Abstract
Sub-acute mastitis (SAM) is a prevalent disease among lactating women, being one of the main reasons for early weaning. Although the etiology and diagnosis of acute mastitis (AM) is well established, little is known about the underlying mechanisms causing SAM. We collected human milk samples from healthy and SAM-suffering mothers, during the course of mastitis and after symptoms disappeared. Total (DNA-based) and active (RNA-based) microbiota were analysed by 16S rRNA gene sequencing and qPCR. Furthermore, mammary epithelial cell lines were exposed to milk pellets, and levels of the pro-inflammatory interleukin IL8 were measured. Bacterial load was significantly higher in the mastitis samples and decreased after clinical symptoms disappeared. Bacterial diversity was lower in SAM milk samples, and differences in bacterial composition and activity were also found. Contrary to AM, the same bacterial species were found in samples from healthy and SAM mothers, although at different proportions, indicating a dysbiotic ecological shift. Finally, mammary epithelial cell exposure to SAM milk pellets showed an over-production of IL8. Our work therefore supports that SAM has a bacterial origin, with increased bacterial loads, reduced diversity and altered composition, which partly recovered after treatment, suggesting a polymicrobial and variable etiology.
Similar content being viewed by others
Introduction
Human milk is a complex and live fluid, containing a relatively diverse and potential beneficial microbiota under healthy conditions1, which enhances gut microbiota colonization, likely stimulates commensal tolerance and supports the maturation of the immune system2,3,4,5. Occasionally, the lactating mother is afflicted with the development of mastitis, which frequently arises during the first 6 weeks post-partum and is one of the main causes of early weaning6,7,8. According to the World Health Organization (WHO), mastitis affects up to 33% of lactating women9, but this is likely biased by the difficulties for defining the disease. Classically, mastitis is defined as an inflammation of the breast, accompanied of infection or not6,9,10. Most researchers consider that lactational mastitis has an infectious origin. Symptoms appear when a blockage of the milk ducts occur, presumably due to the overgrowth of some bacterial species which form biofilms and trigger inflammation11,12,13,14. According to its course, lactational mastitis can be classified into different types, being acute (AM) and sub-acute mastitis (SAM) the most prevalent among breastfeeding women. AM can be easily identified due to the intensity of its symptoms, namely erythema, pain, swelling, fever and other general symptoms. Staphylococcus aureus is considered the main causative agent in AM, producing toxins responsible for the systemic symptoms12,15,16,17. SAM, albeit courses with milder symptoms, is most prevalent among lactating women and therefore represents one of the principal causes of undesired cessation of lactation. Based on bacterial cultures from human milk samples, Staphylococcus epidermidis has been proposed as the predominant species responsible of SAM18,19,20, as well as other coagulase negative staphylococci (CNS) and viridans streptococci12,21. These are frequently encountered in healthy skin microbiota and human milk, and can occasionally overgrow and form thick biofilms in the milk ducts22,23, leading to milk stasis and opportunistic infections, which result in the symptoms of SAM previously described11,24 Identifying SAM can be challenging, and a poor diagnostic and/or treatment can lead to recurrent or chronic infections. Microbial culture is the standard diagnose procedure, but this technique is time-consuming, and can result in false negatives (as many microbial species cannot be grown under standard laboratory conditions). In addition, most bacteria associated with lactational mastitis can be frequently detected in healthy mother’s milk, which further complicates the diagnosis15. Studies addressing the microbiology of SAM are limited, and information about bacterial loads during human lactational mastitis is scarce. In addition, most human milk microbiota studies based on molecular techniques focus on the total bacterial DNA composition, not considering that part of its DNA may correspond to dead or inactive bacteria, as well as free bacterial DNA. For this reason, RNA-based sequencing of human samples is being used to clarify the elusive aetiology of some diseases with complex microbial origin25,26.
The aim of the current work was to describe the human milk bacterial composition in mothers suffering SAM, taking into account total and active bacteria (as inferred by DNA and RNA 16S rRNA gene sequencing, respectively), and loads, in order to better characterize the etiology of the disease and find potential bacterial biomarkers. In addition, bacterial pellets from human milk were exposed to a mammary epithelial cell line, in order to investigate their potential role in inflammatory processes.
Results
Study population
Fifty-one women were enrolled in the study, including 24 healthy-controls, 24 SAM and 3 AM. From the whole data set, some drop-outs occurred during the study (one mother from the control group, and 5 mothers from the SAM group abandoned the study before collecting the second sample). Characteristics of mothers and infants are summarized in Table 1.
Total and active bacterial load increase in human milk during mastitis
Quantification of the 16S rRNA gene through qPCR of both DNA (total bacterial load) and cDNA (active bacterial load) showed significantly increased bacterial loads in the mastitis samples during the course of the symptoms (Fig. 1). Mean total bacterial load in the control samples was 610,127 cells/ml (SEM = 110,218) and 828,850 cells/ml (SEM = 100,692) at first and second time point, respectively. Mean total load in the mastitis samples (including SAM and AM) during the course of the symptoms reached 3,137,000 cells/ml (SEM = 956,632), which was significantly higher as compared to controls at the same time point (non-parametric Kruskal Wallis test, p < 0.01). After the symptoms had disappeared, mean total load in the mastitis group decreased to 1,430,000 cells/ml (SEM = 259,037), although the values were still significantly higher as compared to controls at time 0, and thus, bacterial load did not fully return to healthy levels at this time point (non-parametric Kruskal–Wallis test, p < 0.01). Mean active bacterial load was significantly lower as compared to total bacterial load in all groups (non-parametric Kruskal–Wallis test, p < 0.001), except in the mastitis group at time 1. Similar values were observed in the control samples at the two studied time points (time 0 = 67,064 cells/ml [SEM = 27,505]; and time 1 = 84,808 cells/ml [SEM = 21,117]). Mean active bacterial load increased in the mastitis group during the course of symptoms, up to 598,395 cells/ml (SEM = 373,253) although this difference was not significant, perhaps as a consequence of the larger data variation, which could be affected by RNA instability. Mean active load in the mastitis group after symptoms disappeared increased up to 1,601,000 cells/ml (SEM = 229,296), and this difference was significant when compared to all the other groups (non-parametric Kruskal–Wallis test, p < 0.001).
