Abstract
Nicotine in electronic cigarette (ECIG) liquids can exist in a free-base or protonated (or “salt”) form. Protonated nicotine is less aversive upon inhalation than free-base nicotine, and many ECIG manufacturers have begun marketing protonated nicotine products, often with high nicotine concentrations. Regulations intended to control ECIG nicotine delivery limit nicotine concentration but do not consider nicotine form. In this study, we systematically examined the effect of nicotine form on nicotine yield for varying powers and liquid vehicles. A Kanger Subox Mini-C tank ECIG (0.5 Ω) was used to generate aerosols at varying powers (5–45 W) from liquid solutions that contained either free-base or protonated nicotine at 15 mg/g concentration, with a liquid vehicle consisting of either propylene glycol (PG) or vegetable glycerin (VG), resulting in four different solutions (free-base/PG, free-base/VG, protonated/PG, and protonated/VG). Nicotine yield was quantified using gas chromatography-mass spectrometry. Nicotine yields were not influenced by nicotine form under any condition investigated. At each power level, PG-based liquids resulted in approximately double the nicotine yield of VG-based liquids. Nicotine concentrations in the aerosols matched those of the parent liquids for both the PG and VG conditions. Increasing power led to greater nicotine yield across all conditions. The amount of nicotine emitted by an ECIG is independent of whether the nicotine is free-base or protonated, however the liquid vehicle has a strong effect on yield. Regulations intended to limit nicotine emissions must consider not only nicotine concentration, but also liquid vehicle and device power.
Similar content being viewed by others
Introduction
Nicotine in tobacco products can be found in a free-base or protonated (“salt”) form, depending on the pH of the product. In internal tobacco industry documents, nicotine form has been long recognized as central to the sensory experience of tobacco use, particularly in what is known as “impact”1. In the 1960s, Philip Morris began manipulating the ratio of free-base to protonated nicotine in cigarette smoke, a factor that is described as key to the ascension of the Marlboro brand to the status of the world’s top-selling cigarette1. In 2014, PAX Labs, the original maker of the JUUL electronic cigarette (ECIG), obtained a patent for mixing free-base nicotine with an acid to convert it to the salt form2. This formulation reduces the aversiveness associated with inhaling the high free-base nicotine liquids3,4. Using nicotine salts, JUUL was able to employ nicotine concentrations as high as 50 mg/mL at a time when ECIG products available on the market averaged a nicotine concentration of 12 mg/mL, predominantly in the free-base form5,6. The transition to a high salt-based nicotine concentration liquid allowed the JUUL manufacturers to design a device that emits a high nicotine yield in a small puff volume.
Today, the use of nicotine salts is rapidly growing, and many ECIG manufacturers offer nicotine salt-containing ECIGs and refillable solutions7. These devices and liquids vary by liquid composition, i.e., propylene glycol to vegetable glycerin (PG/VG) ratio, the two most common ECIG liquid vehicles5, electrical features, and device design. While ECIG nicotine yield has been shown to increase with PG/VG ratio and power8,9, the influence of nicotine form on yield previously has not been examined directly; the data available to date indicate that for a given pH, nicotine yield is independent of the acid used in the liquid10, and that the protonated to free-base ratio found in the ECIG aerosol matches that of the liquid11,12. This knowledge gap is salient because to date EU and proposed US regulations aiming to limit nicotine delivery focus exclusively on nicotine concentration13, neglecting form, PG/VG ratio, and electrical power, among other factors.
In this study, we examined the effects of free-base vs. protonated nicotine forms on nicotine yield and the amount of liquid aerosolized while varying electrical power and liquid vehicle.
Results
Figure 1 shows the effect of nicotine form on nicotine yield at varying powers and PG/VG ratios. Nicotine yield was not significantly associated with nicotine form (p = 0.67), whereas yield was strongly associated with power and PG/VG ratio (p < 0.01). The regression model was found to explain 93% of the variance in nicotine yield (p < 0.01). We also found that nicotine yield can be predicted accurately from the product of the TPM and the liquid nicotine concentration (R2 = 0.92). A summary of the results is presented in Table 1.
