Abstract
Camptothecin the third most in demand alkaloid, is commercially extracted in India from the endangered plant, Nothapodytes nimmoniana. Endophytes, the microorganisms that reside within plants, are reported to have the ability to produce host–plant associated metabolites. Hence, our research aims to establish a sustainable and high camptothecin yielding endophyte, as an alternative source for commercial production of camptothecin. A total of 132 endophytic fungal strains were isolated from different plant parts (leaf, petiole, stem and bark) of N. nimmoniana, out of which 94 were found to produce camptothecin in suspension culture. Alternaria alstroemeriae (NCIM1408) and Alternaria burnsii (NCIM1409) demonstrated camptothecin yields up to 426.7 ± 33.6 µg/g DW and 403.3 ± 41.6 µg/g DW, respectively, the highest reported production to date. Unlike the reported product yield attenuation in endophytes with subculture in axenic state, Alternaria burnsii NCIM1409 could retain and sustain the production of camptothecin up to ~ 200 μg/g even after 12 continuous subculture cycles. The camptothecin biosynthesis in Alternaria burnsii NCIM1409 was confirmed using 13C carbon labelling (and cytotoxicity analysis on different cancer cell lines) and this strain can now be used to develop a sustainable bioprocess for in vitro production of camptothecin as an alternative to plant extraction.
Similar content being viewed by others
Introduction
Camptothecin, first reported in 19661, is a monoterpene indole alkaloid. It is commercially produced from plants, mainly Camptotheca acuminata and Nothapodytes nimmoniana2. Camptothecin is the third most in-demand alkaloid after taxol and vinca-alkaloids for anti-cancer applications, and inhibits DNA topoisomerase I in cancer cells leading to cell death3. Among several camptothecin derivatives, topotecan, irinotecan and belotecan were approved and marketed as anti-cancer drugs, while others including silatecan, cositecan, lipotecan, simmitecan, chimmitecan, exatecan, namitecan, lurtotecan, elomtecan, diflomotecan, gimatecan, tenifatecan and genz-644282 are under various stages of clinical investigation4,5. Owing to the high demand of camptothecin, both the plants C. acuminata and N. nimmoniana are endangered and overharvested6. In the Western Ghats of India, complete trees of N. nimmoniana are uprooted for camptothecin extraction. To produce 1 ton of camptothecin, around 1000–1500 tons of N. nimmoniana wood chips are needed7. Hence, the annual demand for N. nimmoniana plants has been more than 1000 tons in recent years6. Thus, to prevent such plant sources from extinction and to meet the ever-increasing demand of camptothecin, an alternative means of camptothecin production is needed.
It is well known that the endophytes can have the potential to produce their host plant-based metabolites. Since the discovery of the first taxol producing endophyte8, endophytes in general have gained attention as a potent alternative source of plant metabolites. Though several successful attempts have been made to isolate endophytes, the commercial production of bioactive compounds using endophytes has not yet been established. The bottleneck has been the inconsistent product yield, which is generally reported to be lost with successive subculture under axenic state9. Many camptothecin producing endophytes have been reported in the last decade. However, the yields reported have been low (0.5–45 µg/g) and inconsistent with subculture in axenic state (Table 1).
In this study, we isolated a high camptothecin yielding endophyte from N. nimmoniana, demonstrating sustainable production of camptothecin in suspension culture. Increased relative abundance of 13C labelled camptothecin molecules in the axenic culture extracts, when the fungus was cultivated in the presence of d-[U-13C]-glucose, demonstrated inherent biosynthesis of camptothecin in the isolated organism. Also, the cytotoxic effect of the crude extract of camptothecin from the fungal suspension culture was demonstrated on colon, lung, ovarian and breast cancer cell lines.
Results
Isolation of camptothecin yielding endophytes from Nothapodytes nimmoniana
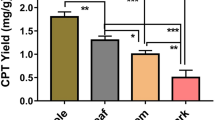
Endophytes residing in the plants may vary with the type of plant tissue10,11. Therefore, leaves, petioles, stem and bark regions from Nothapodytes nimmoniana (J.Graham) Mabb. were used as explants for the comprehensive isolation of endophytes. Endophytes emerged from the cut ends of the explants after 5–7 days of incubation in potato dextrose agar medium (HiMedia, Mumbai). A total of 132 visually distinct endophytic strains were isolated from the explants of N. nimmoniana.
These isolated endophytes were then individually screened for their ability to produce camptothecin in suspension. Suspension cultures were initiated in duplicate and the biomass was harvested and dried for extraction of camptothecin for quantitative analysis using high performance liquid chromatography (HPLC). Among the 132 different endophytic isolates, 94 produced camptothecin in culture, with yields ranging from 0.1 to 400 µg/g DW (dry weight of biomass) (Supplementary Table S1).
