Abstract
Probiotics have been reported to have a positive impact on the metabolic control of patients with type 2 diabetes. We aimed to systematically evaluate the effects of probiotics on cardiometabolic parameters in type 2 diabetes based on randomized controlled studies. MEDLINE, Embase, and CENTRAL databases were reviewed to search for randomized controlled trials that examined the effects of probiotic supplementation on cardiometabolic parameters in patients with type 2 diabetes. 32 trials provided results suitable to be included in the analysis. The effects of probiotics were calculated for the following parameters: BMI, total cholesterol levels, LDL, triglycerides, HDL, CRP, HbA1c levels, fasting plasma glucose, fasting insulin levels, systolic and diastolic blood pressure values. Data analysis showed a significant effect of probiotics on reducing total cholesterol, triglyceride levels, CRP, HbA1c, fasting plasma glucose, fasting insulin levels, and both systolic and diastolic blood pressure values. Supplementation with probiotics increased HDL levels however did not have a significant effect on BMI or LDL levels. Our data clearly suggest that probiotics could be a supplementary therapeutic approach in type 2 diabetes mellitus patients to improve dyslipidemia and to promote better metabolic control. According to our analysis, probiotic supplementation is beneficial in type 2 diabetes mellitus.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is one of the major worldwide unresolved health challenges: it is a major risk factor for a number of common, sometimes potentially lethal diseases, such as hypertension, stroke, coronary heart disease1, or kidney failure and retinopathy2. According to the International Diabetes Federation, the worldwide prevalence of diabetes mellitus was 8.8% in 2015, and by 2040 the prevalence of diabetes in adults is predicted to rise to 10.4%3. The increasing prevalence of obesity provides ground to the rising prevalence of type 2 diabetes4. Even though the main cause of obesity is the imbalanced calorie intake, one intriguing hypothesis links the composition of the human gut microbiome to human energy homeostasis; for instance with their ability to promote adiposity through manipulation of host genes and metabolism, an altered microbiome can lead to predisposition to obesity5. The alteration in the gut microbiota has recently been recognized as a key environmental factor resulting in metabolic diseases, such as type 2 diabetes. In fact, the gut microbiota is involved in the maintenance of host energy homeostasis and in the stimulation of host immunity through a molecular crosstalk6.
Although many drugs have been developed to maintain glycemic control and normalize blood glucose levels either via enhanced insulin production and utilization, suppressed glucose production and absorption, by blocking urine glucose re-absorption and increasing glucose excretion in urine, or the combination of these7, these drugs may cause several adverse effects such as sulphonylureas carry a risk of causing acute severe hypoglycemia; lactic acidosis is also a potentially serious adverse effect associated with the use of biguanides; and gastrointestinal adverse effects may occur with the use of metformin8. Alternatively, the potential role of modifications in the gut microbiome had been explored as a new complementary therapeutic strategy9. Clinical evidence supports the hypothesis that the modulation of the gut microbiota by probiotics could be effective in prevention and management of diabetes10,11.
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. The healthy human body contains such microbes physiologically; and they can be obtained in forms of over-the-counter food supplements as well. Over the last few years, probiotics, especially the lactobacillus species were shown to be effective in the therapy of type 2 diabetes12. In type 2 diabetes, gut microbiome is found to be different from that in the healthy population. In a human study, the amount of Firmicutes bacteria was lower, whereas the number of Bacteroides and Proteobacteria is higher in the gastrointestinal tract of patients with type 2 diabetes compared to non-diabetic persons13. According to the study13, the ratio of Bacteriodes and Firmicutes species had positive correlation with decreased insulin resistance, however, causality has not been proven yet. Following innovative dietary strategies, it seems possible to maintain euglycemia by normalizing the altered microbiome, and to prevent long-term micro- and macrovascular complications of type 2 diabetes9. Although, there have been numerous bacterial species investigated in the therapy of type 2 diabetes, no consensus has been obtained regarding the effectivity and the most effective species. For instance, an earlier meta-analysis suggested, that the intake of certain Lactobacillus species, such as L. fermentum, L. ingluviei and L. acidophilus can lead to weight gain, while the ingestion of L. gasseri and L. plantarum might end up in weight loss both in animal and human studies14. Previous meta-analysis in this field were not conducted with assessment of the evidence quality levels and the number of identified trials that met their inclusion criteria was relatively low (7–12 trials)15,16,17,18,19. Two meta-analysis found no significant effects of probiotics on lipid profile16,19 and two meta-analysis found decreased indexes of lipid profiles17,18. These contradictory reports on the effect of probiotics inspired us to conduct an updated meta-analysis to assess the effect of probiotic therapies in diabetes mellitus type 2 exclusively from randomized controlled trials.
