Abstract
The origin and evolution of solar system bodies, including water on the Earth, have been discussed based on the assumption that the relevant ingredients were simply silicates and ices. However, large amounts of organic matter have been found in cometary and interplanetary dust, which are recognized as remnants of interstellar/precometary grains. Precometary organic matter may therefore be a potential source of water; however, to date, there have been no experimental investigations into this possibility. Here, we experimentally demonstrate that abundant water and oil are formed via the heating of a precometary-organic-matter analog under conditions appropriate for the parent bodies of meteorites inside the snow line. This implies that H2O ice is not required as the sole source of water on planetary bodies inside the snow line. Further, we can explain the change in the oxidation state of the Earth from an initially reduced state to a final oxidized state. Our study also suggests that petroleum was present in the asteroids and is present in icy satellites and dwarf planets.
Similar content being viewed by others
Introduction
Water plays an essential role in various hydrological, geological, and chemical processes in terrestrial planets and is key to discussions of the origins of life and biological activity. Despite its obvious importance, the origin of water on Earth is not fully understood1,2,3,4. Recently, analyses of some isotopes from the comet 67 P/Churyumov–Gerasimenko have shown that the contribution of cometary ice to the Earth’s oceans is less than 1%5, indicating the necessity for other candidates for the source material of terrestrial water. To deliver water to terrestrial-planet embryos, various sources have been proposed, e.g., the adsorption of water vapor on anhydrous silicates6, the incorporation of inward-drifting hydrous silicate grains into planetesimals at approximately 1 AU7, and ice-pebble accretion due to the migration of the snow line8. However, most models fail to explain the fact that the initially accreted Earth was a highly reduced environment9,10. In the late-stage water delivery models known as the classical11 and Grand Tack12,13 models, one problem is that too much water is delivered to the terrestrial planets if it is delivered as hydrous silicates in carbonaceous chondrites from beyond the snow line.
In studies of the origin of water on Earth, much less attention has been paid to “precometary organic matter,” i.e., abiotic hydrocarbons with oxygen-containing functional groups and their derivatives in space, as opposed to ices and silicates. However, astrophysical observations indicate that organic molecules are ubiquitous in space. For example, the space probes used in the missions to the comets 1 P/Halley and 67 P/Churyumov–Gerasimenko have shown that the main components of cometary refractory grains are organic matter and silicates14,15. Further, analyses of interplanetary dusts16,17, cometary dusts collected from the comet 81 P/Wild 217,18, and micrometeorites19 have shown that organic refractory grains are also abundant and ubiquitously distributed in these extraterrestrial materials. Many laboratory experiments suggest that these organic substances are formed in interstellar molecular clouds20 and then processed in the outer parts of the solar system21 via chemical processes such as ultraviolet photolysis, heating, and even the quantum tunneling of simple atoms and molecules22. These studies suggest that the abundant organic matter in space is of particular importance in assessing the origin and evolution of solar system bodies.
To investigate the distribution of precometary organic matter in the solar nebula, researchers have conducted heating experiments on precometary-organic analogs23,24,25. Nakano et al.25 demonstrated that fresh precometary organics evaporate at temperatures between 200 K and 300 K and that carbonaceous materials persist at temperatures higher than 300 K. The distribution of organics in the solar nebula can be determined from this result if we assume a suitable temperature distribution for the solar nebula. These organic-covered silicate grains accreted together with chondrules and other minerals in the solar nebula to form the meteoritic parent bodies. Nakano et al.26 performed experiments on the reactions of precometary organics with water followed by heating in a vacuum using an precometary-organic analog to mimic aqueous alteration and thermal metamorphism in the parent bodies of carbonaceous chondrites. They found that O and N-rich viscous organic matter obtained by aqueous alteration changed to solid carbonaceous materials after heating in a vacuum. However, there have been no experimental investigations with respect to the heating of precometary organics in parent bodies inside the snow line.
In the present investigation, we experimentally studied the potential role of precometary organic matter as a source of water inside the snow line (<2.5 AU) by simulating the heating of organic matter up to several hundreds of degrees Kelvin in the parent bodies of ordinary chondrites27,28,29. Under such high-temperature conditions, the complex organic molecules present in the parent bodies can decompose into small molecules, including water, even inside the snow line, and the produced water may serve as a source material for terrestrial water.
Chemical Compositions of Interstellar-Organic Analogs
Large amounts of precometary organic matter cannot be obtained; therefore, we used chemical reagents to create an analog for precometary organic matter that formed in a molecular cloud24,25. The chemical compositions of organic matter produced by the photolysis of an ice mixture depends on the composition of the ice mixture30,31,32. Briggs et al. used a CO-rich ice mixture (H2O:CO:NH3 = 5:5:1) for photolysis experiments30, while Bernstein et al. used a CH3OH-rich ice mixture (H2O:CH3OH:CO:NH3 = 10:5:1:1)32. Infrared observations33,34 indicate the presence of CO-rich ices in low-mass protostars similar to our Sun and in molecular clouds. Further, the compositions of cometary ices, which are considered to be remnants of interstellar ices, are CO-rich and CH3OH-poor35. Conversely, CH3OH-rich ices are observed primarily in high-mass protostars. Because CH3OH is formed by the hydrogenation of CO in molecular clouds36, one can reasonably expect that the hydrogenation of CO proceeded only to a certain extent (forming only a few percent CH3OH) in the molecular cloud where our solar system was born. Therefore, the ice mixture used by Briggs et al.30 is more relevant for discussions of the organic matter in our solar system than that used by Bernstein et al.32. Furthermore, although the ice-composition dependence of the organic residues’ composition has been previously investigated37,38, a recipe based on these works is precluded by the deficiency of analytical data.