Bacterial load in human milk of healthy mothers and mothers suffering lactational mastitis. Plots show means with standard errors. (a) Bacterial load, as inferred from qPCR of the 16S rRNA gene of the bacterial DNA. (b) Active bacterial load, as inferred from qPCR of the 16S rRNA gene of the bacterial RNA (cDNA). Controls_t0, (n = 24); Controls_t1, (n = 23); Mastitis_t0, (SAM, n = 24; AM, n = 3); Mastitis_t1, (SAM, n = 19; AM, n = 3). t0, samples collected during the course of mastitis symptoms, or first sample collected in healthy controls; t1, samples collected after the clinical symptoms disappeared, or samples collected from healthy controls one week after the first sample collection. Acute Mastitis samples are represented with white triangles in the graph. **p < 0.01 and ***p < 0.001, non-parametric Kruskal Wallis test. Created in GraphPad Prism 5 v5.04 (www.graphpad.com).
Bacterial richness and diversity are reduced during lactational mastitis
After sequencing, one DNA sample (SAM group, time 0) and two cDNA samples (control group and SAM group, time 0) were not considered for further analyses due to the small number of sequences yielded. Total bacterial DNA diversity (as measured by the Shannon Index) and richness (as measured by the Chao1 Index) were lower during the course of mastitis symptoms (Fig. 2a; non-parametric Kruskal–Wallis test, p < 0.05). Diversity levels did not return to control levels after the symptoms had disappeared, although the estimated bacterial richness significantly increased (non-parametric Kruskal–Wallis test, p < 0.001). A similar pattern was observed at the RNA level: Active bacterial diversity was lower during the mastitis symptoms (Fig. 2b; non-parametric Kruskal–Wallis test, p < 0.05), and the bacterial richness in the RNA group increased after symptoms had disappeared (non-parametric Kruskal–Wallis test, p < 0.05). Although there was a trend towards lower richness in the mastitis group during the symptoms, it did not reach statistical significance.
Bacterial diversity and richness in human milk samples from healthy and mastitis-suffering women. Plots show human milk microbiota genus-level diversity and richness (here presented with Shannon and Chao1 indices), with means and standard errors. (a) Represents microbiota DNA Shannon and Chao1 indices. Controls, n = 24; Mastitis_t0, n = 26; Mastitis_t1, n = 22. (b) Shows microbiota RNA Shannon and Chao1 indices. Controls, n = 23; Mastitis_t0, n = 26; Mastitis_t1, n = 22. Acute mastitis samples are represented with white circles, n = 3. t0 = samples during the course of the symptoms; t1 = samples after symptoms disappeared. *p < 0.05 and ***p < 0.001, non-parametric Kruskal Wallis test. Created in GraphPad Prism 5 v5.04 (www.graphpad.com).
Total and active bacterial composition change during and after the course of sub-acute mastitis
Streptococcus and Staphylococcus were the two most abundant bacterial genera, both in the DNA (67.12% and 8.00%, respectively) and RNA sequences (51.23% and 14.57%, respectively) (Fig. 3). No statistically significant effect of maternal antibiotics intake, maternal and infant age, delivery mode nor maternal weight gain during pregnancy, were detected on human milk microbial composition (p > 0.05, MaAsLin test). At genus level, SAM samples at time 0 had lower levels of Pseudomonas than healthy controls (unpaired Wilcoxon test, adjusted p-value = 0.003), and lower levels of Acinetobacter than SAM at time 1 (paired Wilcoxon test, adjusted p-value = 0.031). When looking at the active (RNA-based) bacterial composition, Firmicutes phylum was higher in the SAM group at time 0, as compared to controls (Wilcoxon test, p-value = 0.01), at the expense of a depletion in Proteobacteria (Wilcoxon test, p-value = 0.001) and to SAM at time 1 (Wilcoxon test, p-value = 0.05). Peptoniphilus, Prevotella, and Finegoldia were at higher levels in the active portion of SAM at time 1 as compared to healthy controls (adjusted p-value = 0.0087, 0.019 and 0.042, respectively), while healthy controls were enriched in Neisseria (adjusted p-value = 0.042), suggesting that the bacterial composition was not fully recovered after the clinical symptoms had disappeared. Relative abundances of bacterial genera per group are summarized in Table S1. At OTU (species) level, the impact of health status on human milk microbiota was also reflected in differences in bacterial composition between healthy controls, mastitis during the course of symptoms (time 0) and mastitis after symptoms cessation (time 1), both at DNA level (Adonis p-value = 0.015, CCA analysis), and active RNA level (Adonis p-value = 0.04, CCA analysis) (Fig. 4). As inferred from DNA analysis, controls appeared more dispersed in the CCA plot, while mastitis groups were more similar in composition and clustered closer to each other. At RNA level, although there was also some overlap, the three groups clustered separately, and the highest divergence was explained by axis 1, which separates controls from mastitis groups. Thus, the CCA plots also support a different bacterial composition at the species level in mastitis and control groups, with a partial recovery after the symptoms disappeared. Streptococcus mitis/oralis, Streptococcus salivarius, Acinetobacter johnsonii, Streptococcus lactarius and Rothia mucilaginosa were the most abundant species detected in the human milk samples at DNA level (Table S2). Streptococcus mitis/oralis, Streptococcus salivarius, Staphylococcus epidermidis, Rothia mucilaginosa and Streptococcus lactarius were the most abundant active bacteria detected (RNA level) (Table S3). Staphylococcus aureus was more abundant in SAM, both at time 0 (adjusted p-value = 0.001), and time 1 (adjusted p-value = 0.0003, Wilcoxon test), as compared to healthy controls. Porphyromonas endodontalis (adjusted p-value = 0.003) and Streptococcus peroris (adjusted p-value = 0.003) were more prevalent in SAM group at time 1, as compared to controls. Paired Wilcoxon test showed that Acinetobacter johnsonii was more abundant in SAM group at time 1 as compared to SAM at time 0 (adjusted p-value = 0.025). Staphylococcus aureus and Streptococcus lactarius were also more active in SAM samples both at time 0 (unpaired Wilxocon test, adjusted p-value = 0.023; p = 0.013, respectively) and time 1 (unpaired Wilxocon test, adjusted p-value = 0.005; P = 0.018, respectively), as compared to controls. Streptococcus peroris was significantly more active in SAM group at time 1, as compared to controls (unpaired Wilxocon test, adjusted p-value = 0.006) and to SAM time 0 samples (paired Wilcoxon test, adjusted p-value = 0.012).
