Abstract
The use of intraoperative margin revision to achieve margin clearance in patients undergoing pancreatoduodenectomy for pancreatic cancer is controversial. We performed a systematic review and meta-analysis to summarize the evidence of intraoperative margin revisions of the pancreatic neck and its impact on overall survival (OS). Nine studies with 4501 patients were included. Patient cohort was stratified in an R0R0-group (negative margin on frozen and permanent section), R1R0-group (revised positive margin on frozen section which turned negative on permanent section), and R1R1-group (positive margin on frozen and permanent section despite margin revision). OS was higher in the R1R0-group (HR 0.83, 95% CI 0.72–0.96, P = 0.01) compared to the R1R1-group but lower compared to the R0R0-group (HR 1.20; 95% CI 1.05–1.37, P = 0.008), respectively. Subgroup analyses on the use of different margin clearance definitions confirmed an OS benefit in the R1R0-group compared to the R1R1-group (HR 0.81; 95% CI 0.65–0.99, P = 0.04). In conclusion, intraoperative margin clearance of the pancreatic neck margin is associated with improved OS while residual tumor indicates aggressive tumor biology. Consensus definitions on margin terminologies, clearance, and surgical techniques are required.
Similar content being viewed by others
Introduction
Pancreatic cancer represents the seventh-leading cause of cancer deaths worldwide1. At present, treatment with curative intent encompasses surgical resection in combination with chemotherapy2. Despite recent advances in multi-disciplinary therapy, relapse rates are as high as 70% within 2 years3. The risk of tumor recurrence depends on the unique and aggressive biology of pancreatic cancer4,5. Pathological characteristics such as lymphovascular (LVI) and perineural invasion (Pn1), nodal metastases (N+), and positive resection margins (R+) were identified to be associated with a worse prognosis following resection6. The association of microscopic residual tumor at the resection margins with survival has remained a topic of controversial debates among clinicians7,8. In a more radical approach the aim of potentially curative surgery is to achieve clear margins, even if synchronous resections of adjacent organs or a complete pancreatectomy is required9. Therefore, at many centers intraoperative frozen section (FS) analyses of resection margins are routinely conducted and margin revision is performed in case of positive margins. An alternative approach considers margins to reflect the tumor biology with positive margins indicating an aggressive disease. In fact, the literature on the topic of intraoperative margin clearance and outcome following pancreatoduodenectomy (PD) for pancreatic cancer remains conflicting. Previous meta-analyses included up to five studies and therefore did not consider the entire currently available evidence10,11,12. The detailed location of positive margins on FS were not further outlined as well as the attempts of re-resection in case of positive FS. In addition, there were neither analyses regarding the applied definition of margin clearance between the resection groups, nor the rate of margin-clearance after resection in the previous reviews.
Therefore, the aim of the present systematic review and meta-analysis was to summarize the current evidence of intraoperative margin revisions of the pancreatic neck to achieve tumor clearance for patients undergoing PD for pancreatic cancer and its impact on survival.
Methods
This systematic review and meta-analysis was reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines13. The study was performed according to guidance provided by the Cochrane Handbook for Systematic Reviews of Interventions14.
Search strategy
A systematic database search of the MEDLINE, EMBASE, and Cochrane databases was done on March 27th, 2020 for all studies reporting on intraoperative FS evaluation during PD for pancreatic cancer. The detailed search strategy is provided as supplemental data. There were no language or time restrictions for the initial search. The retrieved title and abstract lists were independently scrutinized by two reviewers (EB, ER) to determine full-text eligibility. A third author (NNR) was consulted in case of disagreements between the two reviewers. Additional studies were added after a manual review of reference lists in relevant articles.
