Abstract
Orb-weaving spiders have two main methods of prey capture: cribellate spiders use dry, sticky capture threads, and ecribellate spiders use viscid glue droplets. Predation behaviour is a major evolutionary driving force, and it is important on spider phylogeny whether the cribellate and ecribellate spiders each evolved the orb architecture independently or both strategies were derived from an ancient orb web. These hypotheses have been discussed based on behavioural and morphological characteristics, with little discussion on this subject from the perspective of molecular materials of orb web, since there is little information about cribellate spider-associated spidroin genes. Here, we present in detail a spidroin catalogue of six uloborid species of cribellate orb-weaving spiders, including cribellate and pseudoflagelliform spidroins, with transcriptome assembly complemented with long read sequencing, where silk composition is confirmed by proteomics. Comparative analysis across families (Araneidae and Uloboridae) shows that the gene architecture, repetitive domains, and amino acid frequencies of the orb web constituting silk proteins are similar among orb-weaving spiders regardless of the prey capture strategy. Notably, the fact that there is a difference only in the prey capture thread proteins strongly supports the monophyletic origin of the orb web.
Similar content being viewed by others
Introduction
An orb-weaving spider generally has morphologically distinct types of abdominal silk glands, and each silk gland synthesizes specialized silk proteins (spidroins) and produces up to seven different silks with diverse mechanical properties1,2,3,4. These varied mechanical properties enable spiders to utilize silks in several situations, including egg protection, dispersal, locomotion, and prey capture5,6. In particular, the evolution of prey capture threads has had a strong effect on spider diversity3,4,7,8.
Capture threads can be categorized into glue or mechanical stickiness. Almost all orb-weaving spiders use adhesive capture threads coated with viscid glue. Viscid glue is an aqueous droplet produced in an aggregate gland, with flagelliform silk used for the core fibre3,9,10,11,12,13. The aggregate gland glue is composed of adhesive glycoproteins surrounded by an aqueous solution of low molecular mass compounds (LMMCs)14. Therefore, the difference in droplet size changes the proportion of water and achieves different viscosity15. Spiders that use a dry capture thread with mechanical stickiness instead of the viscid capture silk are called cribellate spiders. A cribellate spider spins the mechanically sticky silk produced by cribellar spidroin (CrSp) from a cribellum in the plate-like spinning organ. The cribellate silk is generated by a small acinous-shaped cribellate silk gland16,17. Typical cribellate spiders, such as deinopids and uloborids, use a pseudoflagelliform silk produced by pseudoflagelliform spidroin (Pflag) instead of flagelliform silk produced by flagelliform spidroin (Flag) for core fibres18,19,20, which are coated with finely brushed cribellate silks17,21,22 as is found in ecribellate orb-weavers. The cribellate capture threads are composed of materials with different mechanical properties, the core fibre is stiff, and the cribellate nanofibrils is extensible23. This composite has a synergistic mechanical function that the core fibres are broken intermittently at the early stage of tensile deformation, and the threads gradually unravel and elongate to increase thread extension24. The cribellate capture threads are dry and, rather than adhering to prey, entangle prey25,26,27. The stickiness of cribellar fibre is achieved through interactions with the epicuticular waxes of insects28. The cribellate spider Progradungula otwayensis (family Gradungulidae) varies the spinning behaviour of cribellate silk and produces various prey capture threads with different mechanical properties29. It is thought that viscid threads produced by ecribellate orb-weavers are coated with aqueous droplets of the aggregate gland glue and are less expensive to produce than cribellate capture silk9,30,31,32,33,34. On the other hand, although it takes a long time to build a cribellate orb web, these webs are long lasting35.
The transition from dry cribellate capture silk to viscid silk is difficult to understand because they require different conditions to function. Thus, the fundamental differences might indicate that cribellate and ecribellate spiders have evolved each orb architecture independently36. On the other hand, the shared synapomorphy of web architecture and building sequences suggests a monophyletic origin of the orb web (ancient orb web hypothesis)37,38,39,40. This ancient orb web hypothesis has been discussed based on a molecular phylogeny with high resolution across broad taxonomic scale. This hypothesis is supported when combined with morphological and behavioral characters41, but is rejected from pure transcriptomic phylogenomic analysis42.
To understand the origin of the orb web, we need to look not only at the morphological and behavioural traits but also at molecular information of spidroin material that makes-up the orb-web. However, contrary to the ecribellate spiders, there is still little molecular information about the spidroins in cribellate spiders. Regarding the CrSp gene, only partial sequences of candidate genes in Tengella perfuga (family Zoropsidae) and Stegodyphus mimosarum (family Eresidae, KFM60634.1) have been reported43, and the Pflag gene sequence is also only known in Deinopis spinosa (family Deinopidae)44.
Here, we prepare the spidroin data in cribellate orb-weaving spider (family Uloboridae) with multiple omics approaches and conduct phylogenetic analysis. The Uloboridae cribellate spider family is an appropriate target for this study. Uloborids are cosmopolitan cribellate spiders belonging to 18 genera (world spider catalog: https://wsc.nmbe.ch/), and the general eastern Asian uloborid is in the genus Octonoba45. Using six uloborid species (Octonoba sybotides, O. yesoensis, O. grandiprojecta, O. varians, O. grandiconcava, and O. okinawensis), we curate the spidroin data set with transcriptome assembly complemented with long read sequencing, where silk composition is confirmed by proteomics. The cribellate data set provides the insight into the evolution of the spider web.