Four of the highest yielding endophytes were selected for further analysis and their camptothecin yield was confirmed again in freshly initiated cultures (in triplicate). Camptothecin yields obtained from the suspension cultures of these strains are shown in Table 2. Strain P4-6-PE2, isolated from the petiole region of a young plant gave the highest yield of camptothecin in suspension (426.7 ± 33.6 µg/g), followed by strain P4-4-LE2 isolated from the leaf region of the plant, with a yield of 403.3 ± 41.6 µg/g. It is to be noted that the yield of camptothecin obtained from the natural plant of C. acuminata varies from 0.5 to 2.5 mg/g12 and the yield from N. nimmoniana ranges from 0.8 to 3 mg/g13.
Camptothecin production was confirmed in the suspension cultures of these camptothecin yielding endophytic strains using tandem mass spectrometry (LC–MS/MS). As expected, a precursor mass to charge ratio (m/z) of 349.12 corresponding to that of camptothecin was observed in the authentic standard and in the extracts from all the shortlisted (top 4) endophytic strains (Fig. 1). Further, for structural confirmation, the precursor m/z (349.12) was fragmented by applying collision energy of 35 electron Volts (eV) and an m/z of 305.13 (fragment) after the loss of CO2 was witnessed (Supplementary Fig. S1 online), confirming the presence of camptothecin in the extract sample.
The presence of camptothecin in the endophytic extracts was confirmed using thin layer chromatography (TLC). Along with the camptothecin producing culture extract, a non-camptothecin producing culture extract was also spotted on the TLC plate for analysis. As shown in Supplementary Fig. S2 online, all four camptothecin yielding endophytic extracts gave spots corresponding to that of authentic camptothecin. In contrast, the spot was absent from a non-camptothecin producing strain.
Molecular characterization of the selected high camptothecin yielding endophytes
Endophytic strains grown on plates made with PDA medium showed difference in their morphological appearance and all of them formed spores when viewed under a microscope (Supplementary Fig. S3 online). All four isolates were found to be different species of Alternaria genus. Among the four, the highest yielding strain was found to be A. alstroemeriae with a yield of 426.7 ± 33.6 µg/g and the second highest was found to be A. burnsii with a yield of 403.3 ± 41.6 µg/g. Accession numbers from NCBI Genbank and the respective catalogue numbers of each strain deposited in NCIM have been listed in Table 2.
Sustainable production of camptothecin in the highest yielding endophyte
Sustainable production of camptothecin in the two highest camptothecin yielding endophytes was assessed in suspension culture generated from the 1st through the 12th generation slants. The highest yielding strain (A. alstroemeriae NCIM1408) demonstrated a sharp decrease in the camptothecin yield from 426.7 ± 33.6 µg/g DW in culture developed from its 1st generation slant to 17.9 ± 0.7 µg/g DW in culture from its 12th generation slant. This result was in accordance with the well-known product yield attenuation observed in axenic cultures of endophytes (Table 1). Interestingly, unlike the highest yielding strain, the second-highest yielding strain (A. burnsii NCIM1409) could demonstrate sustainable production of camptothecin (up to ~ 200 μg/g) in culture even from its 12th generation slant used as inoculum. As shown in Fig. 2, after an initial decrease in the yield to 254.1 ± 17.9 µg/g DW in cultures developed from the second generation slant, this endophyte demonstrated a steady yield of ~ 200 µg/g DW in cultures from 3rd till 12th generation slant.
These results demonstrate that the highest yielding strain may not always be a sustainable producer, and it is equally important to consider sustainable production along with product yield of the metabolite during the screening and selection of a potential endophyte for successful bioprocess development.
Confirmation of camptothecin biosynthesis by A. burnsii via carbon metabolism
Existence of host independent biosynthetic machinery in endophytes has been doubted due to product yield attenuation under an axenic state14. To confirm the endophytic strain has the endogenous ability to synthesize camptothecin, the cultures were fed with d-[U-13C]-glucose and 13C label incorporation into camptothecin was monitored. The fungal cultures fed with d-glucose and d-[U-13C]-glucose showed similar growth and camptothecin production, evident from the biomass (~ 8 g/l DW) and camptothecin yield (~ 200 µg/g DW) respectively, achieved after 8 days under similar cultivation conditions. An m/z of 349.12 corresponds to that of camptothecin with all unlabelled carbon atoms and the presence of one 13C labeled carbon would increase the molecular weight of camptothecin by 1 dalton. Since camptothecin has 20 carbons, there is a possibility that 1–20 carbon atoms in the camptothecin molecule produced can get labelled in the crude extract of camptothecin when grown on d-[U-13C]-glucose. This was verified by tandem mass spectrometry confirming the increase in the molecular mass of camptothecin corresponding to the number of carbon atoms labelled in it. The mass spectrum revealed majority of carbon atoms (2–20) getting labelled in camptothecin. This was further supported by the MS–MS fragmentation pattern of the most abundantly labelled camptothecin molecules in the sample after the removal of labelled CO2 (Supplementary Table S2, Supplementary Fig. S4 online). Moreover, the absence of unlabelled camptothecin mass peak in d-[U-13C]-glucose fed sample (Fig. 3c) and absence of labelled camptothecin mass peak in d-glucose fed sample (Fig. 3a,b) could also substantiate labelled glucose metabolism and its incorporation in the synthesis of camptothecin by the organism.
