Abstract
The asymmetric mandibles of termites are hypothetically more efficient, rapid, and powerful than the symmetric mandibles of snap-jaw ants or termites. We investigated the velocity, force, precision, and defensive performance of the asymmetric mandibular snaps of a termite species, Pericapritermes nitobei. Ultrahigh-speed recordings of termites revealed a new record in biological movement, with a peak linear velocity of 89.7–132.4 m/s within 8.68 μs after snapping, which caused an impact force of 105.8–156.2 mN. High-speed video recordings of ball-strike experiments on termites were analysed using the principle of energy conservation; the left mandibles precisely hit metal balls at the left-to-front side with a maximum linear velocity of 80.3 ± 15.9 m/s (44.0–107.7 m/s) and an impact force of 94.7 ± 18.8 mN (51.9–127.1 mN). In experimental fights between termites and ant predators, Pe. nitobei killed 90–100% of the generalist ants with a single snap and was less likely to harm specialist ponerine ants. Compared with other forms, the asymmetric snapping mandibles of Pe. nitobei required less elastic energy to achieve high velocity. Moreover, the ability of P. nitobei to strike its target at the front side is advantageous for defence in tunnels.
Similar content being viewed by others
Introduction
Some invertebrates’ elastic power-amplifying systems that incorporate latches and springs can overcome the physiological limits of muscle contraction and perform a powerful or rapid movement1,2. For example, mantis shrimps (Crustacea: Stomatopoda) perform high-speed strikes to prey by using elastic energy stored in their raptorial appendages3. Springtails (Hexapoda: Collembola) escape from enemies with quick and powerful jumps by using their springing organ4. Many ants and termites, such as the snap-jaw ant Mystrium camillae Emery (Hymenoptera: Formicidae)5 and the soldiers of termite Termes panamaensis (Snyder) (Blattodea: Termitidae)6, have mandibles that are morphologically specialised for powerful snapping attacks. The snapping speeds of the mandibular attacks of M. camillae (111.1 m/s)5 and T. panamaensis (67 m/s)6 are the most rapid animal movements currently reported1,5,6, followed by the mandible-closing movement of Odontomachus bauri Emery trap-jaw ants (64.3 m/s)7, the diving of gyrfalcons (58 m/s)8, the nematocyst discharge of jellyfish (37 m/s)9, and strike behaviour of mantis shrimps (31 m/s)1.
The snapping mandibles of termite soldiers have two forms: symmetric and asymmetric10,11. T. panamaensis has two narrow and elongated symmetric snapping mandibles that press together until they slide against each other (Fig. 1a). These mandibles strike enemies along their lateral sides6. By contrast, the asymmetric snapping mandibles of some termites are relatively short and wide, with a twisted left mandible and curved right mandible10. The right mandible presses against the left until the two surfaces slide. Moreover, the left mandible snaps in a clockwise motion (Fig. 1b), presumably striking enemies along the front side. Additionally, asymmetric snapping mandibles may perform more violent strikes than symmetric mandibles can by storing elastic energy in the left twisted mandible12. However, no conclusive evidence has supported these hypotheses.
Snapping mandibles of termite soldiers. (a) In the symmetric snapping of Termes panamaensi6,10, elastic energy is stored in both mandibles, as indicated by the deformation and motion of the left (L) and right (R) mandibles. (b) In the asymmetric snapping of Pericapritermes spp.10, elastic energy is stored in only the left mandible when its anterior part rotates about the joint or pivot point O. The posterior part remains stationary during snapping. (c) Morphology of the twisted left mandible of the Pericapritermes nitobei termite used in this study. The length of the anterior part of the left mandible (LA) and the mass of its subsections A1 and A2 (i.e., MA1, MA2) are required to estimate its moment of inertia (IA). The subsections A1 and A2 were prepared by performing two cuts; one cut was made at the pivot point (C1) and the other was made at the centre of the anterior part (C2).
Ants are major natural predators of termites. Predator ants include specialist predators such as Pachycondyla spp. (Formicidae: Ponerinae)13 and generalist predators such as Anoplolepis spp. and Pheidole spp.14,15. The powerful mandibular snapping of termite soldiers is hypothesised to be a specialised defence mechanism against ants16. Termites can snap the ground to leap away from ants17,18 or snap at ants to push them away16 or even kill them6. However, these defensive behaviours have not been studied in termites with asymmetric mandibles.
This study investigated the velocity, force, precision, and defensive behaviours of asymmetric mandibular snaps performed by Pericapritermes nitobei (Shiraki) soldiers. Specifically, we conducted ultrahigh- and high-speed video recordings, ball-strike experiments, and ant-defence assessments to test the following hypotheses: (1) snaps of asymmetric mandibles are more rapid and powerful than snaps of symmetric mandibles are, (2) asymmetric mandibles can precisely strike at the front side, and (3) asymmetric mandibles are effective for defending against ants. In addition to revealing the fastest known animal movement, we compared asymmetric and symmetric termite mandibles and analysed the evolution and mechanical effects of mandible morphology.
