Abstract
Precious coral species have been used to produce jewelry and ornaments since antiquity. Due to the high value and demand for corals, some coral beds have been heavily fished over past centuries. Fishing and international trade regulations were put in place to regulate fishing practices in recent decades. To this date, the control of precious coral exploitation and enforcement of trade rules have been somewhat impaired by the fact that different species of worked coral samples can be extremely difficult to distinguish, even for trained experts. Here, we developed methods to use DNA recovered from precious coral samples worked for jewelry to identify their species. We evaluated purity and quantity of DNA extracted using five different techniques. Then, a minimally invasive sampling protocol was tested, which allowed genetic analysis without compromising the value of the worked coral objects.The best performing DNA extraction technique applies decalcification of the skeletal material with EDTA in the presence of laurylsarcosyl and proteinase, and purification of the DNA with a commercial silica membrane. This method yielded pure DNA in all cases using 100 mg coral material and in over half of the cases when using “quasi non-destructive” sampling with sampled material amounts as low as 2.3 mg. Sequence data of the recovered DNA gave an indication that the range of precious coral species present in the trade is broader than previously anticipated.
Similar content being viewed by others
Introduction
Precious corals are among the most appreciated and oldest known gems. They are valued for their color, texture and workability (polishing, carving), and have thus been collected and used for adornment for millennia1,2,3. Growing demand, particularly in Asia in recent years, has led to an increase in prices of precious corals used in jewelry4,5,6.
The most valuable precious coral species belong to the Coralliidae family within the Octocorallia subclass of the Anthozoa. The precious coral material used for jewelry is the worked (i.e. cut, carved and polished) hard coral skeletal axis, which is a biogenic material created by a biomineralization process7. In this process, closely packed magnesium-rich calcite crystals are secreted by coral polyps (1–2 mm in size) to build up a skeleton over decades. The polyps can thrive on the surface of the skeleton as colonies connected and surrounded by a 0.5–1 mm thick surface tissue (coenenchyme)8. The Coral Commission of The World Jewellery Confederation (CIBJO) lists eight Coralliidae species as significant in the precious coral jewelry industry9,10. Precious coral products are sold worldwide, with production centers located in Italy, Japan and Taiwan and large-scale trade of raw material between these areas5,6,11.
Until recent decades, the populations of these highly coveted marine animals experienced exploitation in boom and bust cycles where the discovery of precious coral beds led to rushes by coral fishers and these beds were exploited as long as it remained economically feasible12,13. Local and international regulations were put in place to control both fishing and international trade of precious corals, among which four Pacific species were listed in Appendix III of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) at the request of China4,13,14,15 (Table 1). It has been reported that traders may often not be aware of the origin and species of their coral jewelry products4,6. At the same time, consumers and jewelers increasingly request specific information about precious corals, particularly their geographic origin and species, mainly due to the perceptions of value that different types of coral have in the market and possible sustainability considerations16.
Therefore, accurate taxonomic identification of precious coral products is of paramount importance for both efficient enforcement of precious coral trade regulations and for the jewelry industry. However, species of polished corals can be extremely difficult to distinguish even for trained experts based on morphological characteristics, and proper analytical tools to conclusively identify the species of worked precious corals are still lacking6,12,16,17.
The various analytical methods tested to distinguish precious coral species based on skeletal material were either unable to provide clear-cut distinction among the different coral species (e.g. trace element analysis, such as X-ray fluorescence spectroscopy, LA-ICP-MS and EMPA18; and Raman spectroscopy19), or were not improved to become a standardized and easy-to-use tool (such as immunolabeling20). As a novel approach, Cartier, et al.21 recently proposed DNA analysis to distinguish species, assuming that coral DNA molecules can be trapped in the organic material or adhered to the CaCO3 crystals during the formation of the skeleton.