Bacterial composition in human milk samples of healthy mothers and mothers suffering sub-acute mastitis. The bar plot shows average percentage of abundance of the genus-level bacteria (DNA) and active bacteria (RNA) detected in the human milk samples by means of Illumina MiSeq sequencing of the 16S rRNA gene. Controls: DNA, n = 24; RNA, n = 23; SAM_t0: DNA, n = 23; RNA, n = 23; SAM_t1; DNA, n = 19; RNA, n = 19. t0 = samples during the course of the symptoms; t1 = samples after symptoms disappeared. Due to the small sample size (n = 3), acute mastitis samples were not included in the bar plots. Created in R software v 3.5.1 (2018–07-02) (https://www.R-project.org).
Human milk microbiota patterns in healthy mothers and mothers suffering lactational mastitis. Constrained correspondence analyses (CCA), which here emphasize variations in microbiota OTU-level patterns, in human milk from healthy mothers and mothers suffering lactational mastitis at two time points, at DNA-level (p = 0.015), and active microbial RNA-level (p = 0.04). The percentage of variation explained by constrained correspondence components is indicated on the axes. t0 = samples during the course of the symptoms; t1 = samples after symptoms cessation. p-values for CCA plots were determined by Adonis, and indicate if health status and/or time can significantly explain data variability. Controls (C): DNA, n = 24; RNA = 23; Mastitis t0: DNA, n = 26; RNA = 26; Mastitis t1: DNA, n = 22; RNA, n = 22. Created in R software v 3.5.1 (2018–07-02) (https://www.R-project.org).
In addition, LEfSe algorithm was applied in order to further examine potential biomarkers of SAM disease (Fig. 5). Bacteria at significantly higher levels in healthy mother’s milk, as compared with mothers suffering from SAM included Acinetobacter johnsonii, Pseudomonas viridiflava, Corynebacterium simulans, Paracoccus marcusii, Pseudomonas fragi and Acinetobacter lwoffii. Conversely, Corynebacterium kroppenstedtii, Staphylococcus aureus, and Prevotella nanceiensis were observed in increased abundance in human milk during SAM. Significant differences in bacterial abundance were also observed when analysing the active bacterial fraction of the samples (Fig. 5b).
Human milk bacterial OTUs associated with sub-acute mastitis. The plots show differentially abundant bacteria between healthy controls and SAM during symptoms, and between SAM during and after symptoms had disappeared, as inferred from: (a) DNA (controls, n = 24; SAM_t0, n = 23; SAM_t1, n = 19); and (b) RNA (controls, n = 23; SAM_t0 = 23; SAM_t1 = 19). The LEfSe algorithm was used for biomarker discovery; the threshold for logarithmic discriminant analysis (LDA) score was 2, and p < 0.05. Created in GraphPad Prism 5 v5.04 (www.graphpad.com).
Under the assumption that pathogens involved in mastitis should be over-represented in the RNA samples relative to their levels in the DNA, an analysis of the bacterial “Activity Index”, corresponding to the ratio between the proportion of each bacteria in the RNA-based and DNA-based sequences, was performed. The average activity indexes for the different genera in each group are shown in Fig. 6. The data show that, in acute mastitis, there is an increase in the activity of Staphylococcus, which decreases (negative index values) after the symptoms subsided. Streptococcus, on the other hand, has a negative activity index during acute mastitis, whereas it shifts to positive activity values when the symptoms disappeared. In SAM samples, only a few patterns can be observed, like an increased activity during mastitis and a decreased activity after recovery.
Average bacterial activity indices in human milk in health and during mastitis. The graph shows average bacterial activity indices, corresponding to the ratio between the proportion of each bacterial genus in the RNA- and DNA-based sequences, and the standard error of the mean (SEM) per groups. Controls, n = 23; Sub-acute mastitis t0, n = 23; Sub-acute mastitis t1, n = 19. Positive ratios are represented in grey, negative ratios in black. Created in GraphPad Prism 5 v5.04 (www.graphpad.com).
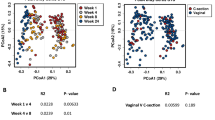
In order to further study potential associations between bacterial activity and mastitis, the activity indices of bacterial species in each samples was compared (Fig. 7). Although some milk samples clustered according to health status, results showed a large overlap in bacterial activity ratios between healthy mothers and those with mastitis before and after treatment. This is in agreement with the above-mentioned biomarker discovery analysis in that the etiology of SAM is complex and probably polymicrobial. In addition, the presence of some bacteria at low activity under health conditions that increase in activity during mastitis suggests that some etiological agents of the disease may already be present in health, and that disease onset may be the outcome of a microbial dysbiosis, whose triggering factors should be identified.
Bacterial activity and cluster analysis in human milk in health and mastitis. The heatmap shows the activity indices corresponding to the ratio between the proportion of each bacterial species in the RNA-based and DNA-based sequences. Controls, n = 23; SAM_t0, n = 23; SAM_t1, n = 19. Indices are represented in different colours depending of their higher or lower values in the samples. Created with R software v 3.5.1 (2018-07-02) (https://www.R-project.org).
Human milk bacteria exposure to mammary epithelial cells and release of IL8
To study the potential pro-inflammatory effect of bacteria associated to SAM, human milk pellets were co-incubated with a mammary epithelial cell line for 24 h. Results showed higher levels of IL8 production in cells exposed to pellets from SAM during the course of the symptoms, which significantly decreased after symptoms disappeared (non-parametric Kruskal–Wallis test, p < 0.01) (Fig. 8). As expected, levels of IL8 from cell supernatants exposed to AM pellets were higher during and after the symptoms, as compared to the other groups, although the small sample size did not allow statistical analyses. Although we did not find any correlation between absolute bacterial loads and the levels of IL8 in the samples, we observed a positive correlation between the genus Staphylococcus’ relative abundance and the levels of IL8 in the DNA group (Spearman’s ρ, 0.363, p value, 0.003). This positive correlation was also observed at the cDNA level, although it was not statistically significant (Spearman’s ρ, 0.229, p value, 0.007).