Results
Octonoba spider cDNA/gDNA sequencing and assembly
De novo transcriptome analyses were performed using cDNA-seq with six species of uloborid spiders (O. sybotides, O. yesoensis, O. grandiprojecta, O. varians, O. grandiconcava, and O. okinawensis) and provided transcriptome references. Total RNA was extracted from each whole body, and synthesized cDNA was sequenced with an Illumina sequencer. 150-bp paired-end sequencing produced a total of 37 M reads on average that passed the quality filter (Table 1). These reads were assembled by each sample, and an average of 41,795 contigs (N50 ranging from 1914 to 2282 bp) were obtained (Table 1). The quality of the assembled gene set was estimated by the BUSCO completeness score, and the completeness score of the Arthropoda gene model ranged from 71.29 to 93.15%. In addition to the transcriptome references, we performed long-read sequencing of genomic DNA (gDNA) with a nanopore sequencer. Spidroin genes are very long and almost entirely composed of the highly repetitive domain between nonrepetitive N/C-terminal domains3,12,46. Therefore, short-read sequencing alone is not sufficient, and hybrid sequencing combined with long-read sequencing is necessary to identify such genes47,48. The gDNA nanopore sequencing produced 133,525 reads with a total of 1.86 G nucleotides on average (Table S1).
Spidroin gene catalog for cribellate spiders
Using assembled transcriptome scaffolds and long reads of gDNA, the spidroin gene sequences were curated, and the whole spidroin gene catalogue of cribellate spiders was produced (Fig. 1a). The full or partial-length gene sequences of all known spidroin types, such as a web radii and drag line silk sequence: major ampullate spidroin (MaSp), a web reinforcement sequence: minor ampullate spidroin (MiSp), a prey-wrapping silk sequence: aciniform spidroin (AcSp), an attachment silk protein sequence: pyriform spidroin (PySp), and an egg case silk sequence: cylindrical spidroin (CySp, also known as TuSp for tubuliform spidroin), were obtained. The lengths of some genes were estimated based on the long reads. Almost all spidroin genes were approximately 10 kbp in length, a size that is common to the spidroin genes in Araneoid spiders48,49. Complete full-length sequences were obtained for MaSp2, CySp, PySp, and MiSp (Fig. S1). To eliminate the possibility of chimeric artefacts, the continuity of each spidroin gene was validated by read mapping, and it was confirmed that the N/C-terminal regions were one continuous molecule (Fig. S2).
Overview of spidroin catalog of six Octonoba spiders. a Spidroin genic architectures obtained from the genome and transcriptome data. The eight circular icons represent spidroin types. The lengths of the NTD (N-terminal domain) and CTD (C-terminal domain) are represented by the red and blue bar lengths, respectively, and grey bars indicate partial domains. The average length of each RTD (repetitive domain) is represented by a green bar, and the estimated iteration number is described in column “n”. b Motifs of CrSp and Pflag in Octonoba spiders and AgSp and Flag in Trichonephila clavipes. c and d Alignment results of the typical repetitive domains of CrSp and Pflag.
The cribellate spider-associated genes (CrSp and Pflag) were also searched based on the partial CrSp candidate sequence in T. perfuga43 and the Flag sequence in Araneus ventricosus48. As a result, a full picture of the genes, including the repetitive and N/C-terminal domains of CrSp and Pflag, was obtained (Fig. 1a). The lengths of the CrSp C-terminal and repetitive domains were 962 bp and 1009 bp on average, respectively. The repetitive domains were relatively long, similar to the architecture of AcSp, which has a repetitive domain with an average length of 1124 bp. The start codon of the CrSp N-terminal domain was determined based on the location of the signal peptide and Kozak rule (Fig. S3). The N-terminal length of other spidroins averages 500 bp, whereas the CrSp N-terminal domain was very long, more than 2 kbp (Fig. 1a). Because this long N-terminal domain was also found in another cribellate spider, S. mimosarum (family Eresidae) (KFM70693.1, Fig. S4), it was confirmed that the CrSp sequence is widely conserved among cribellate spiders. CrSp had a repetitive domain composed of at least 11 tandem copies and no intronic regions. The iteration number of repetitive domains was estimated using the long reads obtained by gDNA sequencing (Fig. S5). On the other hand, the repetitive domains of Pflag were approximately 200 bp in length, which was shorter than the repetitive domains of Pflag in D. spinosa44 (Fig. S6). Our spidroin catalog also revealed the motif variety in the repetitive domain of spidroins associated with prey capture threads. In the CrSp repetitive domain, a β-sheet motif (An), a known characteristic motif of MaSp, was found, and this feature was similar to the viscid glue protein (AgSp: aggregate spidroin) in T. clavipes (Fig. 1b,c). However, as mentioned in a previous study43, other than that, only features commonly found in spacers were observed (TT or SS), and no other noteworthy motif was found. On the other hand, Pflag had similar motifs as Flag, although the motif lengths of Pflag and Flag were different. In addition to the GGX motif reported in the Flag repetitive domain, GPSS and QQ motifs were found in Pflag (Fig. 1d).
Multiple omics profiling for CrSp and Pflag
CrSp and Pflag gene sequences obtained from genome data by homology search with known sequences are guaranteed by the sequence similarity only. Whether they worked in vivo was confirmed by expression profiling of the transcriptome and proteome. Transcriptome analysis was performed on cDNA sequencing samples from six species of Octonoba spiders. cDNA was synthesized from mRNA extracted from whole body total RNA, and two or more biological replicates were prepared for each species, except for O. okinawensis. According to the profile results, all spidroin genes were expressed in all spider bodies (Fig. 2a). Even relatively lowly expressed CrSp showed an expression level of approximately 100 TPM, with an overall trend toward higher expression of MaSp and MiSp observed. Moreover, a slight correlation relationship of gene expression patterns was observed between CrSp and Pflag (Fig. S7).
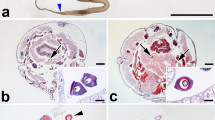
Expression and proteome analysis of Octonoba web threads. (a) Gene expression levels of spidroin genes in the whole body of six Octonoba spiders (Oco: O. grandiconcava; Ova: O. varians; Ook: O. okinawensis; Opj: O. grandiprojecta; Oye: O. yesoensis; Osy: O. sybotides). (b) An Octonoba varians web with the radius and spiral area, including cribellate silk (SEM image c) and stabilimentum (SEM image d). e The coloured amino acids in the CrSp and Pflag protein sequences represent the peptides detected from web threads by mass spectrometry analysis. f emPAI scores of spidroins in each web part (whole web, area without stabilimentum, and stabilimentum area).