Materials and methods
Protocol and registration
This meta-analysis was reported according to the recommendation of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines20. Pre-specified protocol of this meta-analysis was published in the Prospero Center for Reviews and Dissemination (PROSPERO) under the registration number of CRD42019137997.
Search strategy
Meta-analysis was performed using the PICO format: whether an intervention with probiotic supplementation (I) compared with placebo (C) has any effect on metabolic parameters (body mass index (BMI), total-cholesterol, low density lipoprotein (LDL), triglycerides (TG), high density lipoprotein (HDL), high sensitivity C-reactive protein (hs-CRP), haemoglobin A1c (HbA1c), fasting plasma glucose and insulin levels, systolic and diastolic blood pressure (SBP, DBP) (O) in patients with diabetes mellitus type 2 (P). In general, the following search terms were used in all databases: diabetes mellitus type 2 AND (probiotic* OR lactobacillus OR saccharomyces OR enterococcus OR escherichia coli OR streptococcus OR bifidobacterium) AND random*. Trials were identified by searching MEDLINE (via PubMed), EMBASE and CENTRAL databases up to 5th of April 2019. No filters or restrictions were applied. We included human trials without any restriction to language or year of publication.
Eligibility criteria and study selection
Duplicates were removed by the EndNote software first automatically, then manually. Randomized controlled trials in which probiotics in the form of any pharmaceutical formulations or dairy products administered to adult patients with type 2 diabetes were included after title and abstract screening. Combination therapy was not an exclusion criterion. Subsequently, full texts of the articles were reviewed for inclusion of eligible studies. Two review authors (TK and BM) selected the articles fulfilling the inclusion criteria independently, and any disagreement was resolved by consensus.
Data collection
At the end of the screening process, relevant data were independently extracted from studies by the two review authors and any disagreement was resolved by consensus. Data were extracted into a standardized excel sheet form. Data extracted from the papers included: number of participants, dosage, the intervention used, study duration and the outcome parameters including BMI changes as primary outcome and changes in the total-cholesterol, LDL, TG, HDL, hs-CRP, HbA1C, fasting plasma glucose and insulin levels, SBP and DBP as secondary outcomes. The authors of the studies and year of publication were also recorded. Mean values for control and intervention groups, along with the measure of dispersion were extracted.
Risk of bias assessment
Two review authors assessed the risk of bias of the studies independently, and any disagreement was resolved by consensus. The assessment was performed using the updated version of the Cochrane risk-of-bias tool for randomized trials (RoB 2) with the following domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, bias in selection of the reported result, and overall bias21.
Quality of evidence
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to rate the quality of evidence on our primary outcomes.
Statistical analysis
We calculated weighted mean differences (WMD) with 95% confidence intervals (CI) as effect size data based on the difference of before-after values in the intervention and comparator groups. Means were compared by assessing the overlap of CIs. Between-study heterogeneity was tested with (a) chi2 statistics (where p < 0.1 was considered significant) and (b) I2 statistics, where 75–100% was considered considerable22. Due to the methodological differences between interventions, we performed all analysis under the random effect assumption. To assess small study effect, we used visual inspection of funnel plots and Egger's test was performed. If p ≥ 0.1, publication bias is unlikely to occur in the sample. We used trial sequential analysis to investigate if alpha and beta-type errors affect our estimates. All analyses were performed with the Comprehensive Meta-analysis software (Biostat, Inc., Engelwood, MJ, USA) and Stata 11 SE (Stata Corp) software.