Discussion
This study investigated the effects of protonated vs. free-base nicotine on nicotine yield, at varying powers and PG/VG ratios. We found that nicotine yield was not associated with nicotine form, but that yield increased with power and when the liquid vehicle was PG.
The null effect of nicotine form on yield has been reported previously for combustible cigarettes14, and is consistent with the notion that when heated, protonated nicotine undergoes dissociation of the acid/base pair during vaporization and then recombines upon condensation of the aerosol3,10. We have previously shown that the nicotine form in the ECIG aerosol corresponds to that of the liquid11. While form does not impact yield, it likely affects user sensory experience, such as the “throat hit” of the inhaled aerosol3.
The strong effect of liquid vehicle and power is consistent with previously presented theory of ECIG operation8, and with previous empirical studies9,15. In brief, the nicotine vaporization rate depends on the rate at which the liquid vehicle vaporizes from the ECIG heating coil8. The ECIG liquid vaporizes either by evaporation, when the temperature of the liquid is below its boiling temperature, or boiling, when the temperature of the liquid is equal to its boiling temperature. In the evaporation regime, the vaporization rate is governed by the volatility of the liquid. Because PG is more volatile (i.e., has a lower boiling point) than VG, the PG condition will produce a higher vaporization rate at a given temperature and power. In the boiling regime, the amount of liquid vaporized depends on how much of the thermal energy produced by the coil reaches the liquid versus being lost to the surroundings. The rate of thermal energy loss to the surroundings is, in turn, proportional to the temperature difference between the heating coil and the ambient surroundings. Because VG has a higher boiling temperature than PG, the temperature that the system reaches with VG is greater than with PG, and results in greater energy losses to surroundings, and less energy delivery to the liquid. Therefore, in both evaporation and boiling regimes (equivalent to low and high power for a given device), PG vaporizes at a greater rate than VG, carrying more nicotine per unit time8.
However, while PG-based liquids result in higher nicotine yield than VG liquids, the latter are commonly used in ECIG products, particularly in sub-Ohm devices and pod-systems, such as JUUL (30/70 PG/VG ratio16). Apart from nicotine, other factors that contribute to the ECIG desirability include the making possible the ability to exhale “big clouds” of aerosol17. This feature of ECIG operation is associated with the PG/VG ratio15. Compared to PG, VG produces larger particles that are capable of scattering more light, resulting in a more visible aerosol15. This factor, combined with greater throat irritation, likely contributes to the lower overall satisfaction associated with PG-based liquids and higher preference for VG-based liquids18.
In summary, we found that for a fixed puffing protocol, nicotine form is unlikely to influence yield for any current practical scenario (i.e., actual user power levels). However, while form does not affect yield, it may affect nicotine delivery to the blood. To date, research available on the effect of nicotine form on nicotine delivery shows contradictory outcomes19,20, potentially from the different methods used21. In addition, to the extent that nicotine form modifies sensory experience, it may also influence puffing and inhalation behavior and therefore exposure. For example, previous studies have shown that users modified their puffing behavior (i.e., lower puff duration and volume) when using PG instead of VG-based liquids, likely due to sensory experience18. We speculate that nicotine form may play a similar role; for example, users may decrease puffing intensity when using free-base nicotine, and thereby obtain less nicotine. Controlled clinical studies on the impact of form on puffing behavior and exposure could address these questions.
Methods
Aerosol generation
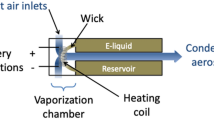
Aerosol was generated using the American University of Beirut Aerosol Lab Vaping Instrument (ALVIN), a custom-built digital puffing machine that can replicate in high resolution individual human puffing behavior. A Kanger Subox Mini-C tank ECIG was connected to ALVIN to generate the aerosol samples. The Subox tank was fitted with a coil head from the same manufacturer (SSOC nichrome 0.5 Ω) and powered using a DC power supply. Five power levels were used in the range 5–45 W. The powers were selected to cover a wide range within the device’s operating power output (1–50 W). For each sampling session, the aerosol exiting the mouth end of the ECIG was drawn through a Gelman type A/E 47 mm glass fiber filter pad where the particle phase of the aerosol was trapped. Total particulate matter (TPM) was determined by weighing the filter assembly before and after each session. Puff topography conditions were kept constant across all conditions at 4 s puff duration, 10 s interpuff interval, and 8LPM flow rate, approximating the average flow rate obtained in a clinical setting using the same device as reported by Hiler et al.22. Three new ECIG heater coils were used for each of the four liquid formulations (i.e., 12 coils in total were used for this study). Each coil was used to sample aerosol at all powers. A detailed list of conditions is provided in Table 1.