Genetic analyses have become a powerful analytical tool to elucidate the species identity and trace the geographic origin of various valuable artefacts of biogenic origin. These include processed products of tortoise shell22, snake skin23, fur24,25, ivory26,27 or tiger bones28. Of greatest relevance to this present study, Meyer, et al.29 reported quasi-nondestructive species identification of pearls based on DNA analysis, where so little amount of pearl material was used for the analyses that the market value of the pearl was not compromised. Particular biogenic materials require specific DNA extraction methods, moreover, we anticipate that DNA preserved in precious coral skeletons to be present in very small amounts and highly fragmented due to the lengthy skeleton-formation process and the degradation of the DNA after the death or the coral30,31,32,33. A further challenge of using DNA to distinguish Coralliidae species may arise from the exceptionally slow evolution of the Octocorallia mitochondrial genomes, which causes different species to be genetically highly similar34,35,36,37,38.
In the present proof of concept study, we aim to explore whether precious coral skeleton fragments cut, carved and polished for jewelry can be taxonomically identified through genetic analysis. We compare five different DNA extraction methods to find the method producing the highest purity and quantity of DNA. We then apply the most successful DNA extraction technique using a minimally destructive sampling method and amplify and sequence the recovered DNA to taxonomically identify the coral samples. We demonstrate that genetic analysis of gem-quality precious corals is a promising method to assess the identity of their species.
Results
Comparison of DNA retrieved from worked precious corals with five extraction methods
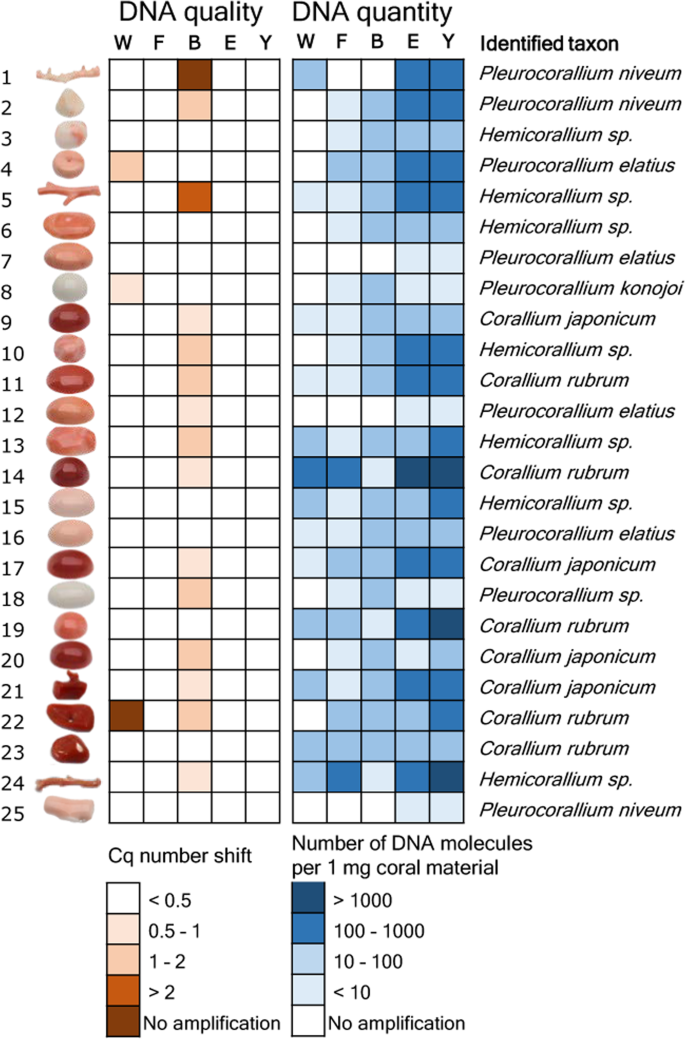
Using a set of 25 worked coral samples, we evaluated which one of five candidate DNA extraction protocols is most suited to retrieve DNA from worked precious coral samples. Each of the five tested methods (abbreviated as “W”, “F”, “B”, “E”, “Y”) have earlier proven to be useful in extracting DNA from biomineralized material29,39,40,41,42,43,44,45,46,47,48. DNA was extracted from each of the 25 worked coral skeletal samples with all five techniques, and DNA purity and quantity were assessed using real-time quantitative PCR (qPCR) technology.