The contributions of CrSp and Pflag proteins to the orb web composition were investigated by proteome analysis. According to a previous study, in the cribellate orb web, the cribellate silks are organized as a mat, are curled and enclose the spiral Pflag axial fibres with a puffy structure50. In addition, uloborid spiders decorate their orb web to reflect UV light and catch the prey insects attracted by the UV light45. This decoration is called stabilimentum and consists of AcSp4. To confirm the presence of CrSp and Pflag, proteome analysis was performed separately for each web part, such as the radius and spiral area, the stabilimentum area, and the whole web (Fig. 2b–d). We expected that the radius and spiral area would mainly include MaSp, MiSp, CrSp, and Pflag and that the stabilimentum area would consist of AcSp. The cribellate orb web sample was collected from the orb web built by O. okinawensis in a laboratory cage. The proteome analysis confirmed the presence of main spidroins, such as MaSp, MiSp, CrSp, Pflag, and AcSp, in the cribellate spider’s web (Fig. S8). The CrSp and Pflag sequences obtained from the genome were also observed as peptide fragments in the web (Fig. 2e). The quantification of the protein amount was calculated as an emPAI (exponentially modified protein abundance index; Fig. 2f and Table S2)51. The AcSp amount in the stabilimentum area was significantly higher than that in the radius and spiral area, and AcSp was shown to be the primary protein of the stabilimentum. On the other hand, for the radius area where CrSp was used as the coating thread, high amounts of CrSp and Pflag were detected. In particular, CrSp was used significantly more in the radius than in the stabilimentum. According to the multiple omics analysis from genomic DNA, RNA, and peptide sequences, we validated the newly found CrSp and Pflag.
Phylogenetic analysis of spidroin genes
Using our Octonoba spidroin catalog evaluated by multiple omics analysis, we compared the N/C-terminal regions with known spidroin genes in other spiders. The known spidroin gene sequences were obtained from a public database (NCBI) without limiting the taxonomic families (Tables S3 and S4). Based on the N/C-terminal regions, each of nine spidroin genes (MaSp, MiSp, AcSp, CySp, PySp, AgSp, Flag, Pflag, and CrSp) belonged to a monophyletic group regardless of the species taxonomy (Figs. 3a and S9). The C-terminal regions of CrSps were classified into a group consisting of previously reported CrSp candidates in T. perfuga and S. mimosarum43. Although Pflags form an independent group without Flags in the phylogenetic tree (Figs. 3a), the alignment analysis showed that the terminal regions are relatively well conserved, and Pflag and Flags seemed to share a monophyletic origin (Fig. 3c,d). On the other hand, since the AgSp clade includes the Flag/Pflag clade, it suggested that the CrSp and AgSp are not derived from a monophyletic lineage. This tendency was also observed for the amino acid compositions. Figure 4 shows the correlation matrix heatmap of amino acid compositions in the repetitive domains of all Octonoba spidroins. Intriguingly, the Octonoba spidroins were separated into two groups: web architecture-associated silks (MaSp, MiSp, and Pflag) and decorative or other silks (CrSp, AcSp, and CySp). Note that the AgSp in T. clavipes and A. ventricosus showed a unique amino acid composition different from that indicated by both groups.
Spidroin phylogeny. (a) Phylogenetic analysis of all spidroin C-terminal regions. Each spidroin type is coloured, and Octonoba spiders are indicated by bold font. Branch labels designate bootstrap support values. All spidroin data are listed in Tables S2 and S3. Alignment results of the CrSp C-terminal domain (b), Flag and Pflag N-terminal domain (c) and C-terminal domain (d).
Hierarchical clustering of spidroins based on amino acid composition in Octonoba spidroin. Hierarchical clustering based on amino acid in repetitive domains of spidroins. Oco: O. grandiconcava; Ova: O. varians; Ook: O. okinawensis; Opj: O. grandiprojecta; Oye: O. yesoensis; Osy: O. sybotides; Ave: A. ventricosus; Ncl: T. clavipes; Smi: S. mimosarum; Tpe: T. perfuga.
Discussion
Our study clarifies the molecular background of cribellate spiders and may enable us to perform large-scale phylogenetic analysis of spidroin variety evolution. So far, the whole spidroin catalog in orb-weaving spider has been reported for T. clavipes, A. ventricosus, and Caerostris darwini48,49,52, and these species are ecribellate orb-weaving spiders belonging to the superfamily Araneoidea. This study targets six spider species that are cribellate orb-weaving spiders belonging to the family Uloboridae and are distantly related to the superfamily Araneoidea. Spidroin catalogues were curated from each of the six species of uloborid spiders. This curation was realized using a previously reported method combining hybrid sequencing and the SMoC algorithm (Spidroin Motif Collection)48,49. Since it is impossible to sequence the heavily repetitive domains in spidroin using a short read sequencer53,54, the hybrid approach with short and long read sequencing is essential. Contig extension with an extremely large k-mer based on the short and long reads provided almost all spidroins. The detailed procedures are described in the “Methods” section.
The Octonoba spidroin catalogue including CrSp and Pflag genes was confirmed by multiple omics approaches, such as genomics, transcriptomics, and proteomics. Previously, partial spidroin genes of cribellate spiders have been found in O. varians, U. diversus (family Uloboridae), T. perfuga (family Zoropsidae), S. mimosarum (family Eresidae), and Deinopis spinosa (family Deinopidae)43,44,55. In O. varians, three approximately 300 aa long segments annotated as the “dragline silk spidroin 1 gene” have been reported (AY666057, AY666058, and AY666059)55. Those sequences matched to the C-terminal region of MaSp in our catalog (Fig. S10). The first CrSp gene sequence was reported by Correa-Garhwal and colleagues in T. perfuga (Zoropsidae; Dictynoidea). According to the alignment with previously reported genes, the C-terminal region of CrSp was conserved among the cribellate spiders (Octonoba, T. perfuga and S. mimosarum, Fig. 3b). In the case of the repetitive domain, although the similarity was not evaluated quantitatively since the reported domains of T. perfuga and S. mimosarum were too short, conservation was observed (Fig. S11).