Confirmation of camptothecin biosynthesis in A. burnsii using d-[U-13C]-glucose. (a) Mass spectrum of the crude extract of camptothecin shows that non labelled camptothecin m/z of 349.12 was visualized only in d-glucose fed samples and (b) m/z corresponding to labelled camptothecin was not present in them. (c) Non labelled camptothecin m/z of 349.12 was not visualized in d-[U-13C]-glucose fed cultures (d) an m/z of 367.17 corresponding to 18 carbons in camptothecin being labelled was seen.
Interestingly, the mass peak corresponding to camptothecin with 18 labelled carbons (13C18C2H16N2O4) showed highest relative abundance (Fig. 3d; Supplementary Fig. S4 online) while m/z corresponding to 20 labelled carbons (13C20H16N2O4) in camptothecin was relatively less abundant. This was presumably due to the fact that potato infusion present in the medium is a source of unlabelled organic carbon which could have diluted the 13C incorporation from d-[U-13C]-glucose.
Cytotoxicity analysis of the camptothecin extract from A. burnsii on cancer cell lines
As the source of camptothecin was novel, the cytotoxicity analysis was also carried out to further substantiate the potential commercial utilization of the microbial strain for various anti-cancer applications. The cytotoxicity of the crude extract of camptothecin from A. burnsii was tested on various cancer cell lines which includes human breast cancer (MCF7), human ovarian adenocarcinoma (SKOV3), human non-small cell lung carcinoma (H1299), colon adenocarcinoma (Caco-2, HT29), and non-cancerous embryonic kidney (HEK293T) cell lines. The IC50 values determined for the camptothecin extract and standard camptothecin against all the cancer cell lines studied have been shown in Table 3 as calculated from the plot of % cell viability vs. log concentration of the extract (Supplementary Fig. S5 online). Apart from MCF7, all other cancer cell lines showed a lower IC50 value for camptothecin extract from A. burnsii than the standard camptothecin (≥ 90% pure), which could be due to the effect of other metabolites present in the crude extract along with camptothecin (Table 3). Among all the cancer cell lines used, camptothecin extract from A. burnsii was found more toxic toward lung and colon cancer cells which is in accordance with the reports on derivatives of camptothecin4.
The cytotoxicity of the camptothecin extract from A. burnsii was further supported by clonogenic (Fig. 4) and wound healing assay (Fig. 5), where colony formation and healing were inhibited on the selected SKOV3 cell line.
Minimal wound healing observed with A. burnsii extract-treated SKOV3 cell line. Control cells showed 29% and 39% healing after 24 and 48 h respectively, while the standard camptothecin treated cells showed 20% and 19% healing after 24 and 48 h respectively. The crude extract-treated cells showed only 1% healing after 48 h.
Discussion
Extensive and invasive natural plant extraction of camptothecin to meet its increasing market demand, has endangered N. nimmoniana plant species in India6. Hence, an alternative method of its production is the need of the hour to prevent the natural plant source from extinction. Endophytes are being explored as one a potential alternative source for high-value plant metabolite production. A wide variation in the metabolite yield in plants due to climatic and geographical factors, slow growth rate of the plant and un-availability of the plant material in the wild, have been the key limitations to meet the market demand of camptothecin. On the contrary, a microbial fermentation process, which is amenable to optimization and scale-up for enhanced yield and productivity, can be an excellent alternative to the natural plant source for large-scale and sustainable production of camptothecin under in vitro conditions.
In this study endophytes were isolated from the plant parts including leaf, petiole, stem and bark regions as the distribution of endophytes in plants can vary with respect to plant tissue type. Also care was taken to surface sterilize the explants for endophyte isolation immediately after excision since the time between excision and isolation can reduce the viability of endophytes residing in the plant part15. Here, we have reported for the first time, a high camptothecin yielding endophyte which could demonstrate sustainable production of camptothecin in axenic state for more than twelve subculture cycles in liquid medium. Production of camptothecin was confirmed by various methods of qualitative and quantitative analysis, like HPLC, TLC and LC–MS/MS.
Though numerous endophytes are present in the plant, only a few of the isolated strains have the potential to produce the host-specific metabolite and many have shown attenuation upon subculturing9,16. Various factors were stated to play a role in the endophyte’s non-sustainability, including their evolutionary relationship with the host plant17, and lack of host stimuli18 or gene silencing under axenic conditions19. Thus, comprehensive bioprospecting and screening are required to identify a sustainable and high product yielding strain of endophyte. In this study, sustainability in camptothecin production was tested in the highest yielding strains (A. alstroemeriae NCIM1408) and as expected the yield attenuated upon subsequent sub-culturing. However, in case of the second highest yielding strain, an initial decline in yield was observed and later the strain sustained the yield at ~ 200 µg/g DW even after 12 subculture cycles. These results demonstrate that the highest yielding strain may not always be a sustainable producer, and it is equally important to consider sustainable production along with product yield of the metabolite during the screening and selection of a potential endophyte for successful bioprocess development.