Selection criteria
Studies were included if they reported about comparative data for intraoperative FS evaluation during PD for pancreatic ductal adenocarcinoma and corresponding survival data allowing for estimation of hazard ratios (HRs). All types of prospective and retrospective studies were included. Studies on patients (i) without data of intraoperative FS evaluation, (ii) without comparative data between the final margin status samples about long-term survival, (iii) with other pathologies than pancreatic cancer or no sufficient comparative data for pancreatic cancer in mixed cohorts, (v) with pancreatic resections other than PD (Whipple or pylorus-preserving pancreatic head resection), and (vi) in languages other than English, German, French or Spanish were excluded. Study protocols, review articles, letters, case reports or clinical series with a total sample size n < 10 were also excluded.
Data extraction
The following data from eligible studies were extracted by two independent investigators (EB, ER): Author, publication date, study period, number of study centers, total number of patients, total number of FS analyses, patients’ age, gender of patients, neoadjuvant therapy, adjuvant therapy, pathological factors, postoperative morbidity, postoperative mortality, follow-up period, overall- and disease-free survival. For data extraction, the patient cohort was stratified as following: R0R0-resection (patients with a negative margin on FS and final histopathology), R1R0-resection (patients with a positive margin on FS, which turned negative on final histopathology after margin revision), R1R1-resection (patients with a positive margin on FS, which remained positive on final histopathology after margin revision). The evaluated margins included following overlapping margin terminologies: common bile duct/hepatic duct, pancreatic neck, peripheral pancreatic, uncinate process, superior mesenteric artery, superior mesenteric vein/portal vein groove, retroperitoneal, periuncinate retroperitoneal/mesopancreatic, circumferential anterior/posterior, posterior/inferior/superior pancreatic soft-tissue margin, enteral, and duodenum or stomach. Disagreements between the two reviewers (EB, ER) were resolved by a third author (NNR).
Assessment of study quality
The Cochrane Collaboration’s ‘Risk of bias’ tool was used for assessing the methodological quality of the included studies14. The risk of bias domains was customized to the review question. The quality of the studies was assessed individually by two authors (EB, ER). Each of the following risk domains of bias was systematically categorized as ’high risk’, ’unclear risk’ and ’low risk’ of bias: selection bias, recall bias, attrition bias, analytical bias and reporting bias.
Statistical analysis
The outcome measure was the hazard ratio (HR) for overall survival. In case of missing HRs and 95% confidence intervals (CIs) or standard errors (SEs), data was estimated according to the available survival data15,16. To perform meta-analyses, a minimum number of three studies was required. Heterogeneity between studies were explored by evaluating following factors, potentially, affecting overall survival: year of publication, study sample size, details of patients’ follow-up, details of histopathological assessments and outcome, applied margin terminology, details of intraoperative margin revision, reporting of false-negative rate and surgical strategy based on margin assessment. Subgroup analyses were performed if three or more studies revealed data for covariates affecting overall survival. Statistical analysis was performed using Review Manager Version 5.3 software (Cochrane Collaboration). The generic inverse-variance method was conducted using a random-effects model. Odds ratios (ORs) with 95% CI were calculated for binary outcomes. The interstudy heterogeneity (I2) was assessed using the I2value17. The significance level was set at P < 0.05. Publication bias was assessed using graphical funnel plot analyses18.
Results
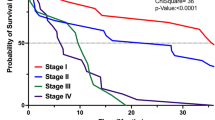
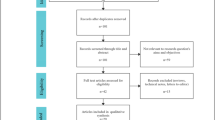
A total of nine articles comprising 4501 patients were included in this meta-analysis19,20,21,22,23,24,25,26,27. Figure 1 displays the results of the search criteria according to the PRISMA guidelines13.
Characteristics of included studies
The studies were published between 2009 and 2020 with a median study sample size of 448 patients. All included studies took place in referral centers for hepato-pancreato-biliary surgery in four different countries. The median follow-up was 19 months. Out of 4501 patients, a total of 1259 (28%) patients had positive tumor margins on final histopathological analysis. Overall, intraoperative FS analyses were performed in 4415 patients with comparative data, representing the final study cohort for meta-analysis. Table 1 outlines the main characteristics of the studies.