Each spidroin was very well conserved among species in the genus Octonoba with respect to the spidroin gene sequence, architecture, and amino acid composition (Figs. 3 and 4). Moreover, except for genes related to prey capture threads (CrSp and AgSp), the spidroin genes in cribellate spiders showed very standard sequence features for orb-weaving spiders and did not differ from the sequence features of ecribellate spiders. Clustering analysis based on spidroin gene sequences showed the clear categorization of spidroin families (Fig. 3). Although Pflag and Flag were not extremely similar (Fig. 3c,d), taking a global phylogenetic view of spidroin, since Flag and Pflag seem to be categorized into the same clade, they may have a monophyletic origin. On the other hand, CrSp and AgSp were individually clustered, and their sequences were different from each other. The motifs and amino acid compositions in the repetitive domain varied completely (Figs. 4 and S11), and computationally predicted protein structures were different from each other (Fig. S12). It is known that the variety of mechanical properties in spider silk is realized by the secondary structure of protein designated by each motif in repetitive domain40. The difference in the secondary structure of CrSp and AgSp shown in Fig. S12 suggests that their mechanical properties are different.
The phylogenetic tree visualizes the evolutionary relationship at various scales and helps us to understand the relationship intuitively. However, the arguments based on the tree must be very cautious. Recently, in opposition to the conventional “ancient orb web hypothesis” that orb-weaving spiders have a single origin37,38,39,56, Fernández and colleagues argued that the orb-weaving spiders have at least three independent origins (Araneoidea, Deinopidae, and Uloboridae) and the prey capture webs evolved multiple times based on genomic-scale data42. They analysed lineage diversification and found that the ANE (superfamily Araneoidea, Nicodamoidea, and Eresoidea) and the UR (family Uloboridae, Deinopidae, Oecobiidae, Hersiliidae, and RTA clade) grades appeared 200–250 million years ago42. In these two grades, only Araneoidea, Hersiliidae, and RTA are ecribellate, and only Uloboridae and a part of Araneoidea construct orb webs. The cribellate and orb-weaving spiders were mixed in each grade. However, the following year, that hypothesis was again challenged. This phylogenetic analysis was thoroughly reviewed by Coddington and colleagues through the selection of appropriate algorithms and rigorous data curation, and it is shown that the spiders have been lost the web 5–7 times, not gained multiple times57. In other words, it was reconfirmed that the single origin was the most likely explanation. Our results based on the spidroin sequences also rejected the convergent evolution of the orb-web and strongly supported the “ancient orb web hypothesis”. The uloborid spidroin catalogue revealed that the cribellate spidroin sequences are well conserved among the cribellate spiders (Fig. S4), and the orb-web-associated spidroin sequences are also conserved among orb-weaving spiders (Fig. 3). Sequence conservation dismisses the possibility of convergent evolution and strongly supports the existence of a common ancestor harbouring cribellate orb webs like uloborid spiders. AgSp has been reported not only from the orb-weaving spiders, but also from Theridiidae spiders that build a cob web54,58,59,60,61. Hence, our molecular analyses suggest the possibility that complex ANE and UR grades appear as a result of the simple evolutionary history of CrSp or orb web losses or AgSp gains. It was suggested that the acquisition of the prey capture strategy and the web structure might be independent.
Methods
Spider sample preparation
Uloborid spider specimens (O. sybotides, O. yesoensis, O. grandiprojecta, O. varians, O. grandiconcava, and O. okinawensis) were collected from Yamagata, Hokkaido, Kanagawa, Kumamoto, Wakayama, Okinawa, and Kagoshima Prefecture, Japan (from May 2015 to December 2018). The samples were stored in a plastic container (PAPM340, RISUPACK CO., LTD.) and transported live back to the laboratory. The collected spiders were initially identified based on morphological characteristics, and identifications were confirmed using the cytochrome c oxidase subunit 1 (COX1) sequence in the Barcode of Life Data System (BOLD: https://www.barcordinglife.org). Based on previously established field sampling protocol62, immediately upon arrival in the laboratory, uloborid spiders were immersed in liquid nitrogen (LN2) for cDNA or genome sequencing and stored at − 80 °C. The SEM images of silk were taken with a scanning electron microscopy Phenom World model Phenom Pro-X using a web built in the laboratory.
Total RNA extraction
Total RNA was extracted from 15 specimens of six uloborid spider species (O. sybotides, O. yesoensis, O. grandiprojecta, O. varians, O. grandiconcava, and O. okinawensis) based on the spider transcriptome protocol62. We immersed the flash frozen specimens into 1 mL TRIzol Reagent (Invitrogen) before homogenizing with a metal cone using the Multi-Beads Shocker (Yasui Kikai). Using an RNeasy Plus Mini Kit (Qiagen) automated with QIACube (Qiagen), the extracted RNA was purified. The total RNA was qualified with a NanoDrop 2000 (Thermo Scientific) and TapeStation 2200 with RNA Screen Tape (Agilent Technologies). The quantity was measured with Qubit Broad Range (BR) RNA assay (Life Technologies).
HMW (high molecular weight) gDNA extraction
According to a previous report48, HMW gDNA was extracted from the whole bodies of flash frozen spiders using Genomic-tip 20/G (QIAGEN). The specimens were quickly homogenized using BioMasher II (Funakoshi) and mixed with 2 mL of Buffer G2 (QIAGEN), including 200 µg/mL RNase A. After the addition of 50 µL of proteinase K (20 mg/mL), the lysate was incubated at 50 °C for 12 h on a shaker (300 rpm). The lysate was centrifuged at 5,000 × g for 5 min at 4 °C to pellet the debris, and the aqueous phase was loaded onto a pre-equilibrated QIAGEN Genomic-tip 20/G (QIAGEN) by gravity flow. The QIAGEN Genomic-tip 20/G (QIAGEN) was then washed three times, and the DNA was eluted with high-salt buffer (Buffer QF) (QIAGEN). The eluted DNA was desalted and concentrated by isopropanol precipitation and resuspended in 10 mM Tris–HCl (pH 8.5). Extracted gDNA was qualified using a NanoDrop 2000 (Thermo Scientific) and TapeStation 2200 with genomic DNA Screen Tape (Agilent Technologies) and quantified using a Qubit Broad Range (BR) dsDNA assay (Life Technologies).