Results
Characteristics of the included studies
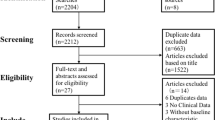
A flow chart of selection for the meta-analysis is shown in Fig. 1.
32 eligible studies were included in the meta-analysis12,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52. Main characteristics of the studies included are shown in Table 1. Fifteen studies administered one bacterial species, while the rest of the studies used a combination of more than one strain: seventeen studies administered two to seven bacterial species. In one article, the flora of the probiotic yoghurt of the intervention group was enriched with specific strains, however placebo yoghurt also contained bacterial flora12. In three articles, probiotics were co-administered with chromium35,51, 52, in one article with selenium42, and in one article with vitamin D43. The duration of intervention ranged from four to 34 weeks. Seventeen of the 32 articles were published from Iran, two from Saudi Arabia, two from Ukraine, two from Brazil, two from India, and the remaining ones from Malaysia, Denmark, Taiwan, Poland, Sweden, Japan, and Greek.
Summary of findings
Data of outcome parameters are summarized in Table 2.
The summary of findings table provides a synopsis of the analysis (Table 3).
Risk of bias within the individual studies
One study had high risk overall40. In seven studies, some concerns were detected; however, we found no articles with any concern about missing outcome data. The quality of the included studies is shown in detail in Fig. 2. Generally, the quality of the studies was good, in most cases with published pre-study protocols. We found three studies that were single-blind37,51,52, three more studies without blinding26,46,48, all the other articles contained double-blind studies.
Probiotics did not change body mass index
Seventeen studies reported BMI changes. Pooled data showed no difference between the probiotic and placebo group. Considerable heterogeneity (I2: 86.6%, p < 0.001) was detected.
Probiotics improved plasma lipid profile
Twenty-one studies included data about the effect of probiotics on total-cholesterol level. Pooled data showed a significant effect of probiotics on reducing total-cholesterol levels with a mean difference of 10.06 mg/dL (95% CI − 15.94, − 4.18, p = 0.001) with a considerable heterogeneity (I2: 93.2%, p < 0.001). Sub-group analysis according to the length of investigation (i.e. duration of treatment) did not reduce the heterogeneity (Fig. 3, short: I2: 93.2%, p < 0.001; long: I2: 93.4%, p < 0.001). Short studies with 8 weeks treatment or shorter showed significant decrease of total cholesterol level (− 14.56 mg/dL, 95% CI − 24.82, − 4.29, p = 0.005), while studies of 12 weeks or longer showed no significant change (p = 0.105).
Forest plot for the effect of probiotics on total cholesterol (T-chol) compared to controls in pooled analysis. The shaded diamonds indicate the effect of probiotics in a particular study (weighted difference in mean). The horizontal lines represent 95% confidence intervals (CIs). The big diamond data marker indicates the pooled effect. The figure shows the summary of studies overall and subdivided by length of intervention. “long”: 12 weeks or longer, “short”: 8 weeks or shorter.
We found a significant difference between these two sub-groups (p = 0.001). Sub-group analysis according to the number of bacterial strains (single or multiple, Fig. 4) did not change heterogeneity, either (multiple: I2: 91.5%, p < 0.001, single: I2: 81.6%, p < 0.001). The beneficial effect of multiple strains probiotics on total cholesterol was significant (− 11.70 mg/dL, 95% CI − 18.60, − 4.79, p = 0.001), however no difference was observed in single bacteria probiotic sub-group (p = 0.611) with significant difference between the two sub-groups (p < 0.001).
Forest plot for the effect of probiotics on total cholesterol (T-chol) compared to controls in pooled analysis. The shaded diamonds indicate the effect of probiotics in a particular study (weighted difference in mean). The horizontal lines represent 95% confidence intervals (CIs). The big diamond data marker indicates the pooled effect. The figure shows the summary of studies overall and subdivided by the number of bacterial species used. “multiple”: combination of bacteria, “single”: one bacterial species used.