Neoadjuvant therapy was administered in 489 of 3674 (13%) patients (Table S1)19,20,22,24,26. Of these, 108 patients (22%) had neoadjuvant chemotherapy, 34 (7%) received radiation therapy, and 347 (71%) underwent chemotherapy and/or radiation therapy, respectively. Adjuvant therapy was administered to 2915 out of 3937 (74%) patients19,20,24,25,26,27.
Five and four studies were single- and multi-center retrospective cohort studies, respectively. The quality assessment revealed a high risk of bias (Figure S1, Table S2). Assessment of the publication bias for the hazard ratio revealed a low asymmetry (Figure S2).
Details of frozen section and permanent section analyses
A total of 3326 patients (74%) had tumor clearance on FS and final histopathological analysis (R0R0-group), 417 patients (9%) had residual tumor margins on FS which turned negative after margin revision on final histopathological analysis (R1R0-group), and 672 patients (15%) had residual tumor margins on final histopathological analysis despite intraoperative margin revision (R1R1-group). Some 100 margins (2%) with initially negative resection margins on FS had a positive resection margin on permanent section (false-negative findings) at the pancreatic neck (Table S3)20,23,24,27. On final histology, different margins were evaluated in all studies ranging between 3 and 8 margin locations.
Details of margin revision
Positive resection margins on FS were detected in 1104 patients (25%). Of these, 887 patients (80%) had an intraoperative margin revision of the pancreatic neck (including other margins), but 420 resected margins (38%) remained positive. To achieve margin clearance, repetitive re-resections were performed in all studies. However, these were limited to the pancreatic neck19,20,21,22,23,24,25,26,27, bile duct19,21,25, and retroperitoneum19 including margins at the superior mesenteric artery and portal vein. Margin locations with persisting residual tumor following repetitive margin revision are detailed in Table S3 and stratified in “transection” and “dissection” margin. The attempt to revise the margin again after a second positive margin was described in four studies as “depending on the surgeon’s decision”20,21,22,24. Out of 1418 patients, 114 patients (8%) underwent total pancreatectomies due to repetitive positive margins at the pancreatic neck24,26,27. Resections of the portal vein or superior mesenteric vein, were performed in 845 out of 4440 patients (19%)19,20,21,22,23,24,25,26. Of these patients, 97 patients (11%) had positive resection margins on FS. One study showed aggressive extended resections due to residual tumors with resections of the hepatic artery and adjacent viscera25.
Details of pathological assessment
The definition of residual tumor varied among included studies. Seven studies designated positive tumor margins as presence of cancer cells at the histological specimen19,20,21,22,25,26,27. Of these, two studies declared the deposits of high-grade dysplastic lesions in addition to cancer cells as positive resection margins19,26. Four studies20,23,24,26 defined residual tumor, if cancer cells were present within 1-mm of the resection margin and three studies19,21,25 if cancer cells were present at the resection edge. One study used both classifications depending on the recruitment period22. To classify the extent of pancreatic cancer, the 6th-8th editions of the American Joint Committee on Cancer Guidelines (AJCC) were used19,20,24,25,26. Two studies23,26 described the expertise of the pathologists involved in the frozen specimen assessment and two further studies19,23 gave details about the techniques of FS analyses.
Meta-analysis of intraoperative margin revision and survival outcomes
Median overall survival ranged between 18 and 29 months in the R0R0-group, 11–25 months in the R1R0-group, and 13 and 21 months in the R1R1-group, respectively (Table S4). Meta-analysis for overall survival revealed a significantly reduced risk of death in the R0R0-group compared to the R1R1-group (HR 0.67; 95% CI 0.60–0.75, P < 0.001) (Fig. 2). Patients in the R1R0-group showed increased survival compared to the R1R1-group (HR 0.83; 95% CI 0.72–0.96, P = 0.01) but decreased survival compared to the R0R0-group (HR 1.20; 95% CI 1.05–1.37, P = 0.008), respectively. Subgroup analyses for the use of the 1-mm margin clearance definition confirmed the survival benefit in the R1R0-group compared to the R1R1-group (HR 0.81; 95% CI 0.65–0.99, P = 0.04) but revealed no significant difference if 0-mm margin clearance was applied (HR 0.86; 95% CI 0.69–1.07, P = 0.18). In addition, patients in the R1R0-group had a comparable survival outcome compared to the R0R0-group in case of a 1-mm margin clearance (HR 1.21; 95% CI 0.92–1.59, P = 0.14) whereas 0-mm margin definitions were associated with a worse outcome (HR 1.26; 95% CI 1.00–1.59, P = 0.05). The subgroup of patients with R0R0-resection had a significantly better survival compared to the R1R1-group with 1-mm and 0-mm margin clearance, respectively (Fig. 3). Data on disease-free survival was reported in two studies19,26.