Library preparation and sequencing of cDNA and gDNA
Library preparation was performed using the same protocol as the previously conducted for cDNA/gDNA sequencing of A. ventricosus and E. variegata47,48. The libraries for cDNA sequencing were prepared using the NEBNext Ultra RNA Library Prep Kit for Illumina (New England BioLabs) according to the manufacture’s protocol. Using NEBNext Oligo d(T)25 beads without a wash step, mRNA was isolated from 100 µg of extracted total RNA. The cDNA synthesis was performed using ProtoScript II Reverse Transcriptase and NEBNext Second Strand Synthesis Enzyme Mix. Synthesized double-stranded cDNA was ligated with a NEBNext Adaptor for Illumina after end repair using NEBNext End Prep Enzyme Mix. After USER enzyme treatment, cDNA amplification by PCR was performed with the following conditions: 20 μL cDNA, 2.5 μL index primer, 2.5 μL universal PCR primer, 25 μL NEBNext Q5 Hot Start HiFi PCR Master Mix 2X; 98 °C for 30 s and 12 cycles each of 98 °C for 10 s, 65 °C for 75 s and 65 °C for 5 min. In the case of nanopore sequencing of gDNA long reads, we purified HMW gDNA by > 8 kb size selection using a BluePippin system (Sage Science) with a 0.75% agarose gel cassette before starting the library preparation with the 1D library protocol (SQK-LSK108, Oxford Nanopore Technologies). The cDNA sequencing was performed with a NextSeq 500 (Illumina, Inc.) using 150 bp paired-end reads with a NextSeq 500 High Output Kit (300 cycles). Sequenced read quality was assessed with FastQC (v0.10.1: https://www.bioinformatics.bbsrc.ac.uk/projects/fastqc/). Nanopore sequencing of gDNA long reads was performed with v9.4 SpotON flow cells (FLO-MIN106) on a GridION instrument (Oxford Nanopore Technologies).
De novo transcriptome assembly
The de novo assembly was performed using Bridger [r2014‐12‐01:63] with the following options: pair_gap_length = 0 and k‐mer = 31. The transcriptome assembly was validated by analysis of BUSCO (Benchmarking Universal Single-Copy Ortholog) completeness with the Arthropoda lineage gene set 64.
Spidroin gene curation
Octonoba spidroin gene curation was performed based on a previously reported SMoC (Spidroin Motif Collection) algorithm48. This algorithm was implemented using the hybrid assembly with short and long reads. Illumina short reads were assembled in contigs by the de Bruijn graph method, and a BLAST search for N/C-terminus and repetitive region candidates was carried out on the contigs. The obtained candidates were used as seeds for screening the short reads harbouring an exact match of extremely large k-mers up to the 5′-end, and the short reads were aligned on the 3′-side of the matching k-mer to build a PWM (Position Weight Matrix). Based on stringent thresholds, the seed sequences were extended until neighbouring repeats appeared. Finally, the collected full-length subsets of the repeat units were mapped onto error-corrected long reads. The data about the spidroin gene length or architecture were curated manually based on the mapped long reads.
Phylogenetic analysis
Phylogenetic reconstructions of the N/C-terminal spidroin regions were performed by MAFFT alignment of the first 90 N-terminal amino acid residues and the last 80 C-terminal amino acid residues of Octonoba spiders with the corresponding amino acid residues of all other available spidroin sequences (Tables S3 and S4). Maximum likelihood gene trees were constructed with 1,000 bootstrap replicates by RAxML v8.2.1165. FigTree v1.4.3 (https://tree.bio.ed.ac.uk/software/figtree/) was used as a viewer for the trees.
Expression analyses
Transcript abundances were estimated by kallisto v0.42.2.1 in transcripts per million (TPM). Each transcriptome data set was obtained from whole-body cDNA sequencing of Octonoba spiders, and our transcriptome assembly was used as the reference.
Orb web thread collection and preparation for proteome analysis
Stabilimentum and other thread components were directly collected from the orb web of Octonoba okinawensis. All silk samples were gently washed with 100 µL of base buffer [50 mM ammonium carbonate in distilled water] with 0.1% SDS per 0.5–1.0 mg of silk at RT for 1–2 min. After the supernatant removal, silk samples were immersed in 10 µL of 6 M guanidine-HCI buffer (pH8.2) and protein extraction was performed by sonication for 10 min with Bioruptor II (BM Equipment Co., Ltd.). The lysates were reacted with DTT (dithiothreitol) for 30 min at 37 ℃ followed by IAA (iodoacetamide) for 30 min at 37 ℃ in the dark. After five times dilution by 50 mM ammonium carbonate in distilled water, the samples were reacted with Lys-C for 3 h at 37 ℃ followed by trypsin for 16 h at 37 ℃ to digest proteins. The enzymatic reaction was quenched by acidification with TFA (trifluoroacetic acid) and the digested samples were desalted using Empore SDB-XC membrane (3 M) packed StageTips.