ECIG liquid preparation
Analytical grade PG (≥ 99.5%, CAS 57-55-6), VG (99.0–101.0%, CAS 56-81-5), nicotine (≥ 99%, CAS number 54-11-5) and benzoic acid (≥ 99.5%, CAS 65-85-0) were procured from Sigma-Aldrich Corporation and used to prepare four different solutions with entirely free-base or protonated nicotine at 15 mg/g concentration, each using a 100/0 and 0/100 PG/VG ratios (Table 1). These ratios were selected to test the maximum range of potential liquid vehicle interaction with the effect of nicotine form on nicotine yield. The protonated nicotine solutions were prepared by adding standard solutions of benzoic acid to free-base nicotine as 1:1 mol ratio.
Nicotine quantification
Nicotine in the aerosol and liquid was determined by GC–MS analysis of samples extracted in an ethyl-acetate solvent as previously described in Talih, et al.23. An extracted calibration curve with concentrations ranging from 1 to 20 ppm and spiked with the internal standard hexadecane was used to interpret the resulting chromatograms. Spiked filter assays of nicotine in PG and VG solutions showed recoveries of > 90%.
Statistical analysis
A multiple linear regression analysis was used to estimate the association between nicotine yield with nicotine form, power, and PG/VG ratio. Statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY, USA). Statistical significance was p < 0.05.
References
Stevenson, T. & Proctor, R. N. The SECRET and SOUL of Marlboro: Phillip Morris and the origins, spread, and denial of nicotine freebasing. Am. J. Public Health 98, 1184–1194. https://doi.org/10.2105/AJPH.2007.121657 (2008).
Bowen, A. & Xing, C. Nicotine salt formulations for aerosol devices and methods thereof. USA patent (2015).
Pankow, J. F. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem. Res. Toxicol. 14, 1465–1481 (2001).
Chen, L. pH of Smoke: A Review, Report Number N-170, Internal Document of Lorillard Tobacco Company; Lorillard Tobacco Company: Greensboro, NC; 18 pp, Bates nos. 00118164-8181, https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=ykhn0101 (1976).
El-Hellani, A. et al. Nicotine and carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design characteristics. Nicotine Tob. Res. 20, 215–223. https://doi.org/10.1093/ntr/ntw280 (2016).
Stepanov, I. & Fujioka, N. Bringing attention to e-cigarette pH as an important element for research and regulation. Tob. Control https://doi.org/10.1136/tobaccocontrol-2014-051540 (2014).
Harvanko, A. M., Havel, C. M., Jacob, P. & Benowitz, N. L. Characterization of nicotine salts in 23 electronic cigarette refill liquids. Nicotine Tob. Res. https://doi.org/10.1093/ntr/ntz232 (2019).
Talih, S. et al. Transport phenomena governing nicotine emissions from electronic cigarettes: model formulation and experimental investigation. Aerosol Sci. Technol. 51, 1–11. https://doi.org/10.1080/02786826.2016.1257853 (2016).
Kosmider, L., Spindle, T. R., Gawron, M., Sobczak, A. & Goniewicz, M. L. Nicotine emissions from electronic cigarettes: individual and interactive effects of propylene glycol to vegetable glycerin composition and device power output. Food Chem. Toxicol. 115, 302–305. https://doi.org/10.1016/j.fct.2018.03.025 (2018).
El-Hellani, A. et al. Carboxylate counteranions in electronic cigarette liquids: influence on nicotine emissions. Chem. Res. Toxicol. 30, 1577–1581. https://doi.org/10.1021/acs.chemrestox.7b00090 (2017).