Forest plots comparing overall survival (A) in patients with secondary R0 resection after margin revision (R1R0-group) and en bloc R0-resection (R0R0-group), (B) in patients with R0 resection after margin revision (R1R0-group) and residual tumor on final assessment (R1R1-group), and in patients with en bloc R0-resection (R0R0-group) and residual tumor on final assessment (R1R1-group). An inverse variance random effects model was used for meta-analysis. Squares and horizontal bars indicate point estimate (hazard ratios) with 95% CI for the individual studies.
Subgroup analysis with forest plots comparing overall survival by using a 0-mm and 1-mm margin clearance (A) in patients with secondary R0 resection after margin revision (R1R0-group) and en bloc R0-resection (R0R0-group), (B) in patients with R0 resection after margin revision (R1R0-group) and residual tumor on final assessment (R1R1-group), and in patients with en bloc R0-resection (R0R0-group) and residual tumor on final assessment (R1R1-group). An inverse variance random effects model was used for meta-analysis. Squares and horizontal bars indicate point estimate (hazard ratios) with 95% CI for the individual studies.
Subgroup analyses for the type of pathological assessments, aggressive treatment with repetitive pancreatic re-resections and extended resections, positivity of other resection margins and neoadjuvant/adjuvant treatment were not feasible due to missing stratified data for these covariates and the margin status as well as the high heterogeneity of reported data across the studies. These included further the use of different metrics (e.g. mean/median age vs. range), variable details of the surgical technique for PD or information regarding the level of lymphadenectomy, and missing stratifications for the study groups and subgroups which eventually precluded meta-regressions.
Meta-analysis of intraoperative margin status as indicator of tumor biology
To test the hypothesis that intraoperative margins reflect patients’ tumor biology, we further assessed the outcomes of histopathological features between the study groups (Table S4). There was a trend for a lower odds-ratio of advanced tumor stage (T \(\ge \) 3) in the R0R0-group compared to the R1R1-group (OR 0.67; 95% CI 0.43–1.04, P = 0.08), however, this did not reach statistical significance. The odds-ratio for nodal metastases in the R0R0-group was significantly lower compared to the R1R1-group (OR 0.61; 95% CI 0.41–0.91, P = 0.02) and higher in the R1R0-group compared to the R0R0-group (OR 1.89; 95% CI 1.00–3.59, P = 0.05), respectively. Further comparisons of advanced tumor stage, N+, poor histological grading (G \(\ge \) 3), LVI, and Pn1 revealed no significant differences between the groups, respectively.