To formulate the recipe for our analog, we referred to analytical data30,39,40 produced by the photolysis of mixed ices (H2O:CO:NH3 = 5:5:1). Briggs et al. analyzed soluble organic matter using gas chromatography-mass spectrometry (GC-MS) and showed that the lower molecular weight compounds were C2–C3 hydroxy acids and hydroxy amides, glycerol, urea, hexamethylenetetramine, and formamidine30. Greenberg and Mendoza-Gomez analyzed the less soluble part of the same sample using high-resolution fast atom bombardment mass spectrometry and proposed the following elemental compositions: C9H8, C12H12, C12H14, C13H16, C13H16O, C14H18O, and C14H18O2 39. Further analyses of the same sample using electron impact mass spectrometry indicated that the sample consists of a complex mixture of hydrocarbon chains (-CH2-)n from C5 to C30 40. Figure 1 shows the recipe used in the present study, called molecular cloud (MC) organics. Some of the compounds in this recipe have been found in interstellar space41,42,43,44 and comets45,46. The chemical compounds in Fig. 1a,b, and 1c are based on the analytical data of Briggs et al.30, Greenberg and Mendoza-Gomez39, and Mendoza-Gomez and Greenberg40, respectively. We used reagents with similar chemical characteristics when we could not obtain the same chemical compounds as in the analyses, and we estimated unidentified chemical compounds from their elemental compositions. In addition, to discuss the formation mechanism of water, we used two specific compositions: alcohols and carbonic acid (Fig. SI 1a) and ketone and amides (Fig. SI 1b).
Composition of the precometary-organic-matter analog (MC). To formulate the recipes in groups a, b, and c, we referred to the analytical data in refs. 30,39,40, respectively. *When we could not obtain the chemical reagents from the analyses, we used reagents with similar chemical characteristics. #Unidentified chemical compounds were estimated from their elemental compositions. IS and C indicate chemical compounds observed in interstellar space41,42,43,44 and comets45,46, respectively.
Results
In-situ observations using a diamond anvil cell
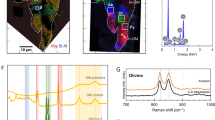
To study the heating of organic matter, we first made in-situ observations using an externally heated diamond anvil cell47 with the temperature profile shown in Fig. SI 2. Figure 2 and Videos SI 1–SI 7 show photomicrographs and videos, respectively, that we obtained during the heating experiments. At temperatures between 24 °C and 150 °C, the sample was uniform, and the color changed from pale yellow to yellow. At approximately 150 °C, phase separation occurred and two organic phases coexisted until 350 °C. One was transparent and had low viscosity, while the other was dark brown and had high viscosity. The latter is hereafter referred to as “highly viscous organic matter.” The viscosity of the highly viscous organic matter increased with the temperature. At approximately 350 °C, the two organic phases became one phase again, the color changed to reddish-black, and the viscosity decreased. From these changes in the color and viscosity, we concluded that polymerization proceeded until 350 °C and that thermal cracking occurred at temperatures higher than 350 °C. This process is nearly the same as the process that forms geopolymer kerogen and subsequently petroleum48. Water droplets formed simultaneously at approximately 350 °C, and the droplets coalesced as temperature rises. At 400 °C, water and black oil coexisted, and they continued to coexist after the sample had been cooled to room temperature. Figure 3a shows the change in the near-infrared spectra with temperature. At temperatures between 24 °C and 200 °C, the sample has rich functional groups, including -CH2, -CH3, -OH, and -NH2. At temperatures higher than 300 °C, two absorption bands originating from water are clearly observed; the bands at 5200 cm−1 and 7100 cm−1 are due to a combination of OH stretching and bending vibrations and to the OH stretching overtone of water, respectively49. Meanwhile, the presence of -CH2, -CH3, and -NH2 became unclear due to the strong H2O bands. At 400 °C, the difference between the oil and the water became clearer. Figure 3b shows the change in the intensity of the 5200-cm−1 water band with time (i.e., temperature), clearly showing that the intensity of water increased at approximately 300 °C. Therefore, we concluded that water formation started at approximately 300 °C. Note that this conclusion is valid only for the laboratory time scale of heating. On astrophysical time scales, the water formation temperature should be lower than 300 °C.
In-situ observations of the heating of an precometary-organic-matter analog (MC) using a diamond anvil cell. Photomicrographs were taken during the heating experiment. A indicates air bubbles, and Qz indicates quartz crystals. White arrows indicate highly viscous organic matter. The scale bars represent 100 μm.
(a) Near-infrared absorption spectra of the precometary-organic-matter analog (MC) at temperatures between 24 °C and 400 °C. Blue arrows indicate absorption bands owing to H2O, gray arrows indicate absorption bands owing to OH, amine, and ammonia (5000 cm−1) and amine and ammonia (6800 cm−1), and HC (3991, 4364, and 4530 cm−1) indicates hydrocarbons. (b) The ratio of the 5200-cm−1 water band peak height at time t to that at t = 0. The upper abscissa shows the corresponding temperatures.
Reactor Experiments
To characterize the water and black oil produced in this experiment in more detail, we also performed heating experiments using an autoclave, as shown in Fig. SI 3. Figure 4a,b show photographs of the starting material and the recovered products after 5 h of heating at 400 °C and 14 MPa, respectively. The color of the aqueous product is a cloudy pale brown, and the oil is black and viscous similar to crude oil. The densities of the oil and the aqueous product measured at 18.4 °C are 997.3 ± 48.2 kg m−3 and 1060.7 ± 42.7 kg m−3, respectively. The pH of the aqueous product was 9.3.
Photographs of (a) the starting material, i.e., the precometary-organic-matter analog (MC) shown in Fig. 1, and (b) the black oil and aqueous products recovered from the MC heating experiment at 400 °C. The diameter of the sample bottle is 30 mm.
