treated with hydroxychloroquine5 or lopinavir/ritonavir5. The small number of patients receiving remdesivir in our cohort did not allow to assess its efficacy, which is still uncertain5,6. In contrast with recent studies7, corticosteroids were not associated with increased survival; this may be partially explained by the heterogeneity in the choice of corticosteroid, its posology, and the timing of treatment in our cohort. Current knowledge on tocilizumab for COVID-19 is highly controversial8. In our study, tocilizumab use was associated with lower mortality in survival analysis; however, this finding was not confirmed by IPW. Finally, HD-LMWH use was associated with prolonged survival in our cohort. This supports previous observational evidence that LMWH use may lower COVID-19 mortality9, in particular among critically-ill patients and at high doses10.
Our study has many limitations: the limited sample size; the observational, retrospective design which implies indication bias for the choice of treatment (including posology of LMWH); and the lack of some relevant variables in our dataset. Robust statistical methods were used to assess the causal relationship between treatment and outcomes: however, the presence of residual, unmeasured confounders cannot be excluded. In conclusion, our results support further study of HD-LMWH and tocilizumab as COVID-19 therapeutics. Randomized controlled trials are needed to assess their role in the management of COVID-19.
Data availability
Data may be made available by contacting directly the corresponding author.
References
Guglielmetti, L., Chiesi, S. COVID-19 in Italy - Passing through bitter waters. Eur. Respir. J. [Internet] European Respiratory Society; 2020 [cited 2020 May 24]; Available from: https://erj.ersjournals.com/content/early/2020/05/19/13993003.01812-2020.
Alicandro, G., Remuzzi, G. & Vecchia, C. L. Italy’s first wave of the COVID-19 pandemic has ended: No excess mortality in May, 2020. Lancet Elsevier 396, e27–e28 (2020).
WHO. Global surveillance for COVID-19 caused by human infection with COVID-19 virus. Interim guidance. 20 March 2020. WHO/2019-nCoV/SurveillanceGuidance/2020.6.
Guglielmetti, L., Kontsevaya, I., Leoni, M.C. Severe COVID-19 pneumonia in Piacenza, Italy – a cohort study of the first pandemic wave. J. Infect. Public Health; In press.
Consortium, W.S., et al. Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. medRxiv Cold Spring Harbor Laboratory Press; 2020; : 2020.10.15.20209817.
Beigel, J. H. et al. Remdesivir for the treatment of Covid-19—final report. N. Engl. J. Med. 2, 2 (2020).
Group TWREA for C-19 T (REACT), W., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA [Internet] 2020 [cited 2020 Sep 18]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2770279.
Parr, J. B. Time to reassess Tocilizumab’s role in COVID-19 pneumonia. JAMA Intern. Med. 2, 2 (2020).
Nadkarni, G.N., et al. Anticoagulation, Mortality, Bleeding and Pathology Among Patients Hospitalized with COVID-19: A Single Health System Study. J Am Coll Cardiol [Internet]; 2020 [cited 2020 Sep 14]; Available from: https://www.onlinejacc.org/content/early/2020/08/24/j.jacc.2020.08.041.
Jonmarker, S. et al. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit Care 24, 653 (2020).
Acknowledgements
The authors are indebted to the members of the COVID-Piacenza Group: Mario Barbera, Luigi Cavanna, Andrea Magnacavallo, Carlo Moroni, Massimo Nolli, Roberta Schiavo, Matteo Silva (Piacenza Hospital, Piacenza, Italy); Cristina Maestri, Massimo Piepoli (Castel San Giovanni Hospital, Piacenza, Italy). The authors are grateful to health care workers of the Guglielmo da Saliceto and Castel San Giovanni Hospitals in Piacenza, Italy, and to all the patients who were included in the study. A special acknowledgement to Marcello Tavio (Ancona, Italy), Marco Rizzi (Bergamo, Italy), and to the Italian Society of Infectious and Tropical Diseases (SIMIT) for supporting the corresponding author for the work in Piacenza.
Funding
Authors have no competing interests to disclose. There is no specific funding to declare for this study. The work of the corresponding author (LG) in the Hospital of Piacenza was supported by the Italian Society of Infectious and Tropical Diseases (SIMIT).
Author information
Authors and Affiliations
Contributions
L.G. made a substantial contribution to the conception and design of the work, to the acquisition, analysis and interpretation of data for the work, performed statistical analysis, wrote the manuscript, critically revised the manuscript for important intellectual content, gave final approval of the current version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. D.A. made a substantial contribution to the conception of the work, to the acquisition, analysis and interpretation of data for the work, wrote the manuscript, critically revised the manuscript for important intellectual content, gave final approval of the current version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. I.K. made a substantial contribution to the analysis and interpretation of data for the work, wrote the manuscript, critically revised the manuscript for important intellectual content, gave final approval of the current version to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. G.T. and M.C. made a substantial contribution to the conception and design of the work, to the analysis and interpretation of data for the work, critically revised the manuscript for important intellectual content, gave final approval of the current version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All other authors gave a substantial contributions to the interpretation of data for the work, revised the manuscript for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guglielmetti, L., Aschieri, D., Kontsevaya, I. et al. Treatment for COVID-19—a cohort study from Northern Italy. Sci Rep 11, 20964 (2021). https://doi.org/10.1038/s41598-021-00243-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00243-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.