Discussion
The present meta-analysis revealed that intraoperative margin clearance of the pancreatic neck was associated with improved overall survival for patients undergoing PD for pancreatic cancer. The survival benefit was even higher in patients with en bloc R0-resections as compared to both, intraoperative margin revisions and incomplete resections. However, we noticed several inconsistencies in the details of pathological assessments across the included studies reflecting the conflicting scientific landscape at its best with the ongoing debate on the correct definition of microscopic margin involvement after PD28. The AJCC defines in their current edition residual tumor as the presence of cancer cells within 1-mm of the margin. In contrast, the last edition of the International Union Against Cancer endorses a 0-mm clearance and considers the presence of tumor cells at the resection edge as a positive margin. Given the fact, that there is not even an international consensus on margin terminologies, none of the margins were reported consistently apart from the transection margins of the “pancreatic neck” and “common bile duct” (Table S3). In detail, Fatima et al. referred the retroperitoneal margin as a composite definition of the “uncinate process/SMA margin, and posterior, inferior, and superior soft tissue pancreatic margins “, whereas Mathur et al. described the retroperitoneal margin as the “SMA margin, and anterior and posterior sections of the uncinate margin”19,21. Kooby, Nitschke and Crippa et al. termed the retroperitoneal margin as the “SMA margin”20,22,26. Pang and Schmidt et al. applied the definition “periuncinate retroperitoneal margin” and “retroperitoneal/uncinate margins” as the retroperitoneal margin, respectively23,27. Hence, these variable designations of margins between the studies hamper definitive conclusions of the oncological impact of the retroperitoneal margin and represents a major source for heterogeneity. The complexity of the retroperitoneal region is further aggravated by the fact, that it is reported to be the most frequently positive margin after PD6,29,30,31. During PD, the tissue is dissected off the retroperitoneal space32. Therefore, FS analyses in this area are limited as are extensions of further resection unless concomitant arterial resections will be conducted33. The attempt to revise a margin varied among the studies. Despite aggressive re-resection approaches in the majority of studies, there was a clear disbalance of reported total pancreatectomies and extended resections24,26,27. The study by Crippa et al. concluded that conversion to total pancreatectomy is not associated with a survival benefit if compared to patients with PD (HR 1.89, 95% CI 1.08–3.31). Yet, more than 80% of their total pancreatectomy cohort had a residual tumor at the medial, retroperitoneal, and circumferential margins (1-mm). In contrast to this, the study by Schmidt et al. demonstrated a survival benefit if isolated intraoperative positive pancreatic neck margins (HR 0.69, 95% CI 0.36–1.32) were present (0-mm), but they excluded patients with R0R0-resections which further limits the validity of total pancreatectomy.
The implementation of standardized protocols for pathological workup rapidly increased the number of reported R1-rates from 20% to around 80%34,35. Moreover, rigorous pathological sectioning and sampling techniques were also associated with a higher rate of detecting R1-resections at permanent section analyses9,36. Although the pooled rate of false-negative results was below 2% across all studies, the study by Schmidt et al. reported a false-negative rate of 20% but outlined no further information of their pathological workup27. Diagnosis of malignant pancreatic lesions on FS can be challenging if chronic fibrosing pancreatitis is present37. The main drawback of its reliability is in case of a negative result as the sensitivity ranges between 33 and 80% while having a specificity of 100%38. As the use of neoadjuvant therapy for borderline resectable pancreatic cancer is further emerging, the accuracy of FS analyses might be restricted39. Nevertheless, the false-negativity rate of FS analyses was 3% and 4% in the studies including neoadjuvant therapies20,24 but the pathological assessment of FS was briefly mentioned in two studies19,23.
Recent studies determined the involvement of multiple margins and individual margins to be an independent prognostic factor40,41. However, positive resection margins might also indicate a biologically more aggressive tumor rather than simply reflecting a meticulous pathological sampling or insufficient surgical technique42. Recurrent disease after surgery is frequently observed, so that pancreatic cancer remains a systemic disease43,44. Although isolated local recurrence is less frequent than distant metastasis, the long-term survival is similar45. We identified distinct pathological characteristics between the study groups suggesting an advanced disease in the R1R1-group and R1R0-group as nodal metastases were more present in these groups compared to the R0R0-group. Moreover, 50% of the margin revisions in the studies failed to achieve negative margins, implying this theory of more advanced tumor biology in the R1R0- and R1R1-group. Notwithstanding that other prognostic histopathological features as lymphovascular and perineural invasion were not significantly different, one has to consider that less than half of the included studies disclosed these pathological characteristics in their analyses which were responsible for heterogeneity between the studies.