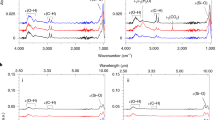
Figure 5 shows the mid-infrared spectra of the starting material, the aqueous and oil products, and a reference spectrum of pure liquid water. The spectrum of the aqueous products clearly shows the OH stretching–vibration band at approximately 3800−2800 cm−1 and the libration band below 800 cm−1. These features are in good agreement with those of pure water, indicating that liquid water is the main component of the aqueous product. The OH2 bending–vibration band of water [δ(OH2)] at approximately 1700–1600 cm−1 appears to be buried within strong bands at 1657 cm−1, 1612 cm−1, and 1550 cm−1 due to other products, which can plausibly be attributed to the amide I and II bands and to the antisymmetric COO− stretching–vibration bands of carboxylate anions, respectively50. The presence of acetamide (CH3CONH2 or C2H5NO), which we used as a reactant, and its alkylated homologues (e.g., CH3CH2CONH2 or C3H7NO), as well as low-molecular weight carboxylic acids such as acetic acid (CH3COOH or C2H4O2), propionic acid (CH3CH2COOH or C3H6O2) and other compounds, are inferred from the high-resolution mass spectrum of the recovered aqueous products (Fig. 6).
Figures SI 4 shows the matrix-assisted laser desorption/ionization (MALDI)-time of flight (TOF) spectrum for the recovered oil during the heating experiment of MC at 400 °C. Many peaks appear in the spectrum, the mass-distribution pattern of which closely resembles that of crude oil51. To identify specific molecules in the oil, we subjected the sample to a further series of column chromatography on silica gel. The total-ion-current chromatograms of the recovered oil are shown in Fig. 7a–c. We detected a series of n-alkanes (C10–C19) and alkylated benzenes (C11–C13) as the main compounds in fraction 1 (Fig. 7a). The n-alkanes were generated by decarboxylation and/or the thermal cracking of fatty acids. This does not contradict the absence of COOH bands in the infrared spectrum of the recovered oil (Fig. 5c) because unsaturated hydrocarbons occurred only in trace concentrations in the oil. This result also shows similar characteristics to the components of typical crude oils. Various aromatic compounds were detected in fraction 2 (Fig. 7b). These were likely generated by thermal alterations of starting materials such as methylation, isomerization, and aromatization. The analytical results for fraction 2 are consistent with the mid-infrared absorption spectrum (Fig. 5c). In addition, several aromatic compounds containing hydroxyl or carbonyl groups were found in fraction 3 (Fig. 7c).
We detected CO, CO2, CH4, H2, C2H6, and C3H8 molecules as major components in the recovered gas (Fig. 8), which shows the significant progress of the decarboxylation and thermal cracking of the C–C bond. Higher concentrations of H2 and trace amounts of the unsaturated hydrocarbons C2H4 and C3H6 suggest a sufficient generation of H2 for the hydrogenation of double bonds. However, we did not detect any nitrogen compounds, because the N-content of MC itself is small and generation of N2 from the nitrogen compounds requires a higher heating temperature. Most of the ammonia (NH3) generated was dissolved in the aqueous solution as NH4+.
Pyrolysis experiments for single molecules52 suggest that water can be formed by the dehydration–condensation reactions of alcohols and carboxylic acids. To check this hypothesis, we performed further heating experiments using simple mixtures (Fig. SI 1): an -OH and -COOH-bearing mixture called MC-1 and a mixture of amides and ketones without -OH and -COOH called MC-2. Figures SI 5a,b show photographs of the recovered products heated to 400 °C, showing that the recovered products are black oil and a relatively transparent liquid. The weight ratios of the gas to the recovered samples in MC-1 and MC-2 were 72:28 and 25:75, respectively, and those of the black oil to the transparent liquid in MC-1 and MC-2 were 6:22 and 52:23, respectively. Figure SI 6 shows infrared spectra of pure water and the recovered transparent products. The spectrum of the sample obtained from MC-1 is nearly the same as that of pure water, confirming that the main component of the transparent product is water. Conversely, the spectrum of the sample obtained from MC-5 differs greatly from that of pure water but resembles that from MC (Fig. 6b). The peak height ratio of the spectrum at 3340 cm−1 to that at 1391 cm−1 of the samples from MC-1, MC, and MC-2 are 5.6, 2.3, and 0.45, respectively. These two peaks were selected because 3340 cm−1 reflects the amount of -OH and because 1391 cm−1 was the strongest peak in the products from MC-2. The OH-contents, defined as the ratio of the OH weight to the total sample weight, of the starting materials of MC-1, MC, and MC-2 were 47.8%, 8.3%, and 0%, respectively. These results clearly indicate that results of pyrolysis experiments for single molecules52 provide useful guidelines for water formation from mixed samples and that the amount of water formed depends on the –OH content of the starting materials. NH3 can be formed by deamination of –NH2. It is usually assumed that petroleum (oil and gas) can be formed by the thermal cracking of polymethyl chains of long-chain hydrocarbons and highly viscous organic matter (Fig. 1a). This explanation may be correct when the MC mixture (Fig. 1) is used as the starting material. However, the results of these additional experiments indicate additional sources of black oil, that is, simple mixtures of ethylene glycol, glycol acid, and glycerol (MC-1) and cyclohexyl phenyl ketone, 4’-cyclohexylacetophenone, acetamide, and urea (MC-2). Heating these molecules may cause polymerization at certain temperatures similar to the highly viscous organic matter; then, these polymers may decompose to form black oil at higher temperatures. Therefore, the possible sources of petroleum are more extensive than previously thought.
Discussion
The atomic composition of the precometary-organic-matter analog used in the present study (MC) is H:C:N:O = 148:100:5:23 (O/C = 0.23, N/C = 0.01). The atomic compositions of organic particles from the comets 1 P/Halley and 81 P/Wild2 are H:C:N:O = 80:100:4:20 (O/C = 0.2, N/C = 0.04)14 and O/C = 0.2 − 0.6, N/C = 0.07 − 0.218, respectively. The atomic compositions of organic particles from interplanetary dust particles and Antarctic micrometeorites, both of which may have originated from comets, are O/C = 0.49, N/C = 0.1617 and O/C = 0.27, N/C = 0.1519, respectively. If we compare these cometary organics with the starting material used in the present study, it is clear that cometary organics are rich in O and N, which shows their primitive nature. Even though we have no information concerning the content of -OH in actual cometary organics, we expect that the amount of water produced by heating organics will likely increase with increasing O content. Therefore, we suggest that the amount of water produced by the heating of actual precometary/cometary organics is likely larger than that obtained in the present experiments.