Methods
Termites and ants
A total of 100 Pe. nitobei termite soldiers were collected by excavating the soil beneath stones and logs in three locations in Taiwan, namely Xiaping Tropical Botanical Garden (23.77°N, 120.67°E), Huisun Forest Station (24.09°N, 121.03°E), and Dakeng (24.19°N, 120.79°E) (Supplementary Information, Table S1). Pe. nitobei is a soil-feeding termite that does not have a centralised nest; they distribute their eggs and larvae in subterranean tunnels beneath stones19. They generally forage on organic matter adjacent to plant roots and rarely appear on the ground surface19. Because Pe. nitobei colonies are difficult to maintain in laboratory conditions, all experiments were conducted within 1 day after collection. The sample sizes and collection sites of subjects used in all the experiments are summarised in Table S1 of the Supplementary Information.
Natural predators of Pe. nitobei were identified on the basis of references and field observations. Pheidole and Anoplolepis spp. are generalist termite predators14,15, and Anochetus and Pachycondyla spp. are specialist predators of termites that perform specialised striking behaviours on termite soldiers or recognise termite odours and raid termite nests20,21. In this study, Ph. megacephala (Formicidae: Myrmecinae) and Anop. gracilipes (Formicidae: Formicinae) were assumed to be generalist predators of Pe. nitobei. Both species have been observed to forage on the ground and prey on termites in Xiaping Tropical Botanical Garden22,23. Two ponerine ant species (Formicidae: Ponerinae), namely Anochetus taiwaniensis and Pa. javanus, were assumed to be specialist predators of Pe. nitobei. Anoc. taiwaniensis was observed foraging for termites in rotting logs at Huisun Forest Station. Pa. javanus was observed foraging in the soil surrounding the subterranean galleries of Pe. nitobei in Xiaping Tropical Botanical Garden, Huisun Forest Station, and Dakeng. The generalist and specialist ants used in the assessments were collected from National Chung Hsing University and Huisun Forest Station, respectively (Supplementary Information, Table S1).
Estimating velocity and force by using an ultrahigh-speed camera
In each experiment, a termite was placed in the centre of a plastic petri dish (diameter: 90 mm) with a filter paper (Advantec No. 1, Toyo Inc.; diameter: 90 mm). Snapping behaviour was then recorded at 460,830 frames per second (fps) by using an ultrahigh-speed video camera (Model: v2512, Phantom, New Jersey, USA) with a resolution of 128 × 128 pixels2. The linear velocity of the mandible tip (VMT) was calculated as follows:
where LA and ωA are the length and angular velocity of the anterior rotating part about the pivot point O (Fig. 1c), respectively. The parameter ωA was calculated from the video footage by using Phantom CV 2.8 (Phantom, New Jersey, USA) for image analysis.
The snapping force (FA) was calculated using the following equation:6,7
where MA and αA are the mass and angular acceleration of the anterior rotating part of the left mandible, respectively. The average αA can be calculated as follows:
where tA is the time required to reach the maximum ωA. The snapping energy (EA) (i.e., the kinetic energy due to rotation) was calculated as follows:
where IA is the moment of inertia of the anterior rotating part about the pivot point O (Fig. 1c). Because the termite’s left mandible cannot be modelled using a common geometrical form, we adjusted the moment of inertia (IA) by using a constant K as follows:
The parameter K was calculated using the location of the anterior left mandible’s centre of mass (LCOM), length of the anterior mandible part (LA), and mass of mandible parts A1 (MA1) and A2 (MA2) (Fig. 1c; Table S3) as follows:
and LCOM was calculated as follows:
The termite’s left mandible is morphologically inhomogeneous. The left mandible is wide in the posterior part and at the pivot point and narrow at the centre of the anterior part (Fig. 1c). To obtain parts A1 and A2, we used a scalpel blade to cut the left mandible first at its pivot point (C1) and then in the middle of the anterior part (C2) (Fig. 1c). To avoid measurement errors, MA1 and MA2 were calculated as the means of 12 A1 and 14 A2 parts, respectively. The total mass of the anterior left mandible (MA) was the sum of MA1 and MA2.
A total of 17 soldiers were used to measure the mass and length of the left mandible (Table S1). Images were captured using a Leica M205 C stereomicroscope with a Leica MC170 HD digital camera (Leica Microsystems, Wetzlar, Germany), and all lengths were calculated using the LAS Image Analysis software module (Leica Application Suite V4.4.0, Leica Microsystems, Wetzlar, Germany). The mass of the termites was measured using an analytical balance (AG245, Mettler Toledo, Greifensee, Switzerland).