There are several limitations to this meta-analysis. All included studies were retrospective studies with a considerable selection bias. The simultaneous positivity of other margins was not amenable to further subgroup analyses or meta-regressions as were other important covariates such as specific neoadjuvant or adjuvant approaches. Although we detected several factors causing heterogeneity in the present study, meta-regressions were not feasible and a random-effects model was assumed as unexplained heterogeneity might still remain between the included studies (e.g. different sampling techniques). Certainly, the main bias in the studies was the use of heterogeneous definitions of margin clearance as well as margin terminologies although recent studies adopted the new R-status of 1-mm clearance46,47. The overall survival benefit of intraoperative margin revision was confirmed by a subgroup analysis of studies applying the 1-mm clearance. This might explain why we observed a survival benefit in our analysis in contrast to the previously published meta-analyses despite the range of reported R1-rates were identical. Still, the effort of intraoperative tumor clearance at the pancreatic neck should respect the tumor biology. Therefore, we recommend at our institution a revision of the pancreatic neck only if none of the other margins remain positive. Ideally, the benefit of intraoperative margin clearance should be addressed in a randomized trial comparing FS analyses to achieve margin clearance in borderline resectable pancreatic cancer with standardized pathological protocols, surgical techniques as well as clearly defined margins. In conclusion, precise surgical techniques combined with standardized pathological assessments are currently the major pillars of a survival benefit in the backdrop of neoadjuvant and immunooncologic approaches following PD48.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pereira, S. P. et al. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol https://doi.org/10.1016/S2468-1253(19)30416-9 (2020).
Adamu, M. et al. Validation of prognostic risk scores for patients undergoing resection for pancreatic cancer. Pancreatology https://doi.org/10.1016/j.pan.2018.05.005 (2018).
Sinn, M. et al. CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol 35, 3330–3337. https://doi.org/10.1200/JCO.2017.72.6463 (2017).
Kleeff, J. et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2, 16022. https://doi.org/10.1038/nrdp.2016.22 (2016).
Yang, C. et al. Postoperative course and prognostic value of circulating angiogenic cytokines after pancreatic cancer resection. Oncotarget 8, 72315–72323. https://doi.org/10.18632/oncotarget.20315 (2017).
Birgin, E., Hablawetz, P., Teoule, P., Ruckert, F. & Wilhelm, T. J. Chronic pancreatitis and resectable synchronous pancreatic carcinoma: a survival analysis. Pancreatology 18, 394–398. https://doi.org/10.1016/j.pan.2018.04.009 (2018).
Raut, C. P. et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann. Surg. 246, 52–60. https://doi.org/10.1097/01.sla.0000259391.84304.2b (2007).
Petrucciani, N., Debs, T., Nigri, G., Gugenheim, J. & Ramacciato, G. What is the impact of intraoperative reresection after a positive pancreatic margin frozen section in the era of perioperative therapies?. Ann. Surg. 267, e55–e56. https://doi.org/10.1097/SLA.0000000000002090 (2018).
Chandrasegaram, M. D. et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br. J. Surg. 102, 1459–1472. https://doi.org/10.1002/bjs.9892 (2015).
Yin, Z. et al. Revision of surgical margin under frozen section to achieve R0 status on survival in patients with pancreatic cancer. J. Gastrointest. Surg. 22, 1565–1575. https://doi.org/10.1007/s11605-018-3806-x (2018).
Barreto, S. G., Pandanaboyana, S., Ironside, N. & Windsor, J. A. Does revision of resection margins based on frozen section improve overall survival following pancreatoduodenectomy for pancreatic ductal adenocarcinoma? A meta-analysis. HPB (Oxford) 19, 573–579. https://doi.org/10.1016/j.hpb.2017.03.006 (2017).
Petrucciani, N. et al. Frozen section analysis of the pancreatic margin during pancreaticoduodenectomy for cancer: Does extending the resection to obtain a secondary R0 provide a survival benefit? Results of a systematic review. Pancreatology 16, 1037–1043. https://doi.org/10.1016/j.pan.2016.09.004 (2016).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. https://doi.org/10.1371/journal.pmed.1000097 (2009).