The present study suggests that liquid water can be formed via the heating of precometary organic matter to approximately 300 °C in the parent bodies of chondrites inside the snow line. Note that this temperature should be much lower on astrophysical time scales. The produced liquid water in the deeper parts of ordinary chondrite parent bodies can move upward and react with anhydrous silicates near the surfaces of their parent bodies to form hydrous silicates, as shown by Alexander et al.53. These parent bodies or their fragments may have served as a source of water for the terrestrial planets. In fact, some evidence for aqueous alteration has been found in primitive ordinary chondrites; for example, various hydrous silicates have been found in ordinary chondrites53,54,55,56. Even in highly heated ordinary chondrites, phosphate minerals have been found57, which might have formed via interactions with fluids during cooling. In addition, the D/H ratio of ordinary chondrites is extremely large58, suggesting isotope fractionation during dehydration. Very recently, water was directly detected in a Hayabusa sample collected from the S-type asteroid Itokawa59, which is equivalent to a parent body of ordinary chondrites. Further, oil produced in the deeper parts of parent bodies could move upward and mix with original precometary organics near the surfaces of such parent bodies. When this mixture is slightly heated in an open system, volatile components can vaporize while solid carbonaceous materials remain, as demonstrated experimentally26. In this way, insoluble organic matter, which has been found in some ordinary chondrites55,60,61, may form. Because the actual parent bodies are heterogeneous and more complex than those described above, water and subsequent hydrous silicates, and petroleum and subsequent insoluble organic matter, might form in localized areas. This inference is supported by the distribution of organic matter and hydrous silicates in carbonaceous chondrites62,63,64. The above observations of ordinary chondrites demonstrate that liquid water and subsequent hydrous silicates exist in the parent bodies of ordinary chondrites. Our experimental results highlight a possible new reservoir of water inside the snow line.
Geochemical evidence suggests that the initially accreted Earth was a highly reduced environment9,10. Most models6,7,8 of early stage water delivery to terrestrial-planet embryos cannot explain this highly reduced state because they assume that materials near Earth orbit were anhydrous silicates and OH (or H2O) introduced via various routes, and this combination does not produce a reduced state. In addition, if we assume that the materials near Earth orbit were completely dry, i.e., that only anhydrous silicates were present, it appears impossible to realize a highly reduced state. Therefore, the Earth’s materials have been simply assumed as mixtures of a highly reduced component and an oxidized component65, and the production mechanism of the highly reduced component has not been discussed. Experimental heating of the precometary-organic-matter analog in a vacuum demonstrated that water and other volatile gases evaporated at temperatures lower than 100 °C and that the remaining solid became carbonaceous matter23,25. From these results, we concluded that anhydrous silicate grains were likely covered by organic matter just inside the snow line or by carbonaceous materials far inside the snow line. Because the heating of carbonaceous materials can produce a highly reduced state25, the presence of such materials can overcome the “highly reduced state problem” for the early Earth. Material accreted later, but not as a late veneer, from ordinary and/or carbonaceous chondrites consist of anhydrous silicates, hydrous silicates, and small amounts of carbonaceous materials. These combinations can produce a more-oxidized state than that seen in the early stage. Earth’s water might have been delivered at this stage, even though the delivery mechanism is still under debate4,11,12,13. In this way, we can explain the change in the oxidation state from an initially reduced state to a final oxidized state. In late-stage water delivery models11,12,13, too much water is delivered to the terrestrial planets because it is delivered as hydrous silicates in carbonaceous chondrites from beyond the snow line. If water can form when precometary organics are warmed by secondary alteration in ordinary chondrites’ parent bodies, it could account for the abundant water brought to Earth by ordinary chondrites inside the snow line.
The formation of abiotic petroleum in some solar system bodies also needs to be considered. In the parent bodies of carbonaceous chondrites, the formation of soluble organic matter such as oil has been demonstrated experimentally via the high-temperature heating of insoluble organic matter with water66,67. The present study suggests the possibility that petroleum formation may have also occurred in the parent bodies of ordinary chondrites via the simple heating of precometary organics, as shown above. However, this petroleum may not remain at the present time because the oil may have changed to solid carbonaceous material (insoluble organic matter) following mild heating near the surfaces of the parent bodies, as discussed above. Cassini found several macromolecular organic compounds68 and hydrogen gas69 during its observations of Enceladus. Even though the formation mechanism of the organic compounds in Enceladus is still unclear, heating of precometary organics with ice may be included in the possibilities. Matson et al.70 estimated the maximum temperature of Enceladus as 1300 K. Therefore, we suggest that the reaction occurring in our experiments is similar to that occurring in the interior of Enceladus. Our density measurements of the oil and aqueous products suggest that an oil layer may exist between the icy crust and the liquid-water ocean of this satellite. Oil may also be concentrated beneath the basin known as Sputnik Planitia on Pluto because the bottom of the icy crust may have a concave shape71 similar to cap rocks on oil reservoirs on the Earth. Further, an oil layer beneath the icy crust could behave as a good thermal insulator to prevent Pluto from cooling, in addition to the recently proposed methane clathrate-hydrate thermally insulating layer72, because the thermal conductivity of crude oil73 is five times smaller than that of methane clathrate-hydrate. For the Earth, an abiotic petroleum formation mechanism has been discussed by Sephton and Hazen74, while Maurette et al. suggested the possibility that kerogen-rich micrometeories75 or cometary organics76 were a source of petroleum for the ancient Earth. The former possibility is supported by laboratory experiments66,67, and the latter may be supported by our present study.
Methods
Starting material
To create an analog of the organic material formed in molecular clouds and then processed in the solar system (Figs. 1 and SI 1), the chemical reagents were weighed and mixed in a mortar without any solvent in an N2 atmosphere.