El-Hellani, A. et al. Free-base and protonated nicotine in electronic cigarette liquids and aerosols. Chem. Res. Toxicol. 28, 1532–1537. https://doi.org/10.1021/acs.chemrestox.5b00107 (2015).
Duell, A. K., Pankow, J. F. & Peyton, D. H. Free-base nicotine determination in electronic cigarette liquids by 1H NMR spectroscopy. Chem. Res. Toxicol. https://doi.org/10.1021/acs.chemrestox.8b00097 (2018).
EuropeanUnion. Directive 2014/40/EU of the European Parliament and of the Council of 3 April 2014 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Concerning the Manufacture, Presentation and Sale of Tobacco and Related Products and Repealing Directive 2001/37/EC, https://eur-lex.europa.eu/eli/dir/2014/40/2015-01-06 (2015).
Seeman, J. I., Fournier, J. A., Paine, J. B. & Waymack, B. E. The form of nicotine in tobacco. Thermal transfer of nicotine and nicotine acid salts to nicotine in the gas phase†. J. Agric. Food Chem. 47, 5133–5145. https://doi.org/10.1021/jf990409b (1999).
Baassiri, M. et al. Clouds and “throat hit”: Effects of liquid composition on nicotine emissions and physical characteristics of electronic cigarette aerosols. Aerosol Sci. Technol. https://doi.org/10.1080/02786826.2017.1341040 (2017).
Talih, S. et al. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control 28, 678–680. https://doi.org/10.1136/tobaccocontrol-2018-054616 (2019).
Kim, H., Davis, A. H., Dohack, J. L. & Clark, P. I. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob. Res. 19, 190–196. https://doi.org/10.1093/ntr/ntw175 (2017).
Spindle, T. R. et al. Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug Alcohol Depend. 188, 193–199. https://doi.org/10.1016/j.drugalcdep.2018.03.042 (2018).
Burch, S. G. et al. Effect of pH on nicotine absorption and side effects produced by aerosolized nicotine. J. Addict. Med. 6, 45–52 (1993).
O’Connell, G. et al. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers. Intern. Emerg. Med. 14, 853–861. https://doi.org/10.1007/s11739-019-02025-3 (2019).
Gholap, V. V., Kosmider, L., Golshahi, L. & Halquist, M. S. Nicotine forms: Why and how do they matter in nicotine delivery from electronic cigarettes?. Expert Opin. Drug Deliv. https://doi.org/10.1080/17425247.2020.1814736 (2020).
Hiler, M. et al. Effects of electronic cigarette heating coil resistance and liquid nicotine concentration on user nicotine delivery, heart rate, subjective effects, puff topography, and liquid consumption. Exp. Clin. Psychopharmacol. https://doi.org/10.1037/pha0000337 (2019).
Talih, S. et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob. Res. 17, 150–157. https://doi.org/10.1093/ntr/ntu174 (2015).
Funding
This research is supported by grant number U54DA036105 from the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
Author information
Authors and Affiliations
Contributions
Conception: S.T., R.S., R.H., N.K., A.H., N.S., A.S.; acquisition and interpretation of data: S.T., R.S., R.H., N.K., A.H., N.S., A.S.; drafted the work: S.T., A.S.; revised the work: S.T., R.S., R.H., N.K., A.H., N.S., A.S.
Corresponding author
Ethics declarations
Competing interests
Dr. Shihadeh is named on a patent application for a device that measures the puffing behavior of electronic cigarette users. The authors have no other interests to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Talih, S., Salman, R., El-Hage, R. et al. Effect of free-base and protonated nicotine on nicotine yield from electronic cigarettes with varying power and liquid vehicle. Sci Rep 10, 16263 (2020). https://doi.org/10.1038/s41598-020-73385-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73385-6
This article is cited by
-
Assessing the impact of protonating acid combinations in e-cigarette liquids: a randomised, crossover study on nicotine pharmacokinetics
Scientific Reports (2023)
-
Sex Differences in Electronic Cigarette Device Use Among College Students
Journal of Community Health (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.