Also the presence of host independent biosynthetic machinery in the endophytes was verified with the sustainable producing strain by feeding them with d-[U-13C]-glucose, as the endogenous potential of endophytes were often doubted14. Mass peaks corresponding to camptothecin with labelled carbons were detected in case of d-[U-13C]-glucose fed samples, while the unlabelled camptothecin mass peak was absent in these samples, which confirmed the innate ability of the endophyte to produce camptothecin via its metabolic processes. This can be further supported by the literature evidence on enhancement and revival of attenuated camptothecin production upon media optimization20, elicitation21 and DNA methyltransferase treatment22. This indicates that potential of metabolite production in endophytes becomes cryptic under certain conditions and it can be restored upon alteration in the culture conditions.
As the source of camptothecin was novel in the study, a cytotoxicity analysis of the crude extract of camptothecin was carried out on various cancerous and a non-cancerous cell lines. Interestingly, the extract was found to be more cytotoxic than the standard on most of the cell lines tested, which could be due to the synergistic effect of other compounds present in the crude extract23. The extract was also found to completely inhibit formation of colonies when clonogenic assay was performed with the selected cell line SKOV3 and just 1% healing after 48 h when wound healing assay was performed. This further substantiates the cytotoxicity of the extract on cancerous cell lines.
In conclusion, a high camptothecin yielding fungal endophyte A. burnsii NCIM1409 was isolated from N. nimmoniana which could demonstrate sustainable production of camptothecin under axenic conditions. Production of camptothecin by the microbial culture was verified via 13C labeled glucose metabolism leading to labeled camptothecin biosynthesis. Interestingly, the crude camptothecin extract from the fungi demonstrated better cytotoxic activity (lower IC50) than the standard camptothecin (> 90% purity) against the tested lung, colon and ovarian cancer cell lines and relatively less cytotoxicity against the non-cancerous cell line. The isolated strain can be used to develop a microbial-based sustainable bioprocess for large scale in vitro production of camptothecin for anti-cancer applications as an alternative to natural plant extraction.
Methods
Isolation of endophytes from Nothapodytes nimmoniana
Endophytes were isolated from 6 different plants of N. nimmoniana cultivated in the University of Agricultural Science, Bengaluru campus (13.0762° N, 77.5753° E). The plant specimen was authenticated as N. nimmoniana (J.Graham) Mabb. of the family Icacinaceae and deposited in Foundation for Revitalisation of Local Health Traditions (FRLH) herbarium, Bengaluru (FRLH 122013). The collected plant material (leaves, petioles, stem and bark) from young and mature plants was washed thoroughly in running tap water to remove the dust particles and then with sterile water. For surface sterilization of the explants, leaves and petioles were treated with 1% (v/v) sodium hypochlorite for 1 min and then washed with sterile distilled water to remove sodium hypochlorite from the plant parts. The washed explants were then treated with 70% (v/v) ethanol for 1 min and then washed again thoroughly with sterile distilled water to remove residual ethanol, if any, from the plant parts. This sterile water wash from the surface sterilized explants was streaked on a potato dextrose agar medium (in petri-plates) to check for the completion/effectiveness of surface sterilization. Absence of any microbial growth on the said medium upon incubation even after 7 days confirmed the effectiveness/completion of surface sterilization. A similar procedure was carried out for the stem and bark regions, except that the treatment with 1% (v/v) sodium hypochlorite was increased to 3 min and that with 70% (v/v) ethanol was up to 5 min. The sterile explants were then cut into small pieces (~ 1 cm2) and placed on the petri dishes having PDA medium (HiMedia, Mumbai) supplemented with streptomycin (100 µg/ml) to prevent any bacterial growth, so as to preferentially isolate the endophytic fungi. The plates with explants were incubated at 28 °C in a biological oxygen demand (BOD) chamber for 7 days to allow the endophytes to emerge and grow from the cut ends. Morphologically distinct endophytes emerged from the cut ends after 5–7 days of incubation and were subsequently removed and plated separately on a fresh medium to obtain pure colonies of the endophytes.
A loop full of the mycelia was streaked on slants made with 5 ml of potato dextrose agar medium (HiMedia, Mumbai) and incubated at 28 °C for 7 days with an initial pH of 5.6. The slants were washed using 5 ml of saline (0.9% (w/v) NaCl) and were used as inoculum (2% v/v) for initiating suspension culture. Suspension cultures (in duplicate) were established with 50 ml of potato dextrose broth (HiMedia, Mumbai) in 250 ml Erlenmeyer flasks at an initial pH of 5.6. The suspension culture was allowed to grow in an incubator shaker at 28 °C and 120 rpm. The shake flasks (in duplicate) were harvested after 8 days of the cultivation period for the estimation of biomass (g/l, DW) and camptothecin yield (μg/g DW) as per the protocol reported elsewhere23,24.
Biomass estimation
The biomass was harvested by centrifuging the cell suspension at 12,857×g for 15 min and discarding the supernatant. The pelleted cells were then washed with distilled water to remove traces of medium components and again separated by centrifugation at 12,857×g for 15 min. Washed biomass was then dried in a hot air oven at 60 °C by spreading it on pre-weighed glass petri plates until constant dry weight was achieved.