Termite behaviours were considered abnormal in ultrahigh-speed recordings under the following conditions: (1) three of five termites died in the petri dish before snapping, (2) the other two termites snapped without being triggered and did not extend their antennae forward to detect targets in front of them, and (3) the termites died immediately after snapping once. We assumed that the bright light required for ultra-speed recording (brightness of approximately 85,000 lux) caused these abnormal behaviours. Therefore, we used a high-speed video camera with less intense light (approximately 8,670 lux) to observe the snapping behaviour and reassess the snapping velocity and force. To minimise the effects of light on ant and termite behaviour, we recorded fights between termites and ants by using a smartphone camera with a light intensity of approximately 850 lux.
Estimating mandible strike accuracy, velocity, and force by using a high-speed camera
Each termite was placed in a plastic petri dish with a filter paper, as in the ultrahigh-speed recordings. Mandible-snapping behaviour was triggered by touching a termite’s antennae from the front by using tweezers. Snapping was recorded at 1,000 fps with a high-speed video camera (MotionPro X3, Integrated Design Tools, Inc., Tallahassee, FL, USA). Behavioural phases were identified, and the duration of each phase was measured using the recorded images.
To measure the striking angle and reassess the velocity and force of mandible snapping, we conducted ball-strike experiments on 15 soldiers. In each experiment, a metal ball (2.25 mg, 0.7 mm in diameter) with a mass similar to that of the termite was obtained from the tip of a ballpoint pen (TOWO OP-100, 0.7 mm) and placed in front of the soldier’s head. The soldiers snapped at the ball 1–2 seconds after it was placed. When the left mandible hit the metal ball, the termite soldier and the ball moved away from each other; movements were recorded by the aforementioned high-speed video camera at 1,000 fps. The diameter of the petri dish was used as a scale for distance measurement. The experiment was repeated until the termite was exhausted and unresponsive to stimuli. For each termite, 3–14 snaps were recorded.
Assuming that no energy loss caused by air resistance or ball rotation occurred, according to the energy conservation principle, the kinetic energy of the rotating anterior part of the left mandible (EA) on striking the ball equals the sum of the linear and angular kinetic energy of the ball and termite as follows:
where MB and MT are the masses of the metal ball (2.25 mg) and termite, respectively; VB and VT are the linear velocities of the ball and termite, respectively; ωT is the angular velocity of the termite; and IT is the moment of inertia of the termite. The parameters VB, VT, and ωT were measured according to the motions of the termite and ball by using video analysis software (Tracker 4.91, http://physlets.org/tracker/). The parameters VT and VB were calculated from the linear displacement of the termite and ball between the first two frames after the termite struck the ball. The variable ωT was calculated using the termite’s number of rotations over a specific time period. By assuming the termite to resemble a cylinder rotating about its centre of mass, its IT was calculated from its mass (MT) and body length (LT) as follows:24
Subsequently, the snapping force (FA) could be calculated using Eqs. (2) and (3). The linear velocity of the tip of the snapping left mandible on striking the ball (VMT) could be calculated using Eqs. (1), (4), and (5) as follows:
where K is a constant for adjusting the moment of inertia (IA) and MA is the total mass of the anterior left mandible. The parameter K was calculated using Eqs. 6 and 7, and EA was calculated using Eq. 8.
We evaluated the precision of mandibular snaps by variations in the ball’s movement directions after a snap. Precise snaps occurred in a particular hitting zone with less variation in movement directions. The ball’s movement directions were calculated as the angle of deviation from the termite’s body axis. To determine whether the snaps had a particular hitting zone, we performed a Shapiro–Wilk test for confirming the normality of angle measurements.
Behavioural phases of Pericapritermes nitobei mandibular snaps. (a) Stages 1–3: High-speed recordings from 10 soldiers at 1,000 frames per second (fps) indicated that the right mandible pressed against the left mandible for 261 ± 43 ms before the mandibles slid across each other to snap, and termites raised their antennae 47.5 ± 22.5 ms before the snap (Stages 2–3). Stage 3–4: Single snap of two soldiers (b and c) recorded by an ultrahigh-speed video camera at 460,830 fps. Mandibular snaps were performed over 21.7–43.4 μs. The peak linear velocity (VMT) of two soldiers at 8.68 μs were 132.4 and 89.7 m/s. Images in (b,c) were obtained by transforming Supplementary Movies S1 and S2, respectively, to frames using codes written in Python language (v. 3.8) with the package opencv-python.
Snap performance of the Pericapritermes nitobei soldier’s asymmetric mandible in ball-strike experiments (92 events from 15 termites, A–O). (a) Motion of the termites and metal ball. When the left mandible hit the metal ball, the termite soldier and ball moved away from each other. Yellow and blue arrows indicate the position of the termite and metal ball, respectively. (b) Linear velocity VMT. (c) Snapping force FA. The maximum recorded VMT and FA values for each soldier are displayed above each bar. (d) Movement directions of the metal ball. Each blue line indicates a record of one moving metal ball. Angle normality was assessed using the Shapiro–Wilk test. Images in (a) were obtained by transforming Supplementary Movie S3 to frames using codes written in Python language (v. 3.8) with the package opencv-python.