Levels if IL8 produced by mammary cells after exposure to bacteria in human milk. The bar plots show levels of IL8 released by cells from a mammary epithelial cell line when exposed to bacterial pellet from healthy controls (Controls_t0, n = 18; Controls_t1, n = 22); sub-acute mastitis (SAM_t0, n = 21; SAM_t1, n = 17); and acute mastitis (AM_t0, n = 2; AM_t1, n = 3). Supernatants from mammary cells exposed only to culture medium were used as negative controls (n = 8). **p < 0.01, non-parametric Kruskal–Wallis test. Created in GraphPad Prism 5 v5.04 (www.graphpad.com).
Discussion
Sub-acute mastitis is a fastidious and common disease among lactating mothers, representing one of the main causes of undesired weaning. Despite its high prevalence and impact on maternal-infant health, SAM is undervalued and under-diagnosed12. Studies performed on human milk during lactational mastitis up to date point to an altered bacterial profile, increased abundance in opportunistic pathogens and lower bacterial diversity14,16,18,20. Classically, AM has been associated to Staphylococcus aureus infections12,15,16,17. SAM, on the other hand, has been associated with increased Staphylococcus epidermidis presence18,19,20, and with lower abundances of other coagulase negative staphylococci (CNS), and viridans streptococci21. Most of these results, however, were derived from culture-dependent analyses, which are known to be biased by false negative rates. Only two studies, up to date, used culture-independent methods to analyse human milk microbial profiles during mastitis18,20. In both studies, lower bacterial diversities in mastitis samples were reported, in addition to enrichment in opportunistic pathogens.
In the present study, both total (DNA-based) and active (RNA-based) bacterial composition and load of the SAM and healthy human milk samples have been studied. Results show that total bacterial load was significantly higher during the time of mastitis symptoms, and decreased after the symptoms ceased, although loads remained significantly higher as compared to healthy controls. Interestingly, active load after the symptoms’ cessation was significantly higher as compared to healthy controls and mastitis during the symptoms. This could indicate increased bacterial activity levels during the process of re-balancing in the bacterial community. After sequencing the 16S rRNA gene amplicons, we observed lower bacterial diversities both at DNA and RNA level during mastitis, in agreement with previously reported data18,20. Richness was also lower during the disease and increased after symptoms had disappeared. However, diversity was not recovered to control levels after the symptoms disappeared. Given that samples were collected short after the symptoms had disappeared (1–4 days after mother’s stopped suffering the symptoms was considered as inclusion criteria), data suggest that a full recovery of bacterial communities takes longer than the remission of clinical symptoms and future studies should collect samples at a later stage. Decreased microbial diversity and/or richness associated to microbial dysbiosis have been previously described in several disease conditions, such as inflammatory bowel disease27,28, colorectal cancer29, tooth decay30 or celiac disease31, among others.
Activity indices, calculated as the ratio between the proportion of each microorganism in the cDNA and the DNA samples from each donor, show differentially active bacteria in the milk from healthy and mastitis-suffering donors. However, milk samples did not cluster according to bacterial activity and maternal health status (controls vs SAM), as observed in the CCA results, and this overlap supports a polymicrobial, variable and dysbiotic etiology of SAM. A plausible explanation for the absence of a unique, clear causal agent responsible of SAM etiology probably lies in the fact that many bacteria associated with SAM are commonly present in human milk under healthy conditions. Thus, contrary to what occurs in AM, SAM may not always be the outcome of an external, or environmental infection but may be caused by a dysbiosis in the pre-existing inhabitants of human milk. Dysbiosis has been introduced as a useful concept to explain disease etiology in cases where classical infection by an external pathogen cannot be identified26. In other inflammatory diseases where pathogenic communities are present under health conditions, an environmental factor, ranging from diet changes to immune alterations, could be the triggering factor leading to a dysbiosis32,33,34, and further studies should identify if any external factor could initiate an unbalance in milk microbial communities.
Despite the high inter-individual bacterial variability of human milk among samples, specific differences between groups were observed. During SAM, Pseudomonas and Acinetobacter were diminished, as compared to healthy controls and after symptoms disappeared (SAM, time 1), respectively. Interestingly, after cessation of the symptoms, SAM (time 1) samples were enriched in typical oral inhabitants, such as Streptococcus and Porphyromonas (DNA); and Prevotella (RNA). In addition, potential anaerobic and opportunistic pathogenic genera like Finegoldia and Peptoniphilus were also increased in SAM at time 1, which could reflect an imbalance in human milk after the mastitis episode. At OTU level, results showed that milk from mothers suffering SAM were enriched in Staphylococcus aureus, even after the symptoms had disappeared, and were also significantly more active as compared to healthy controls. Given that S. aureus was detected in only 1 out of 24 healthy mothers, its higher proportion and prevalence could have been due to an infection from an environmental source. In most other cases, however, the disease-associated bacteria were also found in milk from healthy mothers. Streptococcus lactarius, for instance, was also found to be significantly more active in SAM during and after symptoms cessation, but it was also found at high prevalence in healthy mothers (13 out of 23 samples). In those cases, it is therefore possible that a change in the proportion of pre-existing microorganisms could trigger the inflammatory process. In addition, after symptoms disappeared, samples were enriched in typical oral inhabitants such as Porphyromonas endodontalis and Streptococcus peroris. Given that oral health alterations, especially gingivitis and periodontitis, are very frequent in mothers during pregnancy and lactation35,36, the potential role of oral bacteria in triggering inflammation in the mammary tissue should be considered and tested in future studies.
LEfSe biomarker discovery analyses showed several OTUs associated to health, including Acinetobacter johnsonii, Corynebacterium simulans, and Acinetobacter lwoffiii, among others. Corynebacterium kroppenstedtii, S. aureus and oral species such as Prevotella nanceiensis were among the identified SAM biomarkers. Corynebacterium kroppenstedtii has been previously isolated from granulomatous mastitis and breast abscesses samples37,38. Others like Acinetobacter johnsonii, Propionibacterium acnes, Lactobacillus helveticus and Lactobacillus zeae were significantly more abundant after symptoms had disappeared. Interestingly, P. acnes is a predominant bacterium in the skin microbiome, which has shown anti-S. aureus activity in vitro39,40. Thus, future studies should focus not only on potentially pathogenic organisms, but also on bacteria which could potentially contribute to health conditions, as promoting their growth with pre- or probiotics could prove to be a strategy to prevent or treat SAM11,41.