Extraction of camptothecin
Camptothecin extraction from the fungal biomass was done as per the protocol reported earlier23. Briefly, the dried biomass (0.3 g) was dissolved in 20 ml of distilled water and homogenized using a mortar and pestle, followed by liquid–liquid extraction, repeated thrice using 50 ml of chloroform: methanol solvent mixture (4:1). The organic layer with extracted camptothecin was then collected and evaporated using a rotary evaporator. The dried camptothecin extract was re-suspended in 1 ml of DMSO:methanol (1:50) and filtered through a 0.2 µm filter for further analysis.
Quantification of camptothecin by HPLC
Twenty microliters of the camptothecin extract was injected in a reverse phase HPLC (LC-20AD, Shimadzu, Japan) at a flow rate of 0.8 ml/ min using 25% acetonitrile as the mobile phase. ODS Hypersil gold column (Thermo Scientific) with a particle size of 5 µm was used as the stationary phase at a column temperature of 30 °C. The absorbance of camptothecin was measured at 254 nm by a photodiode array detector25. Concentration of camptothecin in the sample was estimated using a standard calibration curve of peak area vs. known concentration of camptothecin (Supplementary Fig. S6 online), generated using authentic samples of camptothecin (> 90% purity, Sigma Aldrich, MO, USA). The retention time of camptothecin from the standard was found to be 19.8 min and the area of the peak obtained from the endophytic extracts at the same retention time (Supplementary Fig. S7 online) was compared with the standard plot of camptothecin and the corresponding concentration and yield from the different fungal isolates were calculated.
Identification of camptothecin by LC–MS/MS
Camptothecin production in suspension culture of the high yielding isolates was further confirmed using tandem mass spectroscopy. This was based on qualitative identification of camptothecin, extracted from the harvested biomass, using an orbitrap elite mass spectrometer (ThermoScientific, Breman, Germany). A reverse-phase column (ODS Hypersil C18 column, 256 × 4.5 mm, 5 µ) was connected to a heated electrospray ionization (H-ESI) source with the heater temperature maintained at 325 °C. The sheath gas flow rate was set at 40 arb, aux gas flow rate at 20 arb and sweep gas flow rate at 2 arb. The spray voltage for the H-ESI source was set at 4.5 kV and the capillary temperature was maintained at 300 °C. MS grade water with 0.01% formic acid (solvent A) and acetonitrile (solvent B) in the ratio 3:1 was used as mobile phase at a flow rate of 500 µl/min. Samples were injected into a 20 µl sample loop and analyzed for 60 min in the LC coupled with mass spectrometry set up in a mass scanning range of 300–450 m/z. The precursor ions were subjected to collision induced dissociation (CID) of 35 eV to obtain fragment ions. Camptothecin, with a molecular formula of C20H16N2O4, when analyzed in LC–MS under positive mode, yielded an intact mass [M+H] of 349.12. Further, for structural confirmation, by applying CID of 35 eV, the precursor m/z (349.12) could be fragmented to an m/z of 305.13 with a loss of CO23.
Qualitative analysis of camptothecin by TLC
Presence of camptothecin in the endophytic extracts can also be identified using TLC. TLC was performed using silica gel 60 F254 aluminium plates (Merck, Germany). Extracts of camptothecin (10 µl) from selected endophytes were spotted on the TLC plate with the standard camptothecin sample used as positive control. Chloroform–methanol (20:1) solvent system was used as the mobile phase. The plates were placed in the mobile phase in a pre-saturated chamber and the samples were allowed to be drawn upward on the TLC plates via capillary action for the separation of the sample components. As camptothecin is known to exhibit fluorescence under UV light, the TLC plates were dried and visualized at 254 nm in a UV chamber as per the protocol reported elsewhere26,27.
Molecular characterization of the selected endophytes
Freshly grown mycelia (200 mg) from the suspension cultures of the selected endophytes were taken and washed with ethanol before grinding to a fine powder with liquid nitrogen and sterile sea-sand in a mortar and pestle. The homogenized sample was then transferred into a sterile 1.5 ml microcentrifuge tube and 200 µl of cell-lysis buffer (Nucleo-pore DNA Sure Plant Mini Kit, Genetix Biotech, India) was added. This mixture was incubated at 70 °C for 10 min and then chloroform was added (100 μl) and mixed thoroughly. Phase separation was then carried out in the microfuge tube after centrifugation at 20,000×g for 15 min. The top aqueous layer was separated into a sterile 1.5 ml microcentrifuge tube for the isolation of DNA using nucleopore DNA sure plant mini-kit as per manufacturer’s protocol (Genetix Biotech, India). Genomic DNA was isolated from the four highest yielding endophytic strains.