Assessment of termites defending against ants
To observe snapping behaviour against predators, termite soldiers were forced to fight with workers of Anop. gracilipes, Anoc. taiwaniensis, and Pa. javanus and minor workers of Ph. megacephala. The collection locations of ants and the number of termites used in each trial are presented in Table S1. Fights occurred in a petri dish (diameter: 55 mm) layered with a filter paper (Advantec No. 2, Toyo Inc.; diameter: 55 mm) containing 1 mL of water. In each trial, four termites and four ants of the same species were placed on the filter paper, and their fights were recorded at 30 fps for 15 minutes on a smartphone camera (ZenFone 3 ZE520KL, ASUSTeK Computer Inc.). We reconfirmed whether the ants were predators of Pe. nitobei according to the number of encounters with termites and the number of attacks performed by each ant species. To evaluate the termites’ defensive performance, the numbers of mandibular snaps and hits as well as ants killed were recorded. Six trials were performed for each ant species, and the data from all trials were combined to calculate the ants’ attack rates and termites’ hitting and killing probabilities. The attack rate of ants was the ratio of the number of attacks performed to the number of encounters with termites. For termites, the hitting probability was the ratio of the number of hits to the number of snaps and the killing probability was the ratio of the number of ants killed to the number of hits. Pairwise comparisons for hitting and killing probabilities were conducted using Fisher’s exact tests with Bonferroni corrections. All the statistical data were obtained using R software (v. 3.3.1, R Development Core Team, 2013)25.
Results
Mandible-snapping sequences
Figure 2a displays a behaviour schematic. In the high- and ultrahigh-speed recordings, the mandible-snapping behaviour of Pe. nitobei soldiers began with the right mandible pressed against the left mandible to store elastic energy (loading phase). The termites required 261.3 ± 43.1 ms (n = 10) to complete the loading phase: During the first 213.8 ± 43.5 ms, both antennae extended forward as the right mandible continued to press against the left mandible; it took another 47.5 ± 22.5 ms to turn their antennae towards their rear before snap. Mandibular sliding was completed in 21.7–43.4 μs (Fig. 2b,c).
Velocity and force of mandible snaps
In the ultrahigh-speed recordings of mandible snapping, the peak linear velocity occurred at 8.68 μs (tA) after execution and had values of 132.4 (Fig. 2b; Supplementary Movie S1) and 89.7 m/s (Fig. 2c; Supplementary Movie S2) for two termites. The peak snapping energy of these termites was 41.4 and 19.0 μJ respectively, and their peak snapping forces were 156.2 and 105.8 mN, respectively.
In the ball-strike experiments, the energy from the termite’s mandible snap caused the termite and metal ball to move away from each other and sometimes rotate (Fig. 3a, Supplementary Movie S3). On the basis of size and velocity measurements of 15 termite soldiers in 92 events (Tables S2, S3, Supplementary Information), the snapping energy (EA) had a mean of 9.5 ± 3.2 μJ and maximum of 15.8 ± 5.9 μJ (range: 4.6–27.4 μJ). The mean and maximum linear velocities of the mandible tip on striking the ball (VMT) were 60.4 ± 12.0 and 80.3 ± 15.9 m/s (range: 44.0–107.7 m/s), respectively (Fig. 3b). Assuming that the left mandible struck the ball at its maximum linear velocity (tA) 8.68 μs after snapping (Fig. 2b,c), the mean and maximum snapping forces (FA) were 71.3 ± 14.1 and 94.7 ± 18.8 mN (range: 51.9–127.1 mN), respectively (Fig. 3c). The body measurement and snap performance data are summarised in Table 1.
Striking angle of mandible snaps
In the ball-strike experiments, the movement directions of the ball after being snapped by the Pe. nitobei mandible ranged from 2.3° to 122.1° and were normally distributed (Fig. 3d) (Shapiro–Wilk test: W = 0.99, p = 0.70) with a mean of 52.9° ± 22.7°. This result suggests that termites with asymmetric mandibles strike most enemies at the front-left side.
Ant-defence performance
The generalist ants, namely Ph. megacephala and Anop. gracilipes, encountered termites 93 and 107 times and attacked termites 8 (8.6%) and 18 (17.1%) times in the defence experiments, respectively. The specialist ants, namely Anoc. taiwaniensis and Pa. javanus, encountered termites 300 and 143 times and attacked termites 102 (34.0%) and 33 (23.1%) times, respectively. The results indicated that Ph. megacephala and Anop. gracilipes are facultative or generalist predators of termites. Moreover, compared with Ph. megacephala and Anop. gracilipes, Anoc. taiwaniensis and Pa. javanus exhibited higher levels of aggression, which is consistent with them being specialist predators of termites.
The termite soldiers had a significantly high probability of hitting Ph. megacephala (83%), significantly low probabilities of hitting Anop. gracilipes (13%) and Anoc. taiwaniensis (30%), and a moderate probability (41%) of hitting Pa. javanus (Fig. 4a) with a mandible snap. The mandibular snaps of termites killed most generalist ants in a single hit (mortality: 90–100%, Fig. 4b). By contrast, none of the ponerine ants were killed by a mandibular snap (Fig. 4b). Only 2 of 24 Anoc. taiwaniensis (8.3%) individuals were had immobilised left mandibles after being snapped by a termite.