In-situ observations using a diamond anvil cell
To simulate the heating of precometary organic matter in a meteorite parent body that formed inside the snow line (<2.5 AU) up to several hundreds of degrees Kelvin27,28,29, we used an externally heated diamond anvil cell47 at the Institute for Planetary Materials, Okayama University. Diamond anvil cells are commonly used in high-pressure experiments. Because diamonds are transparent at visible, infrared, and X-ray wavelengths, both in-situ optical absorption spectroscopy and X-ray diffraction are possible. We placed the starting material into a 500-μm hole in a 125-μm-thick Ir gasket in air, together with a quartz crystal (Qz in Fig. 1a) for the pressure measurements. The pressures estimated using the method of Schmidt and Ziemann77 were 80 ± 50 MPa and 70 ± 50 MPa at 23 °C and 102 °C, respectively. However, at temperatures higher than 200 °C, we were not able to measure the pressure due to strong fluorescence from the organic matter. The thickness of the Ir gasket after the experiment was 122 μm, indicating that the pressure during the heating experiment was not very high. The temperature profile in the present experiment is shown in Fig. SI 2. During the experiment, we observed changes in the sample in-situ using an optical microscope and recorded these changes using a video recorder. We measured near-infrared spectra of 50 × 50-μm2 sample spots in transmission mode using a micro Fourier-transform spectrometer (Jasco IRT-7000/FTIR-6200). Spectra were acquired with 100 times accumulation at a resolution of 4 cm−1. We estimated the viscosity qualitatively from the movements and velocities of small spheres and interfaces.
Heating experiment using an autoclave
We used an autoclave made of Hastelloy alloy (OM Labotec, MA type), which is shown in Fig. SI 3. After loading a 10-g sample into the autoclave in an N2 atmosphere, we evacuated the residual gas (primarily N2) using an oil-free scroll pump. We then heated the autoclave at a rate of 3 °C/min to 400 °C and maintained that temperature for 5 h. After heating, we cooled the sample to room temperature by turning off the electric power supply. The gas was recovered from the reactor to an evacuated gas bottle by opening a valve between the gas bottle and the reactor (see Fig. SI 3), and then closing a valve attached to the gas bottle. From the mass of the recovered products (oil and aqueous solution), we estimated the weight of the gas. We analyzed the recovered products (gas, oil, and aqueous products) using the following methods.
Mid-infrared spectroscopy
We measured the mid-infrared absorption spectra of the starting material and the recovered oil and aqueous products using a Fourier transform infrared spectrometer (PerkinElmer, Spectrum One). Spectra were acquired with 100 times accumulation at a resolution of 4 cm−1 using an attenuated total reflection attachment.
Aqueous product analysis via high-resolution mass spectrometry
We analyzed the aqueous product via high-resolution mass spectrometry using a Thermo Scientific Exactive with a mass resolution of m/Δm ~ 70,000 at a mass-to-charge ratio (m/z) of 200 (Thermo Fischer Scientific, Inc.). We dissolved a small aliquot of the sample in methanol and used flow injection to introduce approximately 5 μL of the solution into the mass spectrometer. We measured the mass spectrum in both the positive- and negative-electrospray ionization modes over an m/z range of 50–1000 at a spray voltage of ~3 kV. The capillary voltage and the temperature of the ion transfer were 25 V and 300 °C, respectively.
Oil analysis
We analyzed the oil sample via MALDI-TOF mass spectrometry (MALDI-TOF-MS) using a TOF/TOFTM 5800 system (SCIEX). We prepared the sample for the MALDI-TOF-MS analysis in accordance with the previously reported method78,79. Briefly, we dissolved an aliquot (0.1–0.3 mg) of the oil sample in 1 mL of tetrahydrofuran (THF) and mixed it with a matrix reagent (trans, trans-1,4-diphenyl-1,3-butadiene or 9-nitroanthracene). We then transferred the mixture to the MALDI target-plate cell using the water-spotting method. To remove all the THF from the sample, we allowed the entire sample to dry over a one-hour period. We conducted the MALDI-TOF-MS analysis over an m/z range of 0–2000. To confirm the repeatability of the data, we analyzed each sample five times at different spots.
We further subjected the recovered oil (0.5 mL) to column chromatography (5.0 cm × 10 mm i.d.) on a silica gel (Merck silica gel 60, 70–230 mesh, 5.0 g) with elution by n-hexane (30 mL) to isolate the saturated hydrocarbons and alkylated benzenes (fraction 1), with elution by dichloromethane/n-hexane (7:3 v:v, 14 mL) to isolate the aromatic compounds (fraction 2), and with elution by n-hexane/ethyl acetate (9:1 v:v, 10 mL) and ethyl acetate/methanol (1:1 v:v, 10 mL) to isolate the polar compounds (fraction 3). We performed GC-MS (HP6890-HP5973; Hewlett Packard) using a fused-silica DB-5 column (30 m × 0.25 mm i.d.; Hewlett Packard) with helium as a carrier gas at 0.6 mL/min. The oven temperature program was 40 °C (5 min) to 300 °C (maintained for 5 min) at 4 °C/min. We assigned compounds using the NIST Mass Spectral Search Program (National Institute of Standards and Technology), together with data from the literature80,81. We identified 1,4-Diisopropylbenzene by comparing the retention times with an authentic sample (Tokyo Chemical Industry Co.). The detailed analytical procedures are described in Asahina and Suzuki82.
Gas analysis
We measured the composition of the recovered gases from the GC heating experiment using an instrument (7890 A: Agilent) equipped with a pulsed-discharge helium-ionization detector and a micropacked column containing ShinCarbon ST 80/100 (2.0 m × 1.0 mm i.d.; Shinwa Co.). We used ultra-high-purity helium as the carrier gas and introduced the gas at a constant rate into the GC column with a 10-μL sampling loop. The oven temperature program was 40 °C (3 min) to 300 °C (maintained for 15 min) at 15 °C/min. We identified compounds by comparing the retention times with those of reference standards in a gas mixture containing H2, CO, CO2, CH4, C2H4, C2H6, C3H6, C3H8, i-C4H10, and n-C4H10. The detailed analytical procedure is described in Saito et al.83.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Genda, H. Origin of Earth’s oceans: An assessment of the total amount, history and supply of water. Geochem. J. 50, 27–42 (2016).