Isolated genomic DNA was amplified using internal transcribed spacer 1 (ITS1) (5′ TCCGTAGGTGAACCTTGCGG 3′) and internal transcribed spacer 4 (ITS4) (5′ TCCTCCGCTTATTGATATGC 3′) primers28, along with the Phusion High Fidelity DNA polymerase (New England Biolabs, Ipswich). Touch-down method of PCR was used for increased specificity of primer amplification in a Surecycler 8800 (Agilent Technologies, Santa Clara, CA, USA). The upper limit of annealing temperature was set at 65 °C and the lower limit at 53 °C. The PCR conditions used were as follows: initial denaturation for 5 min at 95 °C, 35 cycles of amplification including (1) denaturation at 95 °C for 1 min, (2) primer annealing to DNA at 65–53 °C for 45 s, (3) primer extension at 72 °C for 2 min along with a final extension for 10 min at 72 °C. The purified polymerase chain reaction (PCR) products showed bands between 500 and 700 bp corresponding to that of the conserved ITS regions in fungi. The amplified products were purified using a Qiaquick PCR purification kit as per manufacture’s protocol (Qiagen, California, USA) and the size was confirmed using agarose gel electrophoresis. In the gel made with 0.8% (w/v) agarose, 10 µl of the amplified product and 2 µl of 6X gel loading dye were added in the wells and the system was run at 50 V for 30 min before visualization of the amplicons on the gel under a UV transilluminator (GelDoc, BioRad, Italy). The purified PCR products were then sequenced by Sanger dideoxy method using AB 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Forward and reverse sequences were aligned to identify the consensus sequence consisting of ITS1, 5.8S and ITS4. These sequences were compared against the database of sequences using the nucleotide BLAST algorithm provided by (National Center for Biotechnology Information) NCBI. Also a phylogenetic tree (Supplementary Fig. S8 online) was constructed using maximum likelihood method based on Jukes-Cantor model29 and the analysis was conducted in MEGA730. To increase the level of confidence in the taxonomic identifications, the comparisons were restricted to the sequences from the type material by checking the “sequences from type material” box in the general BLAST page31.The search results that displayed the highest query coverage and maximum score were used to determine the identity of the isolated high yielding endophytes. The sequences were deposited in NCBI and the strains were deposited in the National Collection of Industrial Microorganisms (NCIM) Pune, India.
Investigation of sustainable production of camptothecin in the endophytes
Sustainable production of camptothecin was investigated in the two highest yielding endophytes isolated in the study. Freshly isolated strains (stored as 25% glycerol stocks) were streaked separately as slants made of potato dextrose agar medium (5 ml) (HiMedia, Mumbai) and incubated at 28 °C for 7 days with an initial pH of 5.6. These culture slants were used as inoculum for generating suspension and were considered as the first generation (subculture cycle) slants in the study. After 7 days, single mycelia from the first generation slant was picked and streaked on to the new slants with fresh medium (5 ml of potato dextrose agar) under similar conditions. These were considered as the second generation slants. Similarly, subsequent generation slants (up to 12th) were created after every 7 days of subculture on to fresh medium.
Suspension cultures from each generation slants were initiated as described earlier. After 7 days of growth, the slant-wash with 5 ml of saline (0.9% NaCl) was used as inoculum (2% v/v) for suspension culture. After 8 days of the cultivation period, the shake-flask cultures were harvested (in triplicate) for the estimation of biomass and camptothecin production.
Investigation of camptothecin biosynthesis using 13C labelled glucose
In order to investigate and confirm camptothecin biosynthesis by the endophyte, suspension cultures were initiated using 20 g/l d-[U-13C]-glucose (389374, Sigma Aldrich, MO, USA) as the carbon source in 50 ml of sterilized potato infusion as growth medium in 250 ml Erlenmeyer flasks with 2% (v/v) of inoculum. The suspension cultures were allowed to grow in an incubator shaker at 28 °C and 120 rpm for 8 days of cultivation period before harvest (in duplicate) for the analysis of 13C-labelled camptothecin using LC–MS/MS.
For the preparation of potato infusion, 200 g of fresh potatoes were cleaned, peeled and chopped into smaller pieces and boiled for 15 min in 1000 ml of distilled water at 90 °C. The liquid extract was then filtered through cheesecloth to be used as potato infusion. Extract from the culture grown only in potato infusion was used as a negative control to confirm that no camptothecin production was possible without added glucose in the medium.
Cytotoxicity analysis of extract on cancer cell lines by MTT assay
The crude extract of camptothecin from the selected endophyte A. burnsii was tested for its cytotoxic effect by performing 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) (Sigma) assay on various cancer cell lines including MCF7, SKOV3, H1299, Caco-2, HT29, and HEK293T. All the cell lines were obtained from American Type Culture Collection (ATCC) and were maintained on Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10% fetal bovine serum (FBS) except SKOV3, which was maintained on McCoy's 5A (modified) medium supplemented with 10% FBS at 37 °C in an atmosphere of 5% CO2.
In a 96 well plate, each well was seeded with 2500 cells in a volume of 100 µl of their respective growth medium and incubated for 24 h after which varying concentration (0.01–51.2 µM) of the crude extract of camptothecin and standard camptothecin were added, respectively to the medium. The cells were incubated for 72 h with the drug and then 0.5 mg/ml of MTT reagent in the respective medium was added and incubated for 5 h. Then MTT reagent containing medium was removed and DMSO was added to each well after which the optical density was measured using a 96 well plate reader at a dual-wavelength of 570 and 650 nm. MTT reagent forms formazan crystal within live cells, which then produces purple colour when dissolved in DMSO. The cell viability was calculated by comparing the optical density of the treated wells against the non-treated control wells.