RNA analysis showed that active Lactobacillus iners, Neisseria subflava, Streptococcus lactarius, Streptococcus cristatus and S. aureus were associated with SAM during the symtoms; while others such as P. acnes, Staphylococcus hominis, Acinetobacter lwoffii and Lactobacillus helveticus were associated with health. Some of the potential pathogens deserve further study. Lactobacillus inners, for instance, is a member of the vaginal normal microbiota42,43, although it has been associated to vaginal dysbiosis44. This species can produce a toxin, named as innerlysin, which may cause cell damage45. Thus, its potential role in SAM should be tested in the future. In addition, our results show that pellets from SAM milk samples, containing bacteria, induce pro-inflammatory IL8 release by mammary epithelial cells, supporting an infectious origin of SAM. Although we did not observe an overall correlation between bacterial load and IL8 release in the cells, our results showed a specific correlation between the genus Staphylococcus and IL8 production (only statistically significant at the DNA level), further supporting the potential pro-inflammatory role of this genus.
From a methodological point of view, we propose that the RNA:DNA activity index could be a useful approach to detect pathogenic species in complex human samples, as pathogens would be expected to have higher activity at the disease site than commensal inhabitants.
In summary, our data support a bacterial origin of SAM, with a polymicrobial and variable aetiology, probably the result of a dysbiosis in the milk microbial population.
Materials and methods
Subjects and sampling
A total of 51 mothers participated in the study. Among them, 24 presented symptoms of SAM, 3 presented symptoms of AM and were included for comparison, and the remaining 24 were completely healthy. Human milk samples were collected between 9 and 90 days after delivery, at two time points: during the course of the symptoms (time 0) and after the symptoms cessation (time 1) in the mastitis group; and during a medical consultation to the doctor (time 0), and a second visit a week after (time 1) in the control group. Details of pregnancy and delivery, mother/infant health status, medicines consumption, lactation, and clinical symptoms during mastitis were collected at recruitment through a detailed questionnaire. Mothers (over 18 years old) were recruited at the Breastfeeding Unit of Dr. Peset Hospital and at the Alfafar Health Center (Valencia, Spain). Women were considered to have SAM when presenting breast pain (usually described as profound, needle-like and/or burning pain) accompanied or not by lumps in the breast tissue and without general symptoms18. Women were considered to have AM when presenting profound pain in the breast accompanied by at least two of the following symptoms: local inflammation signs (breast redness, local hyperthermia, or sensitive lump), fever and general discomfort18. Controls were healthy breastfeeding woman who did not present any of the previous symptoms. Exclusion criteria were: suffering from immunological, metabolic or other severe diseases; or having received antibiotics or probiotics 15 days prior to first sample donation. Breastfeeding counselling was offered to all mothers suffering from breast pain. After any other cause of breast pain (such as an incorrect lactating posture, or infant’s short frenulum) was discarded and the diagnosis of sub-acute mastitis was confirmed, mothers were instructed on breastfeeding massage techniques and optimal lactation positioning. Treatments with anti-inflammatory drugs and/or probiotics were prescribed. In fourteen cases from the SAM group, symptoms persisted after 7 days and antibiotics were prescribed. Mothers suffering AM received antibiotics and anti-inflammatory drugs, following the recommended standards. Prior sampling, nipples and mammary areola were cleaned with chlorhexidine soap and sterile water and rinsed with sterile saline solution. After manually discarding the first milk drops, samples were collected with a Medela Symphony breast-pump (Medela, Baar, Switzerland) in sterile collection units. Samples were collected in the morning, at least 1 h after the last feeding. Written, informed consent was obtained from all participants at the time of sample collection. Protocol was approved by the Ethical Committee of Clinical Research from the Dr. Peset University Hospital (Valencia, Spain), with reference number CEIC 19/16. All research was performed in accordance with the relevant guidelines and regulations.
Sample processing and DNA/RNA isolation
Human milk samples (4 ml) were centrifuged at 13,000×g for 10 min at 4 °C, discarding fat and whey. Total DNA and RNA were isolated from pellets by using the MasterPure Complete DNA & RNA Purification Kit (Epicentre, Madison WI, USA) as previously described46. A mix of 150–212 μm and 425–600 μm acid washed glass beads (Sigma-Aldrich) were added and samples were put through two cycles of vigorous mixing (6.5 m/s) for 60 s with 60 s rest period between runs, in a FastPrep-24 5G Instrument (MP Biomedicals, Santa Ana, CA, USA). Nucleic acids were eluted in 30 μl TE buffer. 10 μl of each nucleic acids’ suspension were transferred to a new nuclease-free tube, and treated with the DNA-free DNA Removal Kit (Invitrogen, Carlsbad, CA, USA) to remove DNA and keep only RNA. 0.1 volume of the 10X DNase I Buffer and 1 µl of rDNAse I were added to the tubes, and incubated at 37 °C for 30 min. This step was repeated a total of three times to ensure complete removal of DNA. 0.1 volume of the DNAse Inactivation Reagent was added, incubated for 2 min at RT and centrifuged at 10,000×g and 4 °C for 2 min. Supernatants containing clean RNA were transferred to new Eppendorf tubes. DNA and RNA concentrations were measured in a Nanodrop Spectrophotometer (ThermoScientific).
cDNA synthesis
cDNA was synthesized from RNA by using the Transcriptor First Strand cDNA Synthesis Kit (Roche Life Science, Basel, Switzerland). A mix of: 1 µg of each RNA sample, 1 µl of Anchored-oligo (dT) primer, (2.5 μM); 2 µl of Random Hexamer Primer (60 μM) in a final volume of 13 µl. The primer-template mix was heated at 65 °C for 10 min. 4 µl of Transcriptor Reverse Transcriptase Reaction Buffer (1 × 8 mM MgCl2); 0.5 µl of Protector RNase Inhibitor (20U), 2 µl of Deoxynucleotide Mix (1 mM each) and 0.5 μl (10 U) of the Transcriptor Reverse Transcriptase (final volume: 20 µl) were added to each tube. Tubes were gently mixed and incubated for 10 min at 25 °C, followed by 30 min at 55 °C, and 5 min at 85 °C. Final cDNA products were stored at − 80 °C.