Defensive performance of mandibular snaps by probability (values above each bar) of (a) hitting and (b) killing four predator ant species. Bars with identical letters were not significantly different at p < 0.05 (Fisher’s exact tests with Bonferroni corrections). Termites could not kill their specialist predators Anochetus taiwaniensis and Pachycondyla javanus.
Discussion
The most rapid animal movement
The most rapid recorded animal movement has been updated several times. The cheetah was considered to be the fastest animal before 199826. The newest record was set by the snap-jaw ant M. camillae, whose mandible can achieve a maximum velocity of 93 ± 14.5 m/s5. Our results revealed that the snapping movement speed of Pe. nitobei soldiers’ asymmetric mandibles was similar to or even more rapid than that of snap-jaw ants’ symmetric mandible. Ultrahigh-speed recordings of snap-jaw ants indicated that 3 of 10 individuals had snapping velocities greater than 100.0 m/s, and the maximum recorded velocity was 111.1 m/s5. In our study of Pe. nitobei, the maximum snapping velocity calculated in the ball-strike experiment was 107.7 m/s, which was a conservative estimate because we disregarded the rotational energy of the metal ball and assumed no energy loss due to friction. The peak snapping velocity of Pe. nitobei calculated using ultrahigh-speed recordings reached 132.4 m/s, which was twice the velocity of the symmetric mandibles of T. panamaensis (67 m/s)6 and sets a new record for the most rapid biological movement.
Powerful and efficient snapping mechanism of the asymmetric mandible
The ‘snap-jaw’ mechanism, which combines a spring and tool in one appendage, has been proposed to be more efficient than most other power-amplified appendages with distinct structures2,5. The peak impact force generated by a Pe. nitobei soldier may be approximately 4,600 times their body weight (BW), which is within the range estimated for other snap-jaw systems (3,000–10,000 BW). The aforementioned force is one order of magnitude higher than that reported for trap-jaw ants (approximately 500 BW)7 (Table 1).
Compared with symmetric mandibles, asymmetric mandibles require less energy to achieve super-rapid movement (Table 1). The average snapping velocity of Pe. nitobei soldiers calculated in the ball-strike experiment was similar to that reported for T. panamaensis6 (60.4 m/s vs. 56 m/s); however, the required elastic energy (EA) stored in the Pe. nitobei mandible was only two-thirds that stored in the T. panamaensis mandible (9.5 μJ vs. 15.0 μJ)6 even though Pe. nitobei has a body size twice that of T. panamaensis. At the peak snapping velocity of 132.4 m/s, Pe. nitobei soldiers must store 41.4 μJ of elastic energy, which is less than half of the 109 μJ of elastic energy required by major workers of M. camillae with similar body size and lower snapping velocity (111.1 m/s). Table 1 indicates that the lower energy requirement of Pe. nitobei asymmetric mandibles for achieving super-rapid movement can be attributed to their shorter rotating section (LA) and consequently smaller moment of inertia (IA) than those of symmetric mandibles.
Elastic energy is stored in the deformed shafts of both the symmetric snapping mandibles of T. panamaensis and the snap-jaw ant M. camillae (Fig. 1a)5,6, whereas energy storage in the asymmetric mandibles of Pe. nitobei is confined to the bendable ‘joint’ (or ‘pivot’ in Fig. 1c) at the curved part of the left mandible. The left mandible of a Pe. nitobei soldier is complex in shape, and this mandible is generally laterally compressed but wide along the dorsal–ventral axis (Fig. 1b,c). The location of minimum lateral thickness creates a ‘joint’ in the continuous and single-segmented mandible. The mandible base and its posterior part are more robust than its anterior counterpart is. Assuming homogeneous material composition, the anterior part of the mandible can rotate about the ‘joint’ by lateral pressure from the right mandible. The left mandible twists towards its tip to become laterally wide. Consequently, the left mandible tip becomes more difficult to bend and can effectively transfer impact energy for defence.
We suggest that because of its morphological modifications, the twisted left mandible of a Pe. nitobei soldier has a functional joint for storing elastic energy, which creates a shorter rotating section that requires less stored elastic energy to achieve greater velocity. In other words, asymmetric snapping mandibles are more efficient than symmetric snapping mandibles are.
Antenna extension as an aiming behaviour
Because the direction of movement is unlikely to be corrected during high-speed ballistic movement, an aiming or triggering mechanism before initiating movement is crucial for precision27,28. For example, the archer fish Toxotes jaculatrix can identify the three-dimensional location of their insect target and precisely aim at and shoot it28. The snapping of a Mystrium snap-jaw ant’s mandible is triggered by sensory hairs on its labrum to ensure precise timing for catching rapidly moving prey27. The Odontomachus trap-jaw ant touches its prey using its elongated antennae before snapping, which may be a type of aiming behaviour29.