If we excluded the six articles where probiotics were co-supplemented with either vitamin D or chromium or selenium, and the article where the placebo group also got yoghurt with some bacteria, the heterogeneity did not change, nor the direction of the association (Figure S1).
Twenty studies reported data about LDL levels. No significant difference in LDL levels was observed between probiotic and placebo users (− 3.77 mg/dL, 95% CI − 8.47, 0.93, p = 0.116) with a considerable heterogeneity (I2: 88.6%, p < 0.001). Sub-group analysis according to the length of treatment (Figure S2) did not decrease the heterogeneity (short: I2: 88.9%, p < 0.001, long: I2: 89.5%, p < 0.001). Pooled studies with 8 weeks treatment period or shorter (p = 0.167) and studies of 12 weeks or longer showed no change of total cholesterol level (p = 0.493). We found no significant difference between these two groups (p = 0.555). Sub-group analysis according to the number of bacteria used (single or multiple, Figure S3 did not change heterogeneity, either (multiple: I2: 90.6%, p < 0.001, single: I2: 86.0%, p < 0.001). We found no effect of multiple-strain probiotics on total cholesterol (p = 0.139), and no difference was observed in single bacterium containing probiotic sub-group (p = 0.985), while there was no difference between the two sub-groups (p = 0.119). If we excluded the six articles where probiotics were co-supplemented with either vitamin D or chromium or selenium, and the article where the placebo group also got yoghurt with some bacteria, heterogeneity did not change, either (Figure S4). However, based on our trial sequential analysis, a number of 3,442 observations would be needed to provide sufficient statistical power (vs. the 1,090 patients in the current analysis) (Figure S5).
The meta-analysis of twenty-one trials showed a significant reduction of triglyceride by 17.18 mg/dL (95% CI − 26.17, − 8.19, p < 0.001). Heterogeneity was not substantial (34%, p = 0.065), sub-group analysis was therefore not conducted.
The meta-analysis of twenty-two trials showed a significant increase of HDL by 1.62 mg/dL (95% CI 0.21, 3.04, p = 0.025). I2 test (57.4%, p < 0.001) may represent moderate heterogeneity due to the differences between the interventions.
Probiotics decreased CRP, HbA1c, fasting plasma glucose, fasting insulin, and blood pressure values
The meta-analysis of sixteen trials showed a significant decrease of CRP by 0.43 mg/dL (95% CI − 0.80, − 0.07, p = 0.019). I2 test (64.3%, p < 0.001) represented moderate heterogeneity.
Fourteen studies with reported the effect of probiotics on HbA1c levels. The decrease of HbA1c was slightly but significantly lower in the probiotic groups compared to placebo (− 0.33%, 95% CI − 0.53; − 0.13, p = 0.001). Heterogeneity was substantial (I2: 75.9%, p < 0.001).
Twenty-four studies reported data about fasting plasma glucose. Pooled data showed a significant effect of probiotics in reducing fasting plasma glucose levels with a mean difference of − 16.52 mg/dL, (95% CI − 23.28; − 9.76, p < 0.001) with a substantial heterogeneity (I2: 66.2%, p < 0.001). Sub-group analysis according to the length of investigation (Fig. 5) did not change the heterogeneity in the long-term treatment sub-group (I2: 80.6, p < 0.001), however it decreased significantly in the short period therapy sub-group (I2: 25.8%, p = 0.183).
Forest plot for the effect of probiotics on fasting plasma glucose (FPG) compared to controls in pooled analysis. The shaded diamonds indicate the effect of probiotics in a particular study (weighted difference in mean). The horizontal lines represent 95% confidence intervals (CIs). The big diamond data marker indicates the pooled effect. The figure shows the summary of studies overall and subdivided by length of intervention. “long”: 12 weeks or longer, “short”: 8 weeks or shorter.
Short studies with 8 weeks or shorter showed significant decrease of fasting plasma glucose level (− 15.35 mg/dL, 95% CI − 24.83, − 5.87, p = 0.002), and studies of 12 weeks or longer also showed a significant decrease (− 18.82 mg/dL, 95% CI − 28.58, − 9.06, p < 0.001). We found no significant difference between these two sub-groups (p = 0.723). Sub-group analysis according to the number of applied bacteria strains (single or multiple, Fig. 6) showed an increased heterogeneity in the single strain sub-group, and there was some minor decrease in the multiple strains sub-group (single: I2: 74.5%, p < 0.001; multiple: I2: 60.6%, p < 0.001).