Detection of 16S rRNA gene by qPCR
Total bacterial load (DNA-based) and active bacterial load (cDNA-based) of the samples were analysed through quantitative PCR (qPCR) amplification and detection of the 16S ribosomal RNA gene. Each reaction mixture of 10 μl was composed of: 5 μl of Light Cycler 480 SYBR Green I Master mix (Roche Life Science), 0.25 μl of each specific primer (concentration 10 μM) and 1 μl of template. Amplifications were performed in a Light Cycler 480 Real-Time PCR System (Roche Life Science), using the following conditions: 95 °C for 5 min, (95 °C for 10 s, 60 °C for 20 s, 72 °C for 20 s) (40 cycles), followed by dissociation curve analysis. All amplifications were performed in duplicates and negative controls were included in each qPCR plate. In all, 5 qPCR plates were used for the analyses of all the samples. Primers sequences were as follows: F—5ʹ-CGTGCCAGCAGCCGCGG-3ʹ and R—5ʹ-TGGACTACCAGGGTATCTAATCCTG-3′47. Cq values in each sample were transformed in bacterial cell numbers per ml of milk by comparison with a standard curve obtained with flow cytometry. This standard was generated by using DNA extracted from 10 million bacterial cells from 10 pure cultures of different species commonly found in human milk (Streptococcus epidermidis CECT 231, Bifidobacterium dentium DSM 20436, Acinetobacter lwoffii CECT 453, Corynebacterium matruchotii DSMZ 20635, Lactobacillus casei (lab’s isolate), Lactobacillus acidophilus CECT 4179, S.aureus strain 240, Pseudomonas aeruginosa ATCC 15442, Rothia mucilaginosa (lab’s isolate) and Streptococcus mitis DSMZ 12643). Bacterial cells were quantified and sorted using a BD FACSAria II cytometer (BD, East Rutherford, NJ, USA). DNA from all species were extracted, pooled and diluted in serial ten-fold dilutions to create a single standard curve. Samples that showed Cq values higher than the negative control were considered negative for bacterial detection.
Bacterial composition and active bacterial composition of human milk samples
A total of 75 human milk samples were analysed through sequencing of the 16S rRNA gene. Controls, time 0 (n = 24); Mastitis, time 0 (n = 25; 22 SAM and 3 AM); Mastitis, time 1 (n = 23; 20 SAM, 3 AM). Controls at time 1 were not included in further steps. Prior to sequencing, DNA and cDNA were pre-amplified by using universal bacterial degenerate primers 27F—5ʹ-AGAGTTTGATCMTGGCTCAG-3ʹ and 926R—5ʹ-CCGTCAATTCMTTTRAGT3ʹ, which comprise the hypervariable regions V1–V5 of the gene. This step was performed by using the high-fidelity ABGene DNA polymerase (ThermoScientific, Waltham, Mass., USA) with an annealing temperature of 52 °C and 10 cycles, in order to minimize amplification biases that could arise with longer cycles48. PCR products were purified using Nucleofast 96 PCR filter plates (Macherey–Nagel, Düren, Germany), and concentrations were measured with a Qubit 3 Fluorometer (ThermoScientific). An Illumina amplicon library was performed following the 16S rRNA gene Metagenomic Sequencing Library Preparation Illumina protocol (Part #15044223 Rev. A). The primer sequences used target the 16S rRNA gene V3-V4 regions, resulting in a single amplicon of approximately 460 bp49. After amplification of the 16S rRNA gene, DNA and cDNA were sequenced in an Illumina MiSeq platform according to manufacturer’s instructions (Illumina) using the 2 × 300 bp paired-end protocol, at the FISABIO Institute (Valencia, Spain). No-template controls (NTCs) and negative controls during DNA extraction were included to rule out potential contaminations at the time of DNA extraction or sequencing.
Data analysis and statistics
A quality assessment of the sequences was carried out using the PRINSEQ program50. Sequences were end-trimmed in 20 bp sliding windows, and those with average quality value < 30, and length < 250 bp were not considered for further analyses. Reads were pair-end joined using FLASH program applying default parameters51. Only overlapping paired-end reads were used for further analysis. The negative controls within the sequencing process produced 615 and 348 reads. The DNA and RNA extraction negative controls yielded 1995 and 1293 reads, respectively. Agrobacterium, Brucellaceae and Bradyrhizobiaceae were identified as contaminants and removed from the analyzed datasets52. Operational taxonomic units (OTUs) were generated by clustering reads at 97% of similarity by using VSEARCH53. Centroids (representative OTUs) were taxonomically classified at phylum, class, family, genus and species level using feature-classifier command of QIIME253 version 2017.8 with Greengenes database (version gg_13_5). Sequences belonging to Streptococcus and Staphylococcus genera, whose 16S gene is highly similar among species, were clustered into OTUs at 100% similarity and > 400 bp alignment length by BLASTn analysis54, against a manually curated database for these genera, obtained from RDP Hierarchy Browser55. Streptococcus mitis and Streptococcus oralis were identical in the sequenced region and could not be distinguished from each other.
α-diversity analysis (Shannon and Chao1 indices), were calculated to estimate sample’s diversity and richness; and β-diversity (Bray Curtis dissimilarity index), to quantify the compositional dissimilarity between groups at OTU and genus level, using the R-package vegan56.
Canonical correspondence analysis (CCA) was performed by R software vegan package. In order to control the potential effects of maternal antibiotics intake, maternal age and days postpartum, MaAsLin multivariate analysis with linear model57 was applied. Adonis statistic for permutational multivariate analysis was used to measure differences in variance between groups, and Wilcoxon test implemented in R software was applied to determine significantly different bacterial genera between groups.
Bacterial-OTUs biomarker discovery was performed by linear discriminant analysis effect size (LEfSe) implemented on Galaxy58, in order to detect differentially abundant OTUs characterizing the populations of healthy and mastitis-suffering women. Other analyses and graphs were performed in GraphPad Prism 5 v5.04.