To test DNA extract purity, we assessed PCR inhibition with qPCR using an internal amplification control molecule. Three extraction methods, “F”, “E” and “Y”, resulted in DNA with no detectable PCR inhibition effect from any of the tested 25 samples (Fig. 1, Supplementary Results S1). In contrast, a PCR inhibition effect was observed in 15 out of 25 samples extracted with the “B” method. Of these, complete inhibition of the PCR was observed in one case. Inhibition was also detected in three DNA extracts produced with the “W” method. Of these, no PCR product was observed at all in one sample.
Results of the DNA extract purity and quantity measurement experiment and taxonomic identification of 25 worked precious coral samples. Five methods were used to extract DNA from equal amounts of material from each sample. PCR inhibition measurement and absolute template quantification was performed with quantitative real-time PCR. Two short mitochondrial DNA fragments were sequenced and each specimen was taxonomically assigned. Note that identifications as Corallium japonicum, Pleurocorallium elatius or P. konojoi were possible based on the combination of genetic and morphological assessments.
Absolute quantity of the DNA obtained with the five extraction techniques was tested using qPCR with a standard curve from a dilution series of a standard template DNA molecule with known concentrations. Throughout these analyses, the average qPCR efficiency was 88.5% (± 3.6% standard deviation) and the coefficient of determination for the calibration curve was R2 = 0.9947 (± 0.0035 standard deviation).
The five extraction methods yielded highly varying amounts of DNA (Fig. 1, Supplementary Results S1.). Methods “E” and “Y” both yielded PCR amplifications for all 25 samples. Method “W” yielded PCR product for 13 samples, while methods “F” and “B” both yielded PCR product for 21 samples. Overall, there was concordance among the amplification results; the 13 samples that amplified with method “W” also amplified with methods “F” and “B”, and the latter two methods amplified DNA of the very same 21 samples. Strong significant correlation was found between the copy numbers obtained from the same coral items with the “E” and “Y” methods (r = 0.97, t = 19.223, df = 23, p < 0.001). DNA yield was higher with method “Y” than with method “E” (595 versus 944 molecules per mg coral sample with “E” and “Y”, respectively; paired t-test: t = −2.8832, df = 24, p = 0.008). Focusing on the best performing “Y” method, DNA concentrations ranged between three orders of magnitude: three samples had over 103 DNA copies in each mg of coral skeleton material. In five other samples this value was below 10 (Fig. 1).