Forest plot for the effect of probiotics on fasting plasma glucose (FPG) compared to controls in pooled analysis. The shaded diamonds indicate the effect of probiotics in a particular study (weighted difference in mean). The horizontal lines represent 95% confidence intervals (CIs). The big diamond data marker indicates the pooled effect. The figure shows the summary of studies overall and subdivided by the number of bacterial species used. “multiple”: combination of bacteria, “single”: one bacterial species used.
The beneficial effect on fasting glucose level was significant both in the multiple strains probiotics subgroup (− 19.84 mg/dL, 95% CI − 31.45, − 8.23, p = 0.001) and in the single bacteria probiotic sub-group (− 16.07 mg/dL, 95% CI − 25.88, − 6.26, p = 0.001) with no significant difference between the two sub-groups (p = 0.892). If we excluded the six articles where probiotics were co-supplemented with either vitamin D or chromium or selenium, and the article where the placebo group also got yoghurt with some bacteria, the heterogeneity did not change, either (Figure S6).
The meta-analysis of fifteen trials showed a significant reduction of fasting insulin levels by 1.40 µIU/mL (95% CI − 2.52, − 0.27, p = 0.015). Heterogeneity was not significant (46.8%, p = 0.024), sub-group analysis was therefore not conducted.
Fourteen studies reported data about systolic and diastolic blood pressures. The meta-analysis showed a significant decrease both in systolic blood pressure (− 1.79 mmHg, 95% CI − 3.09; − 0.49, p = 0.007, I2: 0.0%, p = 0.890) and in diastolic blood pressure (− 1.32 mmHg, 95% CI − 2.42; − 0.21, p = 0.019, I2: 0.0%, p = 0.838). Since heterogeneity was not significant, no sub-group analysis was performed.
Discussion
In the present meta-analysis, we aimed to evaluate the effects of probiotics on BMI and metabolic parameters in patients with type 2 diabetes mellitus. Data analysis showed a significant effect of probiotics in reduction of total cholesterol, triglyceride levels, CRP, HbA1c, fasting plasma glucose, fasting insulin levels and both systolic and diastolic blood pressure values. Supplementation with probiotics increased HDL levels however did not have a significant effect on BMI or LDL levels.
Such an evaluation is of high potential importance, as this patient group has especially high risk of cardiovascular diseases. It is crucial to reduce all the modifiable risk factors with efficient and multifactorial therapeutic methods and probiotic supplementation could be a complementary approach.
High total cholesterol levels, high blood pressure and type 2 diabetes mellitus are major risk factors of cardiovascular diseases. Reduction of the high total cholesterol and LDL levels in order to reduce the risk of major cardiovascular events is essential53. Every 1 mmol/L increment in total cholesterol levels increases the risk of cardiovascular diseases by 20% in women and by 24% in men54. Our results show that the consumption of probiotics has a decreasing effect on serum cholesterol levels. The mechanisms behind this reduction are that probiotics seem to be able to reduce serum cholesterol levels by reducing cholesterol absorption in the intestines55 and by the inhibition of HMG-CoA reductase enzyme thereby inhibiting endogenous cholesterol synthesis56.
The exact mechanism of action for the beneficial effects of probiotics on glycemia-related parameters is not fully elucidated. The favorable effects may be due to the immunoregulatory properties of probiotics. Cani et al. demonstrated, that metabolic endotoxemia dysregulates the inflammatory tone and triggers body weight gain and diabetes. Alterations in glucose homeostasis are associated with low-grade inflammation promoted by gut microbiota-derived lipopolysaccharide or endotoxin in mice57. Therefore, lowering plasma lipopolysaccharide concentration could be a strategy for the control of metabolic diseases, such as diabetes mellitus. Naito et al. showed that oral administration of Lactobacillus casei strain to obese mice led to a better insulin resistance through decreasing plasma levels of lipopolysaccharide-binding protein, a marker of endotoxemia58.