Liquid chromatography mass spectrometry analysis
Liquid chromatography mass spectrometry was performed using the same protocol as the previously conducted for dragline silk of A. ventricosus48. We dissolved each silk sample into 12 µL of 0.1% TFA acid and 5% acetonitrile and loaded 5 µL of the solution on a hand-made spray needle column (ACQUITY UPLC BEH C18 materials, 100 µm i.d. Dr. Maisch GmbH, Germany, 5 µm tip i.d., 150 mm length) using an HTC-PAL autosampler (CTC Analytics, Zwingen, Switzerland). Separation of peptide fragments in the samples through the column was performed by reversed-phase chromatography in linear gradient mode using an UltiMate 3000 nanoLC Pump (Dionex Co., Sunnyvale, CA, USA). Two mobile phases, (A) acetic acid/dimethyl sulfoxide/water (0.5:4:96, v/v/v) and (B) acetic acid/dimethyl sulfoxide/acetonitrile (0.5:4:96, v/v/v) were mixed at a flow rate of 500 nL/min. The composition was changed as follows: (A) + (B) = 100%, (B) 0–35% (0–60 min), 35–80% (60–65 min), 80% (65–70 min), and 0% (70.1–95 min). The peptides ionized at 2600 V by the positive electrospray method were injected into an LTQ orbitrap XL ETD (Thermo Electron, San Jose, CA, USA) and detected as peptide ions (scan range: m/z 400–1500; mass resolution: 60,000 at m/z 400). The top 10 peaks of multiply charged peptide ions were subjected to collision-induced dissociation (isolation width: 2, normalized collision energy: 35 V, activation Q: 0.25, activation time: 30 s) to identify the amino acid sequences.
Database search for protein identification
The peak lists were created from LC–MS raw data files with msconvert.exe, which was provided by ProteoWizard64, and analysed with Mascot server version 2.5 (Matrix Science, Boston, MA, USA)65 to identify the peptides and proteins in each sample. Our transcriptome assembly was used in the analysis with the following conditions: precursor mass tolerance; 6 ppm, production mass tolerance; 0.5 Da, enzyme; trypsin, max missed coverage; 2, fixed modification; carbamidomethylation at Cys, variable modification; N-acetylation at protein N-term and oxidation at Met, criteria for identification; p < 0.05 (MS/MS ion search). The protein amount was estimated based on the number of sequenced peptides per protein (emPAI)51.
Bioinformatics analysis
All bioinformatics analyses were performed using Perl custom scripts with the G-language Genome Analysis Environment (v1.9.1)66. The statistical analyses and visualizations were implemented using the R package (v 3.2.1)67. The homology search was carried out with BLASTP. The sequence logo was generated by WebLogo 368, and the sequence alignment figure was produced by MUSCLE69 and MView70. The signal peptide was predicted by SignalP (v 5.0)71 and PrediSi72, and the initiation codon in cDNA was predicted based on Kozak’s rule by ATGpr73.
Data accessibility
Raw sequence reads used for genome assembly and expression analysis have been submitted to DDBJ SRA (sequence read archive). Accession numbers of transcriptome short reads are DRR155974 (Octonoba yesoensis), DRR156101 (Octonoba sybotides), DRR155866 (Octonoba okinawensis), DRR156045 (Octonoba varians), DRR157004 (Octonoba grandiconcava), and DRR157173 (Octonoba grandiprojecta), and nanopore long reads of genomic DNA are DRR238369 (Octonoba okinawensis), DRR238370 (Octonoba varians), DRR238371 (Octonoba sybotides). The assembled contigs are available at the DDBJ under the accession numbers IAQA01000001-IAQA01044186 (Octonoba yesoensis), IAUX01000001-IAUX01039949 (Octonoba sybotides), IALW01000001-IALW01038452 (Octonoba okinawensis), IAST01000001-IAST01041438 (Octonoba varians), ICDQ01000001-ICDQ01046746 (Octonoba grandiconcava), and ICKD01000001-ICKD01039972 (Octonoba grandiprojecta). Each spidroin sequence is available at the DDBJ under the accession number LC570186-LC570252.
References
Vollrath, F. Spider webs and silks. Sci. Am. 266, 70–76 (1992).
Guerette, P. A., Ginzinger, D. G., Weber, B. H. & Gosline, J. M. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science 272, 112–115 (1996).
Gatesy, J., Hayashi, C., Motriuk, D., Woods, J. & Lewis, R. Extreme diversity, conservation, and convergence of spider silk fibroin sequences. Science 291, 2603–2605 (2001).
Foelix, R. F. Biology of Spiders 2nd edn. (Oxford University Press, Oxford, 1996).
Shultz, J. W. The origin of the spinning apparatus in spiders. Biol. Rev. Camb. Philos. Soc. 62, 89–113 (1987).
Hu, X. et al. Molecular mechanisms of spider silk. Cell. Mol. Life Sci. 63, 1986–1999 (2006).
Vollrath, F. Biology of spider silk. Int. J. Biol. Macromol. 24, 81–88 (1999).
Vollrath, F. & Selden, P. The role of behavior in the evolution of spiders, silks, and webs. Annu. Rev. Ecol. Evol. Syst. 38, 819–846 (2007).
Vollrath, F. et al. Compounds in the droplets of the orb spider’s viscid spiral. Nature 345, 526–528 (1990).
Sahni, V., Blackledge, T. A. & Dhinojwala, A. Viscoelastic solids explain spider web stickiness. Nat. Commun. 1, 19 (2010).
Opell, B. D. & Hendricks, M. L. The role of granules within viscous capture threads of orb-weaving spiders. J. Exp. Biol. 213, 339–346 (2010).
Hayashi, C. Y. & Lewis, R. V. Molecular architecture and evolution of a modular spider silk protein gene. Science 287, 1477–1479 (2000).
Hayashi, C. Y. & Lewis, R. V. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J. Mol. Biol. 275, 773–784 (1998).
Opell, B. D., Jain, D., Dhinojwala, A. & Blackledge, T. A. Tuning orb spider glycoprotein glue performance to habitat humidity. J. Exp. Biol. 221, jeb161539 (2018).
Diaz, C. et al. Supersaturation with water explains the unusual adhesion of aggregate glue in the webs of the moth-specialist spider, Cyrtarachne akirai. R. Soc. Open. Sci. 5, 181296 (2018).
Peters, H. M. On the spinning apparatus and the structure of the capture threads ofDeinopis subrufus (Araneae, Deinopidae). Zoomorphology 112, 27–37 (1992).