In all samples, a bacterial ‘Activity Index’ was calculated as the ratio between the proportion of each microorganism in the cDNA and the DNA samples from each donor. This ratio would allow the identification of those bacteria whose activity is higher or lower than expected based on their presence, as inferred by sequencing of the 16S rRNA gene. Data were then log-transformed so bacteria which were over- or under-represented in the RNA relative to the DNA fraction of each individual would be assigned positive or negative values of the Activity Index, respectively. Activity Indexes were represented with R in a heatmap, grouped by health status (Controls, SAM and AM) and time (t0, t1), eliminating bacteria at a proportion < 0.1 in more than 90% of the samples.
Exposure of milk bacteria to a mammary gland epithelium cell line
The mammary epithelial cell line MCF7 (ATCC HTB-22), was seeded onto 96-well plates (30,000 viable cells per well) in complete growth medium (DMEM high glucose (Gibco, ThermoScientific) supplemented with 10% v/v inactivated fetal bovine serum (Sigma), 1 mM sodium pyruvate (Gibco), 0.1 mM non-essential amino acids (Gibco), 10 mM HEPES (Gibco), 2 mM l-glutamine (Gibco) and antibiotics (100 U/ml penicillin, 100 g/ml streptomycin (Gibco)). The cells were grown at 37 °C and 5% CO2 in an incubator for 2 days, and the integrity of the cell culture was checked with an inverted microscope. The medium was replaced with 200 μl fresh complete growth medium without antibiotics, containing the bacterial pellet from 500 μl of centrifuged milk samples. A total of 18 healthy controls, 21 SAM and 2 AM milk samples obtained at time 0; and 22 controls, 17 SAM and 3 AM milk samples obtained at time 1, were analysed in duplicates in the same experiment. Negative controls consisted of MCF7 cells incubated without bacteria. Co-incubation of mammary gland MCF7 epithelial cells with human milk bacteria was maintained for 24 h at 37 °C and 5% CO2 in an incubator. After co-incubation, culture supernatants were aspirated from wells and kept at 4 °C for measuring human IL8 concentration by ELISA (Invitrogen) using 25 uL of supernatants, following the manufacturer’s instructions.
Data availability
The datasets generated and analysed during the current study are available in the European Nucleotide Archive (ENA) repository, (“https://www.ebi.ac.uk/ena”). Sequence and meta-data are accessible under the study identifier PRJEB34421, and samples were deposited under the accession numbers: ERS3744372-ERS3744511.
References
Fitzstevens, J. L. et al. Systematic review of the human milk microbiota. Nutr. Clin. Pract. 32, 354–364 (2016).
Jost, T., Lacroix, C., Braegger, C. P., Rochat, F. & Chassard, C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 16, 2891–2904 (2014).
Marcobal, A. et al. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 58, 5334–5340 (2010).
Fernández, L. et al. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 69, 1–10 (2013).
Toscano, M., De Grandi, R., Grossi, E. & Drago, L. Role of the human breast milk-associated microbiota on the newborns’ immune system: A mini review. Front. Microbiol. 8, 2100 (2017).
Amir, L. H. & Academy of Breastfeeding Medicine Protocol Committee. ABM Clinical Protocol #4: Mastitis, Revised March 2014. Breastfeed. Med. 9, 239–243 (2014).
Odom, E. C., Li, R., Scanlon, K. S., Perrine, C. G. & Grummer-Strawn, L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics 131, e726–e732 (2013).
Berens, P. D. Breast pain: Engorgement, nipple pain, and mastitis. Clin. Obstet. Gynecol. 58, 902–914 (2015).
World Health Organization. Mastitis: Causes and Management (WHO, Geneva, 2011).
Berens, P., Eglash, A., Malloy, M. & Steube, A. M. ABM clinical protocol #26: Persistent pain with breastfeeding. Breastfeed. Med. 11, 46–53 (2016).
Fernández, L. et al. Probiotics for human lactational mastitis. Benef. Microbes 5, 169–183 (2014).
Contreras, G. A. & Rodríguez, J. M. Mastitis: Comparative etiology and epidemiology. J. Mamm. Gland Biol. Neoplasia 16, 339–356 (2011).
Angelopoulou, A. et al. The microbiology and treatment of human mastitis. Med. Microbiol. Immunol. 207, 83–94 (2018).
Marín, M., Arroyo, R., Espinosa-Martos, I., Fernández, L. & Rodríguez, J. M. Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front. Microbiol. 8, 1258 (2017).
Kvist, L. J., Larsson, B. W., Hall-Lord, M. L., Steen, A. & Schalén, C. The role of bacteria in lactational mastitis and some considerations of the use of antibiotic treatment. Int. Breastfeed. J. 3, 6 (2008).
Delgado, S. et al. Characterization of Staphylococcus aureus strains involved in human and bovine mastitis. FEMS Immunol. Med. Microbiol. 62, 225–235 (2011).
Osterman, K. L. & Rahm, V.-A. Lactation mastitis: Bacterial cultivation of breast milk, symptoms, treatment, and outcome. J. Hum. Lact. 16, 297–302 (2000).
Jiménez, E. et al. Metagenomic analysis of milk of healthy and mastitis-suffering women. J. Hum. Lact. 31, 406–415 (2015).
Delgado, S., Arroyo, R., Martín, R. & Rodríguez, J. M. PCR-DGGE assessment of the bacterial diversity of breast milk in women with lactational infectious mastitis. BMC Infect. Dis. 8, 51 (2008).
Patel, S. H. et al. Culture independent assessment of human milk microbial community in lactational mastitis. Sci. Rep. 7, 7804 (2017).
Martín, V., Mediano, P., del Campo, R., Rodríguez, J. M. & Marín, M. Streptococcal diversity of human milk and comparison of different methods for the taxonomic identification of Streptococci. J. Hum. Lact. 32, 84–94 (2016).
Delgado, S. et al. Staphylococcus epidermidis strains isolated from breast milk of women suffering infectious mastitis: Potential virulence traits and resistance to antibiotics. BMC Microbiol. 9, 82 (2009).