In our meta-analysis, probiotics significantly reduced total cholesterol, triglyceride levels, CRP levels, HbA1c levels, fasting plasma glucose levels, fasting insulin, and blood pressure together with the increase of the HDL levels. The observed small changes may not seem to be clinically significant, however the beneficial changes in many parameters can add up leading to a reduction in the severity of type 2 diabetes-related complications, and, as a consequence in lower mortality. The main strength of our study is that we included exclusively randomized clinical trials for evaluation and the number of the included trials were much higher than in other meta-analyses in this field. Some of our outcomes (triglyceride levels, systolic and diastolic blood pressure values) included a homogenous data set, so confounding factors are unlikely to distort our results. Waist to hip ratio was not measured in most of the articles, so that we could not pool the data.
We attempted to determine whether the observed heterogeneity in our outcomes was due to the differences in the length of treatment or in the number of probiotics used. However, according to our subgroup analyses high heterogeneity still remained unknown. We need more randomized clinical trials to be able to determine the most beneficial bacteria, the optimal dosage and treatment period. The identified significant heterogeneity is due to the significant differences between the intervention of the selected articles.
There are considerable limitations in our study. The diversified settings made it impossible to assess the effect of specific probiotic strains on the analyzed parameters. Many of the analyzed studies used probiotic mixtures or dairy products containing several probiotic strains. The data of diversity and richness of gut microbiota are absent in some of the included studies. The number of the probiotic species used in the included trials varied between the studies included in the analysis. The duration of probiotic intervention differed between the included trials. Consequently, substantial heterogeneity was observed between trials within this meta-analysis. No subgroup analysis was possible to assess which particular probiotic preparation could be the most effective to improve metabolic parameters in diabetic patients. Differences in population or differences in outcome were not considerable. The study aim was to test different cardiometabolic parameters in patients with diabetes mellitus type 2 in all included studies. However, differences in intervention were substantial, due to the fact, that different species or different probiotic combinations were used. This fact is worth to mention, because we are not able to have high quality evidence due to the very high indirectness.
In conclusion, according to our meta-analysis the administration of probiotics has a beneficial role in the management of type 2 diabetes regarding metabolic profile. We have shown a significant effect of probiotics in reducing total cholesterol, triglyceride levels, CRP, HbA1c, fasting plasma glucose, fasting insulin levels and both systolic and diastolic blood pressure values. Supplementation with probiotics increased HDL level and it did not had a significant effect on BMI or LDL levels. The practical implication of our study is that probiotic administration as a supportive intervention of type 2 diabetes could be incorporated into diabetes guidelines to beneficially modify cardiometabolic risk factors. Further studies are needed to investigate the combined effects of the different antidiabetic drugs and probiotic species.
References
Kannel, W. & McGee, D. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 2, 120–126 (1979).
Papatheodorou, K., Papanas, N., Banach, M., Papazoglou, D. & Edmonds, M. Complications of diabetes 2016. J. Diabetes Res. 2016, 6989453 (2016).
Ogurtsova, K. et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50 (2017).
Abarca-Gómez, L. et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017).
Turnbaugh, P. J. & Gordon, J. I. The core gut microbiome, energy balance and obesity. J. Physiol. 587, 4153–4158 (2009).
Clarke, G. et al. Minireview: gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 28, 1221–1238 (2014).
Chaudhury, A. et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front. Endocrinol. 8, 6 (2017).
Campbell, I. W. Antidiabetic drugs present and future. Drugs 60, 1017–1028 (2000).
Burcelin, R., Serino, M., Chabo, C., Blasco-Baque, V. & Amar, J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol. 48, 257–273 (2011).
Rad, A. et al. The future of diabetes management by healthy probiotic microorganisms. Curr. Diabetes Rev. 13, 582–589 (2017).
Sun, Z. et al. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 60, 1–14 (2019).
Ejtahed, H. et al. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 94, 3288–3294 (2011).