Julian, P. T. H. & Sally, G. (eds) Cochrane Handbook for Systematic Reviews of Interventions (Wiley, Hoboken, 2011).
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S. & Sydes, M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16. https://doi.org/10.1186/1745-6215-8-16 (2007).
Parmar, M. K., Torri, V. & Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 17, 2815–2834 (1998).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560. https://doi.org/10.1136/bmj.327.7414.557 (2003).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Fatima, J. et al. Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch. Surg. 145, 167–172. https://doi.org/10.1001/archsurg.2009.282 (2010).
Kooby, D. A. et al. Value of intraoperative neck margin analysis during Whipple for pancreatic adenocarcinoma: a multicenter analysis of 1399 patients. Ann. Surg. 260, 494–501. https://doi.org/10.1097/SLA.0000000000000890 (2014).
Mathur, A. et al. Margin status impacts survival after pancreaticoduodenectomy but negative margins should not be pursued. Am. Surg. 80, 353–360 (2014).
Nitschke, P. et al. Impact of intraoperative re-resection to achieve R0 status on survival in patients with pancreatic cancer: a single-center experience with 483 patients. Ann. Surg. 265, 1219–1225. https://doi.org/10.1097/SLA.0000000000001808 (2017).
Pang, T. C. et al. Frozen section of the pancreatic neck margin in pancreatoduodenectomy for pancreatic adenocarcinoma is of limited utility. Pathology 46, 188–192. https://doi.org/10.1097/PAT.0000000000000072 (2014).
Zhang, B. et al. Revision of pancreatic neck margins based on intraoperative frozen section analysis is associated with improved survival in patients undergoing pancreatectomy for ductal adenocarcinoma. Ann. Surg. https://doi.org/10.1097/SLA.0000000000003503 (2019).
Hernandez, J. et al. Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann. Surg. 250, 76–80. https://doi.org/10.1097/SLA.0b013e3181ad655e (2009).
Crippa, S. et al. Positive neck margin at frozen section analysis is a significant predictor of tumour recurrence and poor survival after pancreatodudenectomy for pancreatic cancer. Eur. J. Surg. Oncol. https://doi.org/10.1016/j.ejso.2020.02.013 (2020).
Schmidt, C. M. et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery 142, 572–578. https://doi.org/10.1016/j.surg.2007.07.016 (2007).
Neoptolemos, J. P., Ghaneh, P., Kleeff, J., Halloran, C. M. & Buchler, M. W. Response to comment on “The Impact of Positive Resection Margins on Survival and Recurrence Following Resection and Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma” by Niccolo Petrucciani, MD, PhD, FACS, Laura Antolino, MD, Giovanni Moschetta, MD, Giovanni Ramacciato, MD FACS. Ann. Surg. 270, e130–e131. https://doi.org/10.1097/SLA.0000000000003371 (2019).
Weitz, J., Rahbari, N., Koch, M. & Buchler, M. W. The, “artery first” approach for resection of pancreatic head cancer. J. Am. Coll. Surg. 210, e1-4. https://doi.org/10.1016/j.jamcollsurg.2009.10.019 (2010).
Verbeke, C. S. & Menon, K. V. Redefining resection margin status in pancreatic cancer. HPB (Oxford) 11, 282–289. https://doi.org/10.1111/j.1477-2574.2009.00055.x (2009).
Menon, K. V., Gomez, D., Smith, A. M., Anthoney, A. & Verbeke, C. S. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB (Oxford) 11, 18–24. https://doi.org/10.1111/j.1477-2574.2008.00013.x (2009).
Birgin, E. et al. Early postoperative pancreatitis following pancreaticoduodenectomy: what is clinically relevant postoperative pancreatitis?. HPB (Oxford) 21, 972–980. https://doi.org/10.1016/j.hpb.2018.11.006 (2019).
Mollberg, N. et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann. Surg. 254, 882–893. https://doi.org/10.1097/SLA.0b013e31823ac299 (2011).