We observed that Pe. nitobei had a specific striking angle of 52.9° ± 22.7°, and it could hit active ants with a moderate probability of 8.6–34.0%. These results implied that Pe. nitobei aims before snapping to adjust its striking angle and distance from the target. We suggest that the forward extension of antennae observed in termite soldiers is an aiming behaviour and that the backward movement of the antennae beginning 45.9 ± 10.1 ms before snapping is a synergic behaviour (Fig. 2a). Because the snapping termite soldiers of Pe. nitobei lack eyes30, they likely locate enemies by touching with their antennae instead of by vision, as observed in Odontomachus trap-jaw ants29 and T. panamaensis6. This hypothesis was supported by the observation that the snapping behaviour of Pe. nitobei was triggered by touching their antennae tips with tweezers. However, forward-extended antennae may be at risk of being cut by the rapidly moving left mandible. Therefore, moving the antennae backward immediately before snapping can reduce this risk.
Functional adaptation of asymmetric mandible snapping
The defensive strategies of termite soldiers are highly diversified and adapted to their habitats10,31. For example, Cryptotermes termite soldiers that live in single pieces of wood or dead tree branches use their heavy phragmotic heads to block enemies12. Nasutitermes termite soldiers, which construct arboreal nests and forage in trees, eject viscous secretions to defend the foraging party from ant attacks32,33. Biting or slashing mandibles are most commonly observed among primitive wood-feeding termites, such as most kalotermitids and rhinotermitids34,35.
Snapping mandibles have evolved in four independent clades of Termitidae and in one clade of Kalotermitidae10,34,36,37. These mandibles have been hypothesised to be a defensive weapon, especially in tunnels6,12, where both termites and their enemies are confined to narrow spaces (e.g., approximately 2-mm wide, as reported for Pe. nitobei and Sinocapritermes mushae19,38). Narrow tunnels limit termite movement as well as the gape of biting or slashing mandibles, thereby reducing their defensive performance. This study demonstrated that termites with asymmetric snapping mandibles strike at the front-left side without moving forward or opening their mandible wide. We suggest that asymmetric snapping mandibles are more advantageous than symmetric mandibles are when termites fight with intruding ants in tunnels.
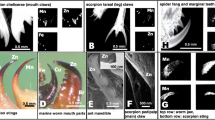
The hypothesised function of termite mandibular snaps is to kill or push out ants6,16. We observed that the defensive function of Pe. nitobei mandibular snaps varied with the encountered ant species. Mandibular snaps could kill generalist predators but barely harmed specialist predators, particularly Pa. javanus, which is observed in most subterranean gallery systems of Pe. nitobei in the field (4/5 localities, unpublished data) and is likely the main predator of Pe. nitobei. Pa. javanus may be able to resist mandibular snaps for the following reasons: (1) Pa. javanus is larger than other ants (head width of approximately 2.5 mm for Pa. javanus vs. approximately 0.6–1.5 mm for other species); (2) the exoskeleton of Pa. javanus is thicker than that of most generalist and ponerine ants;39 and (3) Pa. javanus initiated long-distance attacking behaviour approximately 6.8 mm away from the mandible tip of Pe. nitobei (Fig. 5d), which was considerably longer than the attacking distances observed in Ph. megacephala (approximately 0.7 mm, Fig. 5a), Anop. gracilipes (approximately 0.6 mm, Fig. 5b), and Anoc. taiwaniensis (approximately 1.4 mm, Fig. 5c). Moreover, we observed that Pa. javanus could clamp the termite’s mandibles (Fig. 5d) to prevent counterattacks. Thus, we hypothesised that the aforementioned characteristics and behaviours of Pa. javanus were selected to withstand the stress of termites’ defensive behaviours, particularly powerful mandibular snaps. We also propose that the snapping mandible of Pe. nitobei can function as weapon to defend against their generalist but not specialist ant predators.
Attack behaviours of (a) Pheidole megacephala, (b) Anoplolepis gracilipes, (c) Anoc. taiwaniensis, and (d) Pa. javanus on Pe. nitobei. The attacks of Ph. megacephala, Anop. gracilipes, and Anoc. taiwaniensis were initiated after their antennae touched the mandibles or head of Pe. nitobei (0 ms), with a short distance between the heads of these three species and the mandible tip of Pe. nitobei (approximately 0.6–1.5 mm). By contrast, Pa. javanus initiated an attack when its head was approximately 6.8 mm away from the mandible tip of Pe. nitobei (0 ms). This attack was followed by rapid movement toward the termite’s head (33.3 ms) and clamping at its mandibles (66.6 ms).
In conclusion, we provided evidence that the asymmetric snapping mandibles of Pe. nitobei are a rapid, powerful, and precise ballistic weapon. Asymmetric snapping behaviour was particularly advantageous in defending against intruding ants by striking them at their front side in subterranean tunnels.