Peslier, A. H., Schönbächler, M., Busemann, H. & Karato, S.-I. Water in the Earth’s interior: Distribution and origin. Space Sci. Rev. 212, 743–810 (2017).
Alexander, C. M. O. ’D., McKeegan, K. D. & Altwegg, K. Water reservoirs in small planetary bodies: Meteorites, asteroids, and comets. Space Sci. Rev. 214, 36 (2018).
O’Brien, D. P. et al. The delivery of water during terrestrial planet formation. Space Sci. Rev. 214, 47 (2018).
Marty, B. et al. Origins of volatile elements (H, C, N, noble gases) on Earth and Mars in light of recent results from the ROSETTA cometary mission. Earth Planet. Sci. Lett. 441, 91–102 (2016).
Stimpfl, M. et al. An Ångström-sized window on the origin of water in the inner solar system: Atomistic simulation of adsorption of water on olivine. J. Cryst. Growth 294, 83–95 (2006).
Ciesla, F. & Lauretta, D. Radial migration and dehydration of phyllosilicates in the solar nebula. Earth Planet. Sci. Lett. 231, 1–8 (2005).
Sato, T., Okuzumi, S. & Ida, S. On the water delivery to terrestrial embryos by ice pebble accretion. Astron. Astrophys. 589, A15 (2016).
Wood, B. J., Wade, J. & Kilburn, M. R. Core formation and the oxidation state of the Earth: Additional constraints from Nb, V and Cr partitioning. Geochim. Cosmochim. Acta 72, 1415–1426 (2009).
Rubie, D. C. et al. Heterogeneous accretion, composition and core–mantle differentiation of the Earth. Earth Planet. Sci. Lett. 301, 31–42 (2011).
O’Brien, D. P., Morbidelli, A. & Levison, H. F. Terrestrial planet formation with strong dynamical friction. Icarus 184, 39–58 (2006).
Walsh, K. J. et al. A low mass for Mars from Jupiter’s early gas-driven migration. Nature 475, 206–209 (2011).
O’Brien, D. P. et al. Water delivery and giant impacts in the ‘Grand Tack’ scenario. Icarus 239, 74–84 (2014).
Kissel, J. & Krueger, F. R. The organic component in dust from comet Halley as measured by the PUMA mass spectrometer on board Vega 1. Nature 326, 755–760 (1987).
Bardyn, A. et al. Carbon-rich dust in comet 67P/Churyumov-Gerasimenko measured by COSIMA/Rosetta. Mon. Not. R. Astron. Soc. 469, S712–S722 (2017).
Flynn, G. J. et al. The origin of organic matter in the solar system: Evidence from the interplanetary dust particles. Geochim. Cosmochim. Acta 67, 4791–4806 (2003).
Cody, G. D. et al. Quantitative organic and light-element analysis of comet 81P/Wild 2 particles using C-, N-, and O-μ-XANES. Meteorit. Planet. Sci. 43, 353–365 (2008).
Sandford, S. A. et al. Organics captured from comet 81P/Wild 2 by the stardust spacecraft. Science 314, 1720–1724 (2006).
Yabuta, H. et al. Formation of an ultracarbonaceous Antarctic micrometeorite through minimal aqueous alteration in a small porous icy body. Geochim. Cosmochim. Acta 214, 172–190 (2017).
Agarwal, V. K. et al. Photochemical reactions in interstellar grains photolysis of CO, NH3, and H2O. Orig. Life 16, 21–40 (1985).
Ciesla, F. J. & Sandford, S. A. Organic synthesis via irradiation and warming of ice grains in the Solar nebula. Science 336, 452–454 (2012).
Hama, T. & Watanabe, N. Surface processes on interstellar amorphous solid water: Adsorption, diffusion, tunneling reactions, and nuclear-spin conversion. Chem. Rev. 113, 8783–8839 (2013).
Franchi, I. A. et al. Stable isotope studies of cometary analogue materials. In “Physics and Mechanics of Cometary Materials” ESA-SP 302, 89-92 (1989).
Kouchi, A. et al. Rapid growth of asteroids owing to very sticky interstellar organic grains. Astrophys. J. Lett. 566, L121–L124 (2002).
Nakano, H., Kouchi, A., Tachibana, S. & Tsuchiyama, A. Evaporation of interstellar organic materials in the solar nebula. Astrophys. J. 592, 1252–1262 (2003).
Nakano, H. et al. Alteration of interstellar organic materials in meteorites’ parent bodies: a novel route for diamond formation. Proc. Jpn. Acad. B78, 277–281 (2002).
Miyamoto, M., Fujii, N. & Takeda, H. Ordinary chondrite parent body: An internal heating model. Proc. Lunar Planet. Sci. 12B, 1145–1152 (1981).
Grimm, R. E. & McSween, H. Y. Jr. Heliocentric zoning of the asteroid belt by aluminum-26 heating. Science 259, 653–655 (1993).
Trieloff, M. et al. Structure and thermal history of the H-chondrite parent asteroid revealed by thermochronometry. Nature 422, 502–506 (2003).
Briggs, R. et al. Comet Halley as an aggregate of interstellar dust and further evidence for the photochemical formation of organics in the interstellar medium. Orig. Life Evol. Biospheres 22, 287–307 (1992).
Jenniskens, P. et al. Carbon dust formation on interstellar grains. Astron. Astrophys. 273, 583–600 (1993).
Bernstein, M. P. et al. Organic compounds produced by photolysis of realistic interstellar and cometary ice analogs containing methanol. Astrophys. J. 454, 327–344 (1995).