Angelopoulou, A. et al. Vancomycin and nisin A are effective against biofilms of multi-drug resistant Staphylococcus aureus isolates from human milk. PLoS ONE 15, e0233284 (2020).
Otto, M. Staphylococcus epidermidis pathogenesis. Methods Mol. Biol. 1106, 17–31 (2014).
Yost, S., Duran-Pinedo, A. E., Teles, R., Krishnan, K. & Frias-Lopez, J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 7, 27 (2015).
Simón-Soro, A. & Mira, A. Solving the etiology of dental caries. Trends Microbiol. 23, 76–82 (2015).
Sokol, H. et al. Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048 (2016).
Chehoud, C. et al. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1948–1956 (2015).
Ahn, J. et al. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 105, 1907–1911 (2013).
Simón-Soro, Á., Belda-Ferre, P., Cabrera-Rubio, R., Alcaraz, L. D. & Mira, A. A tissue-dependent hypothesis of dental caries. Caries Res. 47, 591–600 (2013).
Schippa, S. et al. A distinctive ‘microbial signature’ in celiac pediatric patients. BMC Microbiol. 10, 175 (2010).
Mira, A., Simon-Soro, A. & Curtis, M. A. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J. Clin. Periodontol. 44, S23–S38 (2017).
Hajishengallis, G., Darveau, R. P. & Curtis, M. A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725 (2012).
Rodiño-Janeiro, B. K., Vicario, M., Alonso-Cotoner, C., Pascua-García, R. & Santos, J. A review of microbiota and irritable bowel syndrome: Future in therapies. Adv. Ther. 35, 289–310 (2018).
Tettamanti, L. et al. Pregnancy and periodontal disease: does exist a two-way relationship?. Oral Implantol. (Rome) 10, 112–118 (2017).
Wu, M., Chen, S.-W. & Jiang, S.-Y. Relationship between gingival inflammation and pregnancy. Mediat. Inflamm. 2015, 1–11 (2015).
Paviour, S. et al. Corynebacterium species isolated from patients with mastitis. Clin. Infect. Dis. 35, 1434–1440 (2002).
Wong, S. C. Y. et al. Corynebacterium kroppenstedtii is an emerging cause of mastitis especially in patients with psychiatric illness on antipsychotic medication. Open Forum Infect. Dis. 4, 096 (2017).
Grice, E. A. & Segre, J. A. The skin microbiome. Nat. Rev. Microbiol. 9, 244–253 (2011).
Shu, M. et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 8, e55380 (2013).
Espinosa-Martos, I. et al. Milk and blood biomarkers associated to the clinical efficacy of a probiotic for the treatment of infectious mastitis. Benef. Microbes 7, 305–318 (2016).
Shi, Y., Chen, L., Tong, J. & Xu, C. Preliminary characterization of vaginal microbiota in healthy Chinese women using cultivation-independent methods. J. Obstet. Gynaecol. Res. 35, 525–532 (2009).
Zhou, X. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology 150, 2565–2573 (2004).
Petrova, M. I., Reid, G., Vaneechoutte, M. & Lebeer, S. Lactobacillus iners: Friend or Foe?. Trends Microbiol. 25, 182–191 (2017).
Rampersaud, R. et al. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. 193, 1034–1041 (2011).
Boix-Amorós, A., Collado, M. C. & Mira, A. Relationship between milk microbiota, bacterial load, macronutrients, and human cells during lactation. Front. Microbiol. 7, 492 (2016).
Cabrera-Rubio, R., Mira-Pascual, L., Mira, A. & Collado, M. C. Impact of mode of delivery on the milk microbiota composition of healthy women. J. Dev. Orig. Health Dis. 7, 54–60 (2016).
Sipos, R. et al. Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targetting bacterial community analysis. FEMS Microbiol. Ecol. 60, 341–350 (2007).
Klindworth, A. et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41, e1 (2013).
Schmieder, R. & Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27, 863–864 (2011).
Magoc, T. & Salzberg, S. L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963 (2011).
Glassing, A., Dowd, S. E., Galandiuk, S., Davis, B. & Chiodini, R. J. Inherent bacterial DNA contamination of extraction and sequencing reagents may affect interpretation of microbiota in low bacterial biomass samples. Gut Pathog. 8, 24 (2016).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 4, e2584 (2016).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Cole, J. R. et al. The ribosomal database project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, D141–D145 (2009).
Oksanen, J. et al. Vegan: Community ecology package. R Packag. version 2.3 (2016). https://cran.r-project.org/package=vegan.
Morgan, X. C. et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13, R79 (2012).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Acknowledgements
We thank all the volunteers for their participation in this study. We would like to thank José Gimeno for his help with cell culture experiments, and Mar Tárrega, Purificación Rodas Cordón and Maria José San Esteban for helping in the recruitment of mothers and collection of samples. This work was funded by the ERC Starting Grant 639226-MAMI, and the RTI2018-102032-B-I00 Grant from the Spanish Ministry of Science, Technology and Universities. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. Part of this article was included in the PhD thesis “Human milk microbiota and its relationship with milk components in health and during lactational mastitis".
Author information
Authors and Affiliations
Contributions
A.B. conducted the laboratory work and performed data analysis, including bioinformatics and statistics, interpreted the results and drafted the manuscript. M.H.A. coordinated and collected the samples, collected the questionnaires and recruited the subjects. A.M. and M.C.C. supervised the study, participated in the drafting of the manuscript and designed the study. A.A. processed the reads yielded from sequencing the 16S rRNA gene, and performed statistical analyses. All authors reviewed the manuscript and interpreted the results, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boix-Amorós, A., Hernández-Aguilar, M.T., Artacho, A. et al. Human milk microbiota in sub-acute lactational mastitis induces inflammation and undergoes changes in composition, diversity and load. Sci Rep 10, 18521 (2020). https://doi.org/10.1038/s41598-020-74719-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74719-0
This article is cited by
-
Induction of mastitis by cow-to-mouse fecal and milk microbiota transplantation causes microbiome dysbiosis and genomic functional perturbation in mice
Animal Microbiome (2022)
-
The hidden universe of human milk microbiome: origin, composition, determinants, role, and future perspectives
European Journal of Pediatrics (2022)
-
Lactobacillus supports Clostridiales to restrict gut colonization by multidrug-resistant Enterobacteriaceae
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.