Data availability
Ultrahigh-speed video recordings are available as Supplementary Movies S1 and S2. Termite measurements are provided in the Supplementary Information. Video recordings and datasets of the ball-strike experiments and fights between termites and ants are available on request from the corresponding authors.
References
Patek, S. N. The most powerful movements in biology. Am. Sci. 103, 330 (2015).
Patek, S. N., Dudek, D. M. & Rosario, M. V. From bouncy legs to poisoned arrows: Elastic movements in invertebrates. J. Exp. Biol. 214, 1973–1980 (2011).
Patek, S. N., Korff, W. L. & Caldwell, R. L. Deadly strike mechanism of a mantis shrimp. Nature 428, 819–820 (2004).
Brackenbury, J. & Hunt, H. Jumping in springtails: mechanism and dynamics. J. Zool., Lond. 229, 217–236 (1993).
Larabee, F. J., Smith, A. A. & Suarez, A. V. Snap-jaw morphology is specialized for high-speed power amplification in the Dracula ant, Mystrium camillae. R. Soc. Open Sci. 5, 181447 (2018).
Seid, M. A., Scheffrahn, R. H. & Niven, J. E. The rapid mandible strike of a termite soldier. Curr. Biol. 18, R1049–R1050 (2008).
Patek, S. N., Baio, J. E., Fisher, B. L. & Suarez, A. V. Multifunctionality and mechanical origins: Ballistic jaw propulsion in trap-jaw ants. Proc. Natl. Acad. Sci. USA 103, 12787–12792, https://doi.org/10.1073/pnas.0604290103 (2006).
Tucker, V. A., Cade, T. J. & Tucker, A. E. Diving speeds and angles of a gyrfalcon (Falco rusticolus). J. Exp. Biol. 201, 2061–2070 (1998).
Nüchter, T., Benoit, M., Engel, U., Özbek, S. & W, H. T. Nanosecond-scale kinetics of nematocyst discharge. Curr. Biol. 16, R316–318 (2006).
Scholtz, O. I., Macleod, N. & Eggleton, P. Termite soldier defence strategies: a reassessment of Prestwich’s classification and an examination of the evolution of defence morphology using extended eigenshape analyses of head morphology. Zool. J. Linnean Soc. 153, 631–650 (2008).
Weesner, F. M. External anatomy. In Krishna, K. & Weesner, F. M. (eds.), Biology of Termites Vol. I (Academic Press, 1969).
Deligne, J., Quennedey, A. & Blum, M. S. The enemies and defense mechanisms of termites. In Herman, H. R. (eds.), Social Insects Vol. II (Academic Press, 1981).
Hölldobler, B. & Wilson, E. O. The Ants. (Springer-Verlag, 1990).
Dejean, A., Kenne, M. & Moreau, C. S. Predatory abilities favour the success of the invasive ant Pheidole megacephala in an introduced area. J. Appl. Entomol. 131, 625–629 (2007).
Fotso Kuate, A., Tindo, M., Hanna, R., Kenne, M. & Goergen, G. Foraging activity and diet of the ant, Anoplolepis tenella Santschi (Hymenoptera: Formicidae), in southern Cameroon. African Entomol. 16, 107–114 (2008).
Krishna, K. & Araujo, R. L. A revision of the neotropical termite genus Neocapritermes (Isoptera, Termitidae, Termitinae). Bull. Am. Mus. Nat. Hist. 3, 83–130 (1968).
Silvestri, F. Note preliminari sui Termitidi e Termitifili sudamericani. Boll. Mus. Turino 17, 24–24 (1902).
Silvestri, F. Contribuzione alla conoscenza dei termiti e termitofili dell’ America Meridionale. Portici. Redia 7, 1–234 (1903).
Chiu, C.-I., Yang, M.-M. & Li, H.-F. Structure and function of subterranean gallery systems of soil-feeding termites Pericapritermes nitobei and Sinocapritermes mushae. Ins. Soc. 62, 393–400 (2015).
Leal, I. R. & Oliveira, P. S. Behavioral ecology of the neotropical termite-hunting ant Pachycondyla (= Termitopone) marginata: colony founding, group-raiding and migratory patterns. Behav. Ecol. Sociobiol. 37, 373–383 (1995).
Schatz, B., Orivel, J., Lachaud, J. P., Beugnon, G. & Dejean, A. Sitemate recognition: the case of Anochetus traegordhi (Hymenoptera; Formicidae) preying on Nasutitermes (Isoptera: Termitidae). Sociobiology 34, 569–580 (1999).
Chiu, C.-I. et al. Foraging phenology of the fungus-growing termite Odontotermes formosanus (Blattodea: Termitidae). Environ. Entomol. 47, 1509–1516 (2018).
Chiu, C.-I., Yeh, H.-T., Tsai, M.-J. & Li, H.-F. Naturalization and control of Coptotermes gestroi (Blattodea: Rhinotermitidae) in a Taiwanese forest. J. Econ. Entomol. 109, 1317–1325 (2016).