Ehrenfreund, P. & Charnely, S. B. Organic molecules in the interstellar medium, comets, and meteorites: A voyage from dark clouds to early earth. Ann. Rev. Astron. Astrophys. 38, 427–483 (2000).
Gibb, E. L., Whittet, D. C. B., Boogert, A. C. A. & Tielens, A. G. G. M. Interstellar ice: The Infrared Space Observatory legacy. Astrophys. J. Suppl. 151, 35–73 (2004).
Bockelée-Morvan, D., Crovisier, J., Mumma, M. J. & Weaver, H. A. “The composition of cometary volatiles” in Comets II, Festou, M. C., Keller, H. U. & Weaver, H. A. Eds. (Arizona, 2004), pp. 391–423.
Watanabe, N. & Kouchi, A. Efficient formation of formaldehyde and methanol by addition of hydrogen atoms to CO in H2O-CO ice at 10 K. Astrophys. J. Lett. 571, L173–L176 (2002).
Muñoz Caro, G. M., Meierhenrich, U., Schutte, W. A., Thiemann, W. H.-P. & Greenberg, J. M. UV-photoprocessing of interstellar ice analogs: Detection of hexamethylenetetramine-based species. Astron. Astrophys. 413, 209–216 (2004).
Danger, G. et al. Characterization of laboratory analogs of interstellar/cometary organic residues using very high resolution mass spectrometry. Geochim. Cosmochim. Acta 118, 184–201 (2013).
Greenberg, J. M. & Mendoza-Gómez, C. X. “Interstellar dust evolution: A reservoir of prebiotic molecules” in The Chemistry of Life’s Origin, Greenberg, J. M., Mendoza-Gómez, C. X. & Pirronello, V. Eds. (Kluwer, 1993), pp. 1–32.
Mendoza-Gómez, C. X. & Greenberg, J. M. Laboratory simulation of organic grain mantles. Orig. Life Evol. Biospheres 23, 23–28 (1993).
Hollis, J. M., Lovas, F. J., Jewell, P. R. & Coudert, L. H. Interstellar antifreeze: Ethylene glycol. Astrophys. J. Lett. 571, L59–L62 (2002).
Hollis, J. M. et al. Detection of acetamide (CH3CONH2): The largest interstellar molecule with a peptide bond. Astrophys. J. Lett. 643, L25–L28 (2006).
Remijan, A. J. et al. Observational results of a multi-telescope campaign in search of interstellar urea [(NH2)2CO]. Astrophys. J. 783, 77 (2014).
Belloche, A. et al. Re-exploring moleculer complexity with ALMA (ReMoCA): Interstellar detection of urea. Astron. Astrophys. 628, A10 (2019).
Bockelée-Morvan, D. et al. New molecules found in comet C/1995 O1 (Hale-Bopp). Investigating the link between cometary and interstellar material. Astron. Astrophys. 353, 1101–1114 (2000).
Crovisier, J. et al. Ethylene glycol in comet C/1995 O1 (Hale-Bopp). Astron. Astrophys. 418, L35–L38 (2004).
Chertokova, N. & Yamashita, S. In situ spectroscopic study of water speciation in the depolymerized Na2Si2O5 melt. Chem. Geol. 409, 149–156 (2015).
Vandenbroucke, M. & Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 38, 719–833 (2007).
Jin, Y. & Ikawa, S.-I. Near-infrared spectroscopic study of water at high temperatures and pressures. J. Chem. Phys. 119, 12432–12438 (2003).
Silverstein, R. M., Webster, F. X., Kiemle, D. J. & Bryce, D. L. Spectrometric Identification of Organic Compounds 8th Edition (Wiley, 2015).
Robins, C. & Limbach, P. A. The use of nonpolar matrices for matrix-assisted laser desorption/ionization mass spectrometric analysis of high boiling crude oil fractions. Rapid Commun. Mass Spectrom. 17, 2839–2845 (2003).
Moldoveanu, S. C. Pyrolysis of Organic Molecules with Applications to Health and Environmental Issues (Elsevier, 2010).
Hutchison, R., Alexander, C. M. O. ’D. & Barber, D. J. The Semarkona meteorite: First recorded occurrence of smectite in an ordinary chondrite, and its implications. Geochim. Chosmochim. Acta 51, 1785–1882 (1987).
Alexander, C. M. O. ’D., Barber, D. J. & Hutchison, R. The microstructure of Semarkona and Bishunpur. Geochim. Cosmochim. Acta 53, 3045–3057 (1989).
Quirico, E., Raynal, P. I. & Bourot-Denise, M. Metamorphic grade of organic matter in six unequilibrated ordinary chondrites. Meteorit. Planet. Sci. 38, 795–811 (2003).
Dobrică, E. & Brearley, A. J. Widespread hydrothermal alteration minerals in the fine-grained matrices of the Tieschitz unequilibrated ordinary chondrite. Meteorit. Planet. Sci. 49, 1323–1349 (2014).
Jones, R. H. et al. Phosphate minerals in LL chondrites: A record of the action of fluids during metamorphism on ordinary chondrite parent bodies. Geochim. Cosmochim. Acta 132, 120–140 (2014).
Alexander, C. M. O. ’D. et al. The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 337, 721–723 (2012).
Jin, Z. & Bose, M. New clues to ancient water on Itokawa. Sci. Adv. 5, eaav8106 (2019).
Alexander, C. M. O. ’D. et al. The origin of chondritic macromolecular organic matter: a carbon and nitrogen isotope study. Meteorit. Planet. Sci. 33, 603–622 (1998).
Alexander, C. M. O. ’D. Fogel, M.,Yabuta, H. & Cody, G. D. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 71, 4380–4403 (2007).
Busemann, H. et al. Interstellar chemistry recorded in organic matter from primitive meteorites. Science 312, 727–730 (2006).