Peters, H. M. The spinning apparatus of Uloboridae in relation to the structure and construction of capture threads (Arachnida, Araneida). Zoomorphology 104, 96–104 (1984).
Coddington, J. Cladistics and spider classification: Araneomorph phylogeny and the monophyly of orbweavers (Araneae: Araneomorphae, Orbiculariae). Acta Zool. Fenn. 190, 75–87 (1990).
Charles, E. G., Jonathan, A. C., Norman, I. P. & Raymond, R. F. Towards a phylogeny of entelegyne spiders (Araneae, Araneomorphae, Entelegynae). J. Arachnol. 27, 53–63 (1999).
Hayashi, C. Y. & Lewis, R. V. Spider flagelliform silk: Lessons in protein design, gene structure, and molecular evolution. BioEssays 23, 750–756 (2001).
Joel, A. C., Scholz, I., Orth, L., Kappel, P. & Baumgartner, W. Morphological adaptation of the calamistrum to the cribellate spinning process in Deinopoidae (Uloboridae, Deinopidae). R. Soc. Open Sci. 3, 150617 (2016).
Opell, B. Changes in spinning anatomy and thread stickiness associated with the origin of orb-weaving spiders. Biol. J. Linn. Soc. Lond. 68, 593–612 (1999).
Blackledge, T. A. & Hayashi, C. Y. Unraveling the mechanical properties of composite silk threads spun by cribellate orb-weaving spiders. J. Exp. Biol. 209, 3131–3140 (2006).
Piorkowski, D. et al. Uncoiling springs promote mechanical functionality of spider cribellate silk. J. Exp. Biol. 223, jeb215269 (2020).
Griswold, C. E., Coddington, J. A., Hormiga, G. & Scharff, N. Phylogeny of the orb-web building spiders (Araneae, Orbiculariae: Deinopoidea, Araneoidea). Zool. J. Linn. Soc. Lond. 123, 1–99 (2008).
Opell, B. D. & Schwend, H. S. Persistent stickiness of viscous capture threads produced by araneoid orb-weaving spiders. J. Exp. Zool. A Ecol. Genet. Physiol. 309, 11–16 (2008).
Piorkowski, D. & Blackledge, T. A. Punctuated evolution of viscid silk in spider orb webs supported by mechanical behavior of wet cribellate silk. Naturwissenschaften 104, 67 (2017).
Bott, R. A., Baumgartner, W., Braunig, P., Menzel, F. & Joel, A. C. Adhesion enhancement of cribellate capture threads by epicuticular waxes of the insect prey sheds new light on spider web evolution. Proc. Biol. Sci. 284, 20170363 (2017).
Michalik, P., Piorkowski, D., Blackledge, T. A. & Ramirez, M. J. Functional trade-offs in cribellate silk mediated by spinning behavior. Sci. Rep. 9, 9092 (2019).
Opell, B. & Bond, J. Changes in the mechanical properties of capture threads and the evolution of modern orb-weaving spiders. Evol. Ecol. Res. 3, 567–581 (2001).
Bond, J. E. & Opell, B. D. Testing adaptive radiation and key innovation hypotheses in spiders. Evolution 52, 403–414 (1998).
Opell, B. Economics of spider orb-webs: The benefits of producing adhesive capture thread and of recycling silk. Funct. Ecol. 12, 613–624 (1998).
Opell, B. The Material Cost and Stickiness of Capture Threads and the Evolution of Orb-Weaving Spiders. Vol. 62 (1997).
Townley, M., Bernstein, D. T., Gallagher, K. S. & Tillinghast, E. K. Comparative study of orb web hygroscopicity and adhesive spiral composition in three araneid spiders. J. Exp. Zool. 259, 154–165 (1991).
Kawamoto, T. & Ferreira Japyassú, H. Tenacity and silk investment of two orb weavers: Considerations about diversification of the Araneoidea. J. Arachnol. 36, 418–424 (2008).
Kaston, B. J. The evolution of spider webs. Am. Zool. 4, 191–207 (1964).
Dimitrov, D. et al. Rounding up the usual suspects: A standard target-gene approach for resolving the interfamilial phylogenetic relationships of ecribellate orb-weaving spiders with a new family-rank classification (Araneae, Araneoidea). Cladistics 33, 221–250 (2017).
Bond, J. E. et al. Phylogenomics resolves a spider backbone phylogeny and rejects a prevailing paradigm for orb web evolution. Curr. Biol. 24, 1765–1771 (2014).
Garrison, N. L. et al. Spider phylogenomics: Untangling the Spider Tree of Life. PeerJ 4, e1719 (2016).
Blackledge, T. A., Kuntner, M. & Agnarsson, I. In Advances in Insect Physiology Vol. 41 (ed Jérôme Casas) 175–262 (Academic Press, 2011).
Blackledge, T. A. et al. Reconstructing web evolution and spider diversification in the molecular era. Proc. Natl. Acad. Sci. USA 106, 5229–5234 (2009).
Fernandez, R. et al. 2018 Phylogenomics, diversification dynamics, and comparative transcriptomics across the spider tree of life. Curr. Biol. 28, 1489-1497 e1485 (2018).
Correa-Garhwal, S. M. et al. Silk genes and silk gene expression in the spider Tengella perfuga (Zoropsidae), including a potential cribellar spidroin (CrSp). PLoS ONE 13, e0203563 (2018).
Garb, J. E., Dimauro, T., Vo, V. & Hayashi, C. Y. Silk genes support the single origin of orb webs. Science 312, 1762 (2006).
Watanabe, T. Prey attraction as a possible function of the silk decoration of the uloborid spider Octonoba sybotides. Behav. Ecol. 10, 607–611 (1999).
Xu, M. & Lewis, R. V. Structure of a protein superfiber: Spider dragline silk. Proc. Natl. Acad. Sci. USA 87, 7120–7124 (1990).
Kono, N. et al. The bagworm genome reveals a unique fibroin gene that provides high tensile strength. Commun. Biol. 2, 148 (2019).