Hewitt, P. G. Conceptual Physics. (Pearson Education Limited, 2014).
R: A language and environment for statistical computing v. 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria, 2013).
Sharp, N. Timed running speed of a cheetah (Acinonyx jubatus). J. Zool. 241, 493–494 (1997).
Gronenberg, W. The trap-jaw mechanism in the dacetine ants Daceton armigerum and Strumigenys sp. J. Exp. Biol. 199, 2021–2033 (1996).
Schuster, S., Wohi, S., Griebsch, M. & Klostermeier, I. Animal cognition: How archer fish learn to down rapidly moving targets. Curr. Biol. 16, 378–383 (2006).
Ehmer, B. & Gronenberg, W. Antennal muscles and fast antennal movements in ants. J. Comp. Physiol. B 167, 287–296 (1997).
Krishna, K., Grimaldi, D. A., Krishna, V. & Engel, M. S. Treatise on the Isoptera of the world. Bull. Am. Mus. Nat. Hist. 377, 1–2704, https://doi.org/10.1206/377.4 (2013).
Prestwich, G. D. Defense-mechanisms of termites. Annu. Rev. Entomol. 29, 201–232 (1984).
Eisner, T., Kriston, I. & Aneshansley, D. J. Defensive behavior of a termite (Nasutitermes exitiosus). Behav. Ecol. Sociobiol. 1, 83–125 (1976).
Prestwich, G. D. Chemical defense by termite soldiers. J. Chem. Ecol. 5, 459–480 (1979).
Krishna, K. A generic revision and phylogenetic study of the family Kalotermitidae (Isoptera). Bull. Am. Mus. Nat. Hist. 122, 303–408 (1961).
Li, H.-F., Lan, Y.-C. & Su, N.-Y. Redescription of Prorhinotermes japonicus (Isoptera: Rhinotermitidae) from Taiwan. Ann. Entomol. Soc. Am. 104, 878–885 (2011).
Inward, D. J., Vogler, A. P. & Eggleton, P. A comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol. Phylogenet. Evol. 44, 953–967 (2007).
Scheffrahn, R. H., Bourguignon, T., Akama, D. P., Sillam-Dusses, D. & Sobotnik, J. Roisinitermes ebogoensis gen. & sp. n., an outstanding drywood termite with snapping soldiers from Cameroon (Isoptera, Kalotermitidae). ZooKeys 787, 91–105 (2018).
Chiu, C.-I., Yang, M.-M. & Li, H.-F. Redescription of the soil-feeding termite Sinocapritermes mushae (Isoptera: Termitidae: Termitinae): The first step of genus revision. Ann. Entomol. Soc. Amer. 109, 158–167 (2016).
Peeters, C., Molet, M., Lin, C.-C. & Billen, J. Evolution of cheaper workers in ants: a comparative study of exoskeleton thickness. Biol. J. Linn. Soc. 20, 1–8 (2017).
Acknowledgements
The authors thank Jiunn-Ren Roan for suggestions on the energetic perspective of mandible strikes; Wei-Sheng Wang, Ming-Huang Wu, and Ching-Lung Chiu (Utopia Instruments Co., Ltd.) for their assistance with the high-speed videography and analysis; and Wei-Ren Liang for collecting termites and providing suggestions on experimental methods. This work was supported by the Ministry of Science and Technology, Taiwan (MOST 102–2313-B-005-037-MY2 (H.-F.L.), MOST 104-2311-B-005-010-MY3 (K.-J.C.), and MOST 105-2815-C-005-026-B (K.-C.K.)), and partially supported by the Ministry of Education–funded iEGG and Animal Biotechnology Center (MOE 107-S-0023-I (K.-J.C.)) of the Feature Areas Research Center Program within the framework of the Higher Education Sprout Project.
Author information
Authors and Affiliations
Contributions
K.-C.K., C.-I.C., K.-J.C. and H.-F.L. designed and performed the experiments. K.-C.K., C.-I.C. and H.-F.L. collected and identified termites. K.-C.K., M.-C.S. and K.-J.C. conducted mechanical analyses of the snapping behaviour of soldier mandibles. K.-C.K., C.-I.C. and H.-F.L. designed the figures. K.-C.K. and C.-I.C. wrote the manuscript in consultation with K.-J.C. and H.-F.L. All the authors discussed the study results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuan, KC., Chiu, CI., Shih, MC. et al. Termite’s Twisted Mandible Presents Fast, Powerful, and Precise Strikes. Sci Rep 10, 9462 (2020). https://doi.org/10.1038/s41598-020-66294-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66294-1
This article is cited by
-
Alarm communication predates eusociality in termites
Communications Biology (2023)
-
Investigation of the rate-mediated form-function relationship in biological puncture
Scientific Reports (2023)
-
Termite evolution: mutualistic associations, key innovations, and the rise of Termitidae
Cellular and Molecular Life Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.