Le Guillou, C., Bernard, S., Brearley, A. J. & Remusat, L. Evolution of organic matter in Orgueil, Murchison and Renazzo during parent body aqueous alteration: In situ investigations. Geochim. Cosmochim. Acta 131, 368–392 (2014).
Changela, H. G., Le Guillou, C., Bernard, S. & Brearley, A. J. Hydrothermal evolution of the morphology, molecular composition, and distribution of organic matter in CR (Renazzo-type) chondrites. Meteorit. Planet. Sci. 53, 1006–1029 (2018).
Dreibus, G. & Wänke, H. Supply and loss of volatile constituents during the accretion of terrestriall planets. in Origin and Evolution of Planetary and Satellite Atmospheres, Atreya, S. K., Pollack, J. B. & Matthews, M. S. Eds. (Arizona, 1989), pp. 268–288.
Sephton, M. A., Pillinger, C. T. & Gilmour, I. δ13C of free and macromolecular aromatic structures in the Murchison meteorite. Geochim. Cosmochim. Acta 62, 1821–1828 (1998).
Yabuta, H. et al. The insoluble carbonaceous material of CM chondrites: A possible source of discrete organic compounds under hydrothermal conditions. Meteorit. Planet. Sci. 42, 37–48 (2007).
Postberg, F. et al. Macromolecular organic compounds from the depths of Enceladus. Nature 558, 564–568 (2018).
Waite, J. H. et al. Cassini finds molecular hydrogen in the Enceladus plume: Evidence for hydrothermal processes. Science 356, 155–159 (2017).
Matson, D. L. et al. Enceladus’ interior and geysers-possibility for hydrothermal geochemistry and N2 production. Lunar Planet. Sci. 37, 2219 (2006).
Nimmo, F. et al. Reorientation of Sputnik Planitia implies a subsurface ocean on Pluto. Nature 540, 94–96 (2016).
Kamata, S. et al. Pluto’s ocean is capped and insulated by gas hydrates. Nat. Geosci. 12, 407–410 (2019).
Elam, S. K., Tokura, I., Saito, K. & Altenkirch, R. A. Thermal conductivity of crude oils. Exp. Therm. Fluid Sci. 2, 1–6 (1989).
Sephton, M. A. & Hazen, R. M. On the origin of deep hydrocarbons. Rev. Mineral. Geochem. 75, 449–465 (2013).
Dias, F. & Maurette, M. Oil-bearing micrometeorites for an oily-dusty Panthlassa. Meteorit. Planet. Sci. 42, A36 (2007).
Maurette, M. & Brack, A. Cometary petroleum in Haden time? Meteorit. Planet. Sci. 41, A116 (2006).
Schmidt, C. & Ziemann, M. In-situ Raman spectroscopy of quartz: A pressure sensor for hydrothermal diamond-anvil cell experiments at elevated temperatures. Am. Mineral. 85, 1725–1734 (2000).
Edwards, W. F., Jin, L. & Thies, M. C. MALDI-TOF mass spectrometry: Obtaining reliable mass spectra for insoluble carbonaceous pitches. Carbon 41, 2761–2768 (2003).
Burgess, W. A., Pittman, J. J., Marcus, R. K. & Thies, M. C. Structural identification of the monomeric constituents of petroleum pitch. Energy Fuels 24, 4301–4311 (2010).
Radke, M., Leythaeuser, D. & Teichmuller, M. Relationship between rank and composition of aromatic hydrocarbons for coals of different origins. Org. Geochem. 6, 423–430 (1984).
Huang, H. P., Bowler, B. F. J., Oldenburg, T. B. & Larter, S. R. The effect of biodegradation on polycyclic aromatic hydrocarbons in reservoir oils from the Liaohe basin, NE China. Org. Geochem. 35, 1619–1634 (2004).
Asahina, K. & Suzuki, N. Methylated naphthalenes as indicators for evaluating the source and source rock lithology of degraded oils. Org. Geochem. 124, 46–62 (2018).
Saito, H., Suzuki, N. & Takahashi, U. K. Simultaneous and sensitive analysis of inorganic and organic gaseous compounds by pulsed discharge helium ionization detector (PDHID). Geochem. J. 46, 255–259 (2012).
Acknowledgements
We thank Dr. T. Yoshizawa and Mr. T. Hirose, Global Facility Center, Hokkaido University for performing mass analysis of oil and aqueous products. The authors thank four anonymous reviewers for constructive comments. This study was carried out under the Joint Research Program of the Institute for Planetary Materials, Okayama University and the Institute of Low Temperature Science, Hokkaido University. This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology Grants-in-Aid for Scientific Research (KAKENHI; grant JP25108002), and by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI; grant JP17H06087).
Author information
Authors and Affiliations
Contributions
H.N. and A.K. designed and directed the study. H.N., N.H, and Y.M. performed the heating experiments using the autoclave. S.Y., T.O., and N.H. performed the in-situ observations using the diamond anvil cell. T.H. conducted the mid-infrared spectroscopy. K.A., R.T., N.S., H.N., Y.T., Y.O., Y.K., N.W., and A.K. carried out the chemical analyses of the products. A.K., S.T., T.H., K.A., and Y.O. wrote the manuscript. All authors commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakano, H., Hirakawa, N., Matsubara, Y. et al. Precometary organic matter: A hidden reservoir of water inside the snow line. Sci Rep 10, 7755 (2020). https://doi.org/10.1038/s41598-020-64815-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64815-6
This article is cited by
-
Origin of life-forming volatile elements in the inner Solar System
Nature (2022)
-
Aqueous alteration without initial water: possibility of organic-induced hydration of anhydrous silicates in meteorite parent bodies
Earth, Planets and Space (2021)
-
The underexposed effect of elastic electron collisions in dusty plasmas
Communications Physics (2021)
-
Extraterrestrial hexamethylenetetramine in meteorites—a precursor of prebiotic chemistry in the inner solar system
Nature Communications (2020)
-
The NC-CC Isotope Dichotomy: Implications for the Chemical and Isotopic Evolution of the Early Solar System
Space Science Reviews (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.