Kono, N. et al. Orb-weaving spider Araneus ventricosus genome elucidates the spidroin gene catalogue. Sci. Rep. 9, 8380 (2019).
Babb, P. L. et al. The Nephila clavipes genome highlights the diversity of spider silk genes and their complex expression. Nat. Genet. 49, 895–903 (2017).
Joel, A. C., Kappel, P., Adamova, H., Baumgartner, W. & Scholz, I. Cribellate thread production in spiders: Complex processing of nano-fibres into a functional capture thread. Arthropod. Struct. Dev. 44, 568–573 (2015).
Ishihama, Y. et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteom. 4, 1265–1272 (2005).
Garb, J. E. et al. The transcriptome of Darwin’s bark spider silk glands predicts proteins contributing to dragline silk toughness. Commun. Biol. 2, 275 (2019).
Kono, N. & Arakawa, K. Nanopore sequencing: Review of potential applications in functional genomics. Dev. Growth Differ. 61, 316–326 (2019).
Stellwagen, S. D. & Renberg, R. L. Toward spider glue: Long read scaffolding for extreme length and repetitious silk family genes AgSp1 and AgSp2 with insights into functional adaptation. G3 (Bethesda) 9, 1909–1919 (2019).
Tai, P. L., Hwang, G. Y. & Tso, I. M. Inter-specific sequence conservation and intra-individual sequence variation in a spider silk gene. Int. J. Biol. Macromol. 34, 295–301 (2004).
Coddington, J. In Spiders: Webs, Behavior, and Evolution The Monophyletic Origin of the Orb Web (ed W.A. Shear) 319–363 (Stanford University Press, 1986).
Coddington, J. A., Agnarsson, I., Hamilton, C. A. & Bond, J. E. Spiders did not repeatedly gain, but repeatedly lost, foraging webs. PeerJ 7, e6703 (2019).
Hu, X. et al. Egg case protein-1. A new class of silk proteins with fibroin-like properties from the spider Latrodectus hesperus. J. Biol. Chem. 280, 21220–21230 (2005).
Blackledge, T. A., Swindeman, J. E. & Hayashi, C. Y. Quasistatic and continuous dynamic characterization of the mechanical properties of silk from the cobweb of the black widow spider Latrodectus hesperus. J. Exp. Biol. 208, 1937–1949 (2005).
Sahni, V., Blackledge, T. A. & Dhinojwala, A. Changes in the adhesive properties of spider aggregate glue during the evolution of cobwebs. Sci. Rep. 1, 41 (2011).
Collin, M. A., Clarke, T. H. 3rd., Ayoub, N. A. & Hayashi, C. Y. Evidence from multiple species that spider silk glue component ASG2 is a spidroin. Sci. Rep. 6, 21589 (2016).
Kono, N., Nakamura, H., Ito, Y., Tomita, M. & Arakawa, K. Evaluation of the impact of RNA preservation methods of spiders for de novo transcriptome assembly. Mol. Ecol. Resour. 16, 662–672 (2016).
Chang, Z. et al. Bridger: A new framework for de novo transcriptome assembly using RNA-seq data. Genome Biol. 16, 30 (2015).
Simao, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Arakawa, K. et al. G-language Genome Analysis Environment: A workbench for nucleotide sequence data mining. Bioinformatics 19, 305–306 (2003).
Team, R. C. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2015).
Crooks, G. E., Hon, G., Chandonia, J. M. & Brenner, S. E. WebLogo: A sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Brown, N. P., Leroy, C. & Sander, C. MView: A web-compatible database search or multiple alignment viewer. Bioinformatics 14, 380–381 (1998).
Almagro Armenteros, J. J. et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 37, 420–423 (2019).
Hiller, K., Grote, A., Scheer, M., Munch, R. & Jahn, D. PrediSi: Prediction of signal peptides and their cleavage positions. Nucleic Acids Res. 32, W375-379 (2004).
Salamov, A. A., Nishikawa, T. & Swindells, M. B. Assessing protein coding region integrity in cDNA sequencing projects. Bioinformatics 14, 384–390 (1998).
Acknowledgements
The authors thank Akio Tanikawa for the morphological identification of spiders, Anna-Christin Joel for the helpful comments about pictures of cribellate capture threads, and the anonymous reviewers for thoughtful comments and suggestions. The authors also thank Yuki Takai, Naoko Ishi, Hironori Yamamoto, and Kaori Yaosaka for their technical support in the sequencing analysis and the scanning electron microscopy. This work was funded by a Nakatsuji Foresight Foundation Research Grant, the Sumitomo Foundation (190426), the ImPACT Program of Council for Science, Technology and Innovation (Cabinet Office, Government of Japan) and in part by research funds from the Yamagata Prefectural Government and Tsuruoka City, Japan.
Author information
Authors and Affiliations
Contributions
N.K. designed the entire project, performed all analyses, and wrote the manuscript. N.K. and K.A. performed transcriptome analyses. N.K. and M.M. performed proteome analyses. N.K. and H.N. collected spider specimens and silk samples. N.K., M.T. and K.A. managed the computer resources. All authors contributed to editing and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
H.N. is an employee of Spiber Inc., a venture company selling artificial spider silk products. However, all study designs were made by Nobuaki Kono of Keio University, and Spiber Inc. had no role in the study design, data analysis, data interpretation, or writing of the manuscript. N.K., M.M., M.T., and K.A. declare no conflict of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kono, N., Nakamura, H., Mori, M. et al. Spidroin profiling of cribellate spiders provides insight into the evolution of spider prey capture strategies. Sci Rep 10, 15721 (2020). https://doi.org/10.1038/s41598-020-72888-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72888-6
This article is cited by
-
Digestive enzymes and sphingomyelinase D in spiders without venom (Uloboridae)
Scientific Reports (2023)
-
The evolutionary history of cribellate orb-weaver capture thread spidroins
BMC Ecology and Evolution (2022)
-
The balance of crystalline and amorphous regions in the fibroin structure underpins the tensile strength of bagworm silk
Zoological Letters (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.