Larsen, N. et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5, e9085 (2010).
Million, M. et al. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 53, 100–108 (2012).
Zhang, Q., Wu, Y. & Fei,. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina 52, 28–34 (2016).
Kasińska, M. A. & Drzewoski, J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol. Arch. Med. Wewn. 125, 803–813 (2015).
He, J., Zhang, F. & Han, Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: a meta-analysis of RCTs. Medicine 96, e9166 (2017).
Hendijani, F. & Akbari, V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin. Nutr. 37, 532–541 (2018).
Yao, K. et al. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 23, 3044 (2017).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Int. Med. 151, 264–269 (2009).
Sterne, J. A. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Higgins, J. P. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions, 4 (Wiley, New York, 2011).
Abbasi, B., Mirlohi, M., Daniali, M. & Ghiasvand, R. Effects of probiotic soymilk on lipid panel in type 2 diabetic patients with nephropathy: A double-blind randomized clinical trial. Prog. Nutr 20, 70–78 (2018).
Asemi, Z., Zare, Z., Shakeri, H., Sabihi, S.-S. & Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 63, 1–9 (2013).
Bahmani, F. et al. The consumption of synbiotic bread containing lactobacillus sporogenes and inulin affects nitric oxide and malondialdehyde in patients with type 2 diabetes mellitus: randomized, double-blind, placebo-controlled trial. J. Am. Coll. Nutr. 35, 506–513 (2016).
Bayat, A. et al. Effect of cucurbita ficifolia and probiotic yogurt consumption on blood glucose, lipid profile, and inflammatory marker in type 2 diabetes. Int. J. Prev. Med. 7, 30 (2016).
Feizollahzadeh, S., Ghiasvand, R., Rezaei, A., Khanahmad, H. & Hariri, M. Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type ii diabetes mellitus. Probiotics Antimicrob. Proteins 9, 41–47 (2017).
Firouzi, S., Majid, H. A., Ismail, A., Kamaruddin, N. A. & Barakatun-Nisak, M.-Y. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur. J. Nutr. 56, 1535–1550 (2017).
Hariri, M. et al. The effect of probiotic soy milk and soy milk on anthropometric measures and blood pressure in patients with type II diabetes mellitus: a randomized double-blind clinical trial. ARYA Atheroscler. 11, 74 (2015).
Hove, K. et al. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind placebo-controlled study. Eur. J. Endocrinol. 172, 11–20 (2015).
Hsieh, M.-C. et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci. Rep. 8, 1–11 (2018).
Khalili, L. et al. The effects of lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran. Biomed. J. 23, 68 (2019).
Kobyliak, N. et al. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J. Gastrointest. Liver Dis. 27, 41–49 (2018).
Kobyliak, N., Falalyeyeva, T., Mykhalchyshyn, G., Kyriienko, D. & Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 12, 617–624 (2018).
Król, E., Krejpcio, Z., Byks, H., Bogdański, P. & Pupek-Musialik, D. Effects of chromium brewer’s yeast supplementation on body mass, blood carbohydrates, and lipids and minerals in type 2 diabetic patients. Biol. Trace Elem. Res. 143, 726–737 (2011).
Mafi, A. et al. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct. 9, 4763–4770 (2018).
Mazloom, Z., Yousefinejad, A. & Dabbaghmanesh, M. H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran. J. Med. Sci. 38, 38 (2013).
Mobini, R. et al. Metabolic effects of L actobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 19, 579–589 (2017).
Mohamadshahi, M. et al. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. BioImpacts BI 4, 83 (2014).
Moroti, C., Magri, L. F. S., de Rezende Costa, M., Cavallini, D. C. & Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Disease 11, 29 (2012).
Ostadrahimi, A. et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 44, 228 (2015).
Raygan, F., Ostadmohammadi, V. & Asemi, Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 38, 1594–1598 (2019).
Raygan, F., Ostadmohammadi, V., Bahmani, F. & Asemi, Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psych. 84, 50–55 (2018).
Raygan, F. et al. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 10, 51 (2018).
Sabico, S. et al. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 38, 1561–1569 (2019).