IC50 values were calculated using Graphpad Prism 6 (GraphPad Software, San Diego, CA) from the observed cell viability values and SEM32.
Clonogenic and wound healing assay
To investigate the inhibition of the colony formation of these cells in the presence of crude extract of camptothecin, clonogenic assay was performed. Viable cells (5000 per plate) were seeded and allowed to attach overnight in 60 mm plates. SKOV3 cells were incubated with the extract, at a concentration corresponding to their respective IC25 (58.7 nM), for 14 days in medium to facilitate colony formation. The obtained colonies were washed with PBS and fixed using acetic acid:methanol (3:1). The fixed cells were then stained with 0.2% (w/v) crystal violet. The colonies formed were counted and compared with the DMSO controls in triplicate.
Similarly, a wound-healing assay was performed to study the inhibition of these cells’ migration ability in the presence of the crude extract of camptothecin. SKOV3 cells were made to form a confluent monolayer on 60 mm plates by seeding 5 million cells on each plate. Using a 200 µl micropipette tip, a wound or scratch was created on the confluent monolayer. The wounded cells were then rinsed with PBS and treated with the crude extract of camptothecin at a concentration corresponding to their IC25 (58.7 nM). The control plates were treated with an equal volume of DMSO. Using phase-contrast microscopy, the wound closure by cell migration was monitored and imaged at 0, 24 and 48 h at the same spot. The wound area was calculated and the percentage of wound healing was compared between the drug-treated cells and DMSO treated cells as control32.
Statistical analysis
Statistical analyses were performed using Graphpad Prism 6 (GraphPad Software, San Diego, CA, USA). All experiments were performed in triplicates, and the results of MTT and clonogenic assay were analysed with t test and that of wound healing assay was analysed with two-way ANOVA followed by a Dunnett’s posthoc test. The values of *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 were considered as statistically significant.
References
Wall, M. E. et al. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 88, 3888–3890 (1966).
Kusari, S., Zühlke, S. & Spiteller, M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J. Nat. Prod. 72, 2–7 (2009).
Shweta, S. et al. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry 71, 117–122 (2010).
Pizzolato, J. F. & Saltz, L. B. The camptothecins. Lancet 361, 2235–2242 (2003).
Musiol, R. An overview of quinoline as a privileged scaffold in cancer drug discovery. Expert Opin. Drug Discov. 12, 583–597 (2017).
Isah, T. & Mujib, A. Camptothecin from Nothapodytes nimmoniana: Review on biotechnology applications. Acta Physiol. Plant. 37, 1–14 (2015).
Watase, I., Sudo, H., Yamazaki, M. & Saito, K. Regeneration of transformed Ophiorrhiza pumila plants producing camptothecin. Plant Biotechnol. 21, 337–342 (2004).
Stierle, A., Strobel, G. & Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260, 214–216 (1993).
Kusari, S. & Spiteller, M. Are we ready for industrial production of bioactive plant secondary metabolites utilizing endophytes?. Nat. Prod. Rep. 28, 1203–1207 (2011).
Collado, J., Platas, G., Gonzalez, I. & Pelaez, F. Geographical and seasonal influences on the distribution of fungal endophytes in Quercus ilex. New Phytol. 144, 525–532 (1999).
Fisher, P. J., Petrini, O. & Sutton, B. C. A comparative study of fungal endophytes in leaves, xylem and bark of Eucalyptus in Australia and England. Sydowia 45, 338–345 (1993).
Pi, Y. et al. Examination of camptothecin and 10-hydroxycamptothecin in Camptotheca acuminata plant and cell culture, and the affected yields under several cell culture treatments. Biocell 34, 139–143 (2010).
Padmanabha, B. V. et al. Patterns of accumulation of camptothecin, an anti-cancer alkaloid in Nothapodytes nimmoniana Graham, in the Western Ghats, India: Implications for identifying high-yielding sources of the alkaloid. Curr. Sci. 90, 95–100 (2006).
Venugopalan, A. & Srivastava, S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv. 33, 873–887 (2015).
Golinska, P. et al. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 108, 267–289 (2015).
Deepika, V. B., Murali, T. S. & Satyamoorthy, K. Modulation of genetic clusters for synthesis of bioactive molecules in fungal endophytes: A review. Microbiol. Res. 182, 125–140 (2016).
Zhao, J., Zhou, L., Wang, J. & Shan, T. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 1, 567–576. https://doi.org/10.1016/j.phytochem.2012.07.021 (2010).
El-Elimat, T. et al. Flavonolignans from Aspergillus iizukae, a fungal endophyte of milk thistle (Silybum marianum). J. Nat. Prod. 77, 193–199 (2014).
Priti, V. et al. How promising are endophytic fungi as alternative sources of plant secondary metabolites?. Curr. Sci. 97, 477–478 (2009).
Clarance, P., Khusro, A., Lalitha, J., Sales, J. & Paul, A. Optimization of camptothecin production and biomass yield from endophytic fungus Fusarium solani strain ATLOY-8. J. Appl. Pharm. Sci. 9, 35–46 (2019).