Verbeke, C. S. et al. Redefining the R1 resection in pancreatic cancer. Br. J. Surg. 93, 1232–1237. https://doi.org/10.1002/bjs.5397 (2006).
Rau, B. M. et al. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery 152, S103-111. https://doi.org/10.1016/j.surg.2012.05.015 (2012).
Gebauer, F. et al. Resection margin clearance in pancreatic cancer after implementation of the leeds pathology protocol (LEEPP): clinically relevant or just academic?. World J. Surg. 39, 493–499. https://doi.org/10.1007/s00268-014-2808-4 (2015).
Cioc, A. M., Ellison, E. C., Proca, D. M., Lucas, J. G. & Frankel, W. L. Frozen section diagnosis of pancreatic lesions. Arch. Pathol. Lab. Med. 126, 1169–1173. https://doi.org/10.1043/0003-9985(2002)126%3c1169:FSDOPL%3e2.0.CO;2 (2002).
Nelson, D. W., Blanchard, T. H., Causey, M. W., Homann, J. F. & Brown, T. A. Examining the accuracy and clinical usefulness of intraoperative frozen section analysis in the management of pancreatic lesions. Am. J. Surg. 205, 613–617. https://doi.org/10.1016/j.amjsurg.2013.01.015 (2013).
Ferrone, C. R. et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 261, 12–17. https://doi.org/10.1097/SLA.0000000000000867 (2015).
Ghaneh, P. et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann. Surg. 269, 520–529. https://doi.org/10.1097/SLA.0000000000002557 (2019).
Pine, J. K. et al. Prospective assessment of resection margin status following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma after standardisation of margin definitions. Pancreatology https://doi.org/10.1016/j.pan.2020.01.004 (2020).
Kimbrough, C. W., StHill, C. R., Martin, R. C., McMasters, K. M. & Scoggins, C. R. Tumor-positive resection margins reflect an aggressive tumor biology in pancreatic cancer. J. Surg. Oncol. 107, 602–607. https://doi.org/10.1002/jso.23299 (2013).
Jones, R. P. et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surg. https://doi.org/10.1001/jamasurg.2019.3337 (2019).
Rahbari, N. N. et al. AB0 blood group and prognosis in patients with pancreatic cancer. BMC Cancer 12, 319. https://doi.org/10.1186/1471-2407-12-319 (2012).
Kalisvaart, M. et al. Recurrence patterns of pancreatic cancer after pancreatoduodenectomy: systematic review and a single-centre retrospective study. HPB (Oxford) https://doi.org/10.1016/j.hpb.2020.01.005 (2020).
Chang, D. K. et al. Margin clearance and outcome in resected pancreatic cancer. J. Clin. Oncol. 27, 2855–2862. https://doi.org/10.1200/JCO.2008.20.5104 (2009).
Liu, L. et al. Superior mesenteric artery margin of posttherapy pancreaticoduodenectomy and prognosis in patients with pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol. 39, 1395–1403. https://doi.org/10.1097/PAS.0000000000000491 (2015).
Neoptolemos, J. P. et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 15, 333–348. https://doi.org/10.1038/s41575-018-0005-x (2018).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We thank Dr. Nitschke for sharing individual participant data of his study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
E.B. and N.N.R. designed the study; E.B. and E.R. performed the literature searches; E.B., E.R., P.T., F.R., C.R., and N.N.R. conducted the review and extracted the data, E.B. performed the meta-analysis; and E.B. and E.R. performed the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Birgin, E., Rasbach, E., Téoule, P. et al. Impact of intraoperative margin clearance on survival following pancreatoduodenectomy for pancreatic cancer: a systematic review and meta-analysis. Sci Rep 10, 22178 (2020). https://doi.org/10.1038/s41598-020-79252-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79252-8
This article is cited by
-
Utility and diagnostic accuracy of intraoperative frozen sections in hepato-pancreato-biliary surgical pathology
Langenbeck's Archives of Surgery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.