Sato, J. et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: a randomised controlled study. Sci. Rep. 7, 1–10 (2017).
Shakeri, H. et al. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 49, 695–701 (2014).
Sheth, M., Chand, V. & Thakuria, A. Inflated levels of SCFA, Bifidobacteria and Lactobacillus improves the status of pre hypertension and type 2 diabetes mellitus in subjects residing in north east India—a randomized control trial with synbiotic supplementation. Int. J. Curr. Pharm. Res. 7, 33–36 (2015).
Tajadadi-Ebrahimi, M. et al. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann. Nutr. Metab. 65, 34–41 (2014).
Tonucci, L. B., Dos Santos, K. M. O., de Oliveira, L. L., Ribeiro, S. M. R. & Martino, H. S. D. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin. Nutr. 36, 85–92 (2017).
Yanni, A. E. et al. Controlling type-2 diabetes by inclusion of Cr-enriched yeast bread in the daily dietary pattern: a randomized clinical trial. Eur. J. Nutr. 57, 259–267 (2018).
Sharma, S. et al. Beneficial effect of chromium supplementation on glucose, HbA1C and lipid variables in individuals with newly onset type-2 diabetes. J. Trace Elem. Med Biol. 25, 149–153 (2011).
Collaboration, A. P. C. S. Cholesterol, diabetes and major cardiovascular diseases in the Asia-Pacific region. Diabetologia 50, 2289–2297 (2007).
Peters, S. A., Singhateh, Y., Mackay, D., Huxley, R. R. & Woodward, M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: a systematic review and meta-analysis. Atherosclerosis 248, 123–131 (2016).
Lye, H.-S., Kuan, C.-Y., Ewe, J.-A., Fung, W.-Y. & Liong, M.-T. The improvement of hypertension by probiotics: effects on cholesterol, diabetes, renin, and phytoestrogens. Int. J. Mol. Sci. 10, 3755–3775 (2009).
Aggarwal, J., Swami, G. & Kumar, M. Probiotics and their effects on metabolic diseases: an update. J. Clin. Diagn. Res. JCDR 7, 173 (2013).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Naito, E. et al. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J. Appl. Microbiol. 110, 650–657 (2011).
Acknowledgements
This study was funded by “GINOP-2.3.2-15-2016-00048 - STAY ALIVE” co-financed by the European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020, and by the Human Resources Development Operational Programme Grant, Grant Number: EFOP 3.6.2‐16‐2017‐00006 – LIVE LONGER which is co-financed by the European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020, and the New National Excellence Program of the Hungarian Ministry of Human Capacities (UNKP-19-4-PTE-236).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to this study. T.K.: search, selection, data extraction, and writing the first draft of the manuscript; B.M.: search, selection and data extraction; D.N.: data analysis and data interpretation; A.S.: data analysis; P.H. and Z.S.: study design and methodology, interpretation of the data; A.B., A.G., and K.M.: reviewed all content and made significant contributions to the final draft; M.S.: was the supervisory author and reviewed all content and made significant contributions to the conception of the study and to the final draft. All authors have approved the submitted version and agree to assume responsibility for this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kocsis, T., Molnár, B., Németh, D. et al. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Sci Rep 10, 11787 (2020). https://doi.org/10.1038/s41598-020-68440-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68440-1
This article is cited by
-
Impact of Dietary Probiotics on the Immune and Reproductive Physiology of Pubertal Male Japanese Quail (Coturnix coturnix japonica) Administered at the Onset of Pre-Puberty
Probiotics and Antimicrobial Proteins (2024)
-
Effects of probiotic/synbiotic supplementation on body weight in patients with diabetes: a systematic review and meta-analyses of randomized-controlled trials
BMC Endocrine Disorders (2023)
-
Maternal microbiota and gestational diabetes: impact on infant health
Journal of Translational Medicine (2023)
-
Effects of gut microbial therapy on lipid profile in individuals with non-alcoholic fatty liver disease: an umbrella meta-analysis study
Systematic Reviews (2023)
-
Roles of the gut microbiome in weight management
Nature Reviews Microbiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.