Pu, X. et al. Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: Isolation, identification, and fermentation conditions optimization for camptothecin production. Appl. Microbiol. Biotechnol. 97, 9365–9375 (2013).
Vasanthakumari, M. M. et al. Restoration of camptothecine production in attenuated endophytic fungus on re-inoculation into host plant and treatment with DNA methyltransferase inhibitor. World J. Microbiol. Biotechnol. 31, 1629–1639 (2015).
Venugopalan, A., Potunuru, U. R., Dixit, M. & Srivastava, S. Effect of fermentation parameters, elicitors and precursors on camptothecin production from the endophyte Fusarium solani. Bioresour. Technol. 206, 104–111 (2016).
Venugopalan, A. & Srivastava, S. Enhanced camptothecin production by ethanol addition in the suspension culture of the endophyte, Fusarium solani. Bioresour. Technol. 188, 251–257 (2015).
Shweta, S., Gurumurthy, B. R., Ravikanth, G., Ramanan, U. S. & Shivanna, M. B. Endophytic fungi from Miquelia dentata Bedd., produce the anti-cancer alkaloid, camptothecine. Phytomedicine 20, 337–342 (2013).
Rehman, S. et al. An endophytic Neurospora sp. from Nothapodytes foetida producing camptothecin. Appl. Biochem. Microbiol. 44, 203–209 (2008).
Lorence, A., Medina-Bolivar, F. & Nessler, C. L. Camptothecin and 10-hydroxycamptothecin from Camptotheca acuminata hairy roots. Plant Cell Rep. 22, 437–441 (2004).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications (eds Innis, M. A. et al.) 315–322 (Academic Press, New York, 1990).
Jukes, T. H. & Cantor, C. R. Evolution of protein molecules. In Mammalian Protein Metabolism (ed. Munro, H. N.) 21–132 (Academic, New York, 1969).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Raja, H. A., Miller, A. N., Pearce, C. J. & Oberlies, N. H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 80, 756–770 (2017).
Tentu, S. et al. DHQZ-17, a potent inhibitor of the transcription factor HNF4A, suppresses tumorigenicity of head and neck squamous cell carcinoma in vivo. J. Cell. Physiol. 233, 2613–2628 (2018).
Gurudatt, P. S. et al. Attenuation of camptothecin production and negative relation between hyphal biomass and camptothecin content in endophytic fungal strains isolated from Nothapodytes nimmoniana Grahm (Icacinaceae). Curr. Sci. 98, 1006–1010 (2010).
Acknowledgements
The authors thank the Department of Biotechnology, Indian Institute of Technology Madras for the LC-MS facility and Department of Science and Technology (DST), Government of India for the financial assistance for research (Project number: EMR/2015/001418). The authors thank National Cancer Tissue Biobank (NCTB) facility at the Department of Biotechnology, Indian Institute of Technology Madras for, helping with the sequencing of the fungal endophytes.
Author information
Authors and Affiliations
Contributions
K.M. isolated endophytes, designed and implemented the experiments, analyzed and compiled the results, formulated the manuscript. S.S. being the principal investigator conceptualized and designed the entire study, and contributed in data analysis and manuscript formulation. R.K. assisted in designing the experiments and analyzing the data for cytotoxicity analysis. S.K.R. provided the facilities and assisted and reviewed cytotoxicity analysis. K.S. assisted in collection of the plant material and in designing the experiment on isolation of endophytes. U.S. provided the facilities for isolation of endophytes and assisted and reviewed the data on the isolation of endophytes.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohinudeen, I.A.H.K., Kanumuri, R., Soujanya, K.N. et al. Sustainable production of camptothecin from an Alternaria sp. isolated from Nothapodytes nimmoniana. Sci Rep 11, 1478 (2021). https://doi.org/10.1038/s41598-020-79239-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79239-5
This article is cited by
-
Camptothecin bioprocessing from Aspergillus terreus, an endophyte of Catharanthus roseus: antiproliferative activity, topoisomerase inhibition and cell cycle analysis
Microbial Cell Factories (2024)
-
Production, bioprocessing and antiproliferative activity of camptothecin from Aspergillus terreus, endophyte of Cinnamomum camphora: restoring their biosynthesis by indigenous microbiome of C. camphora
Microbial Cell Factories (2023)
-
Scutellaria baicalensis enhances 5-fluorouracil-based chemotherapy via inhibition of proliferative signaling pathways
Cell Communication and Signaling (2023)
-
Genomic and transcriptomic analysis of camptothecin producing novel fungal endophyte: Alternaria burnsii NCIM 1409
Scientific Reports (2023)
-
Insights into the functional potential of bacterial endophytes from the ethnomedicinal plant, Piper longum L.
Symbiosis (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.


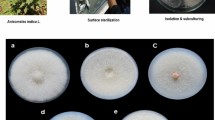


 A. burnsii NCIM1409 and
A. burnsii NCIM1409 and  A. alstroemeriae NCIM1408).
A. alstroemeriae NCIM1408).