DNA extraction with “quasi non-destructive” sampling of worked precious coral samples
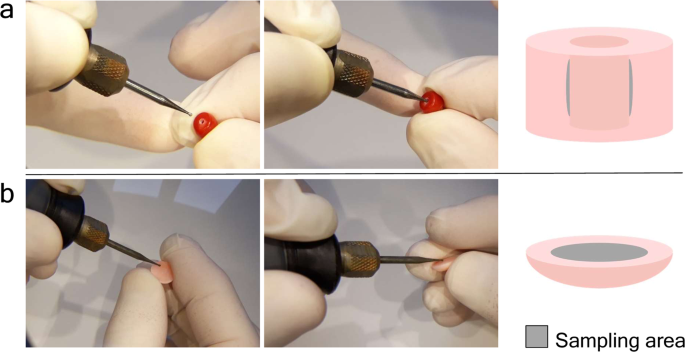
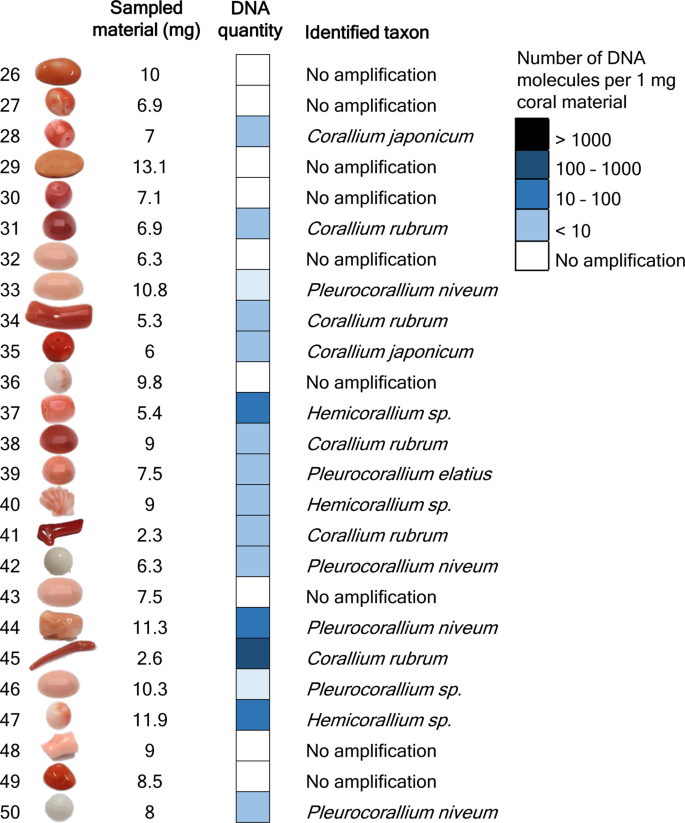
In the previous experiment, 25 samples were completely pulverized and five DNA extractions were carried out with different methods from each. The aim was to select the most suitable technique for extracting DNA from worked coral samples. In the current experiment, the best performing DNA extraction technique was used with “quasi non-destructive” sampling of worked corals. We developed a “quasi non-destructive” technique to take material for analysis from the worked corals with minimal weight loss and virtually invisible effects of the sampling (Fig. 2). A new set of 25 worked coral samples were sampled in this manner; removed material amounts ranged from 2.3 mg to 13.1 mg and were 7.9 mg on average. Modifications were applied to the lysis step of the “Y” extraction method compared to the original protocol, which resulted in an essentially complete dissolution of the coral powder. This allowed the amount of DNA that remained trapped in the undissolved powder to be kept to a minimum. Out of the 25 “quasi non-destructively” sampled worked coral objects, 16 gave qPCR amplicons at least twice (Fig. 3, Supplementary Results S1). Another two samples produced amplification only once and were omitted from further analyses. DNA copy numbers calculated per mg of coral sample were in the same range as in the case of the extractions carried out from ca. 100 mg material using the “Y” method. However, the presence of unsuccessful amplifications and lower average copy number (160 DNA copies) recovered per mg of coral skeletal material indicates that DNA recovery from low amount samples is less effective than from standard material amount, despite the amendments made in the DNA extraction protocol.
Results of DNA quantity measurement and taxonomic identification of 25 worked precious corals sampled by the minimally invasive technique. Absolute template quantification was performed with quantitative real-time PCR. Two short mitochondrial DNA fragments were sequenced and each specimen was taxonomically assigned. Note that identifications as Corallium japonicum, Pleurocorallium elatius or P. konojoi were possible based on the combination of genetic and morphological assessments.
Taxonomic assignment of worked precious corals
We sequenced amplicons of the large ribosomal RNA gene subunit (LR) and the putative mismatch repair protein (MSH) fragments originating from a total of 41 worked coral skeletal samples using massively parallel sequencing. In our entire DNA sequence dataset, the sequence of altogether three OTUs were highly divergent from any of the Coralliidae LR or MSH reference sequences. NCBI BLAST search did not find any sequence entries in the NCBI database with higher than 95% sequence similarity for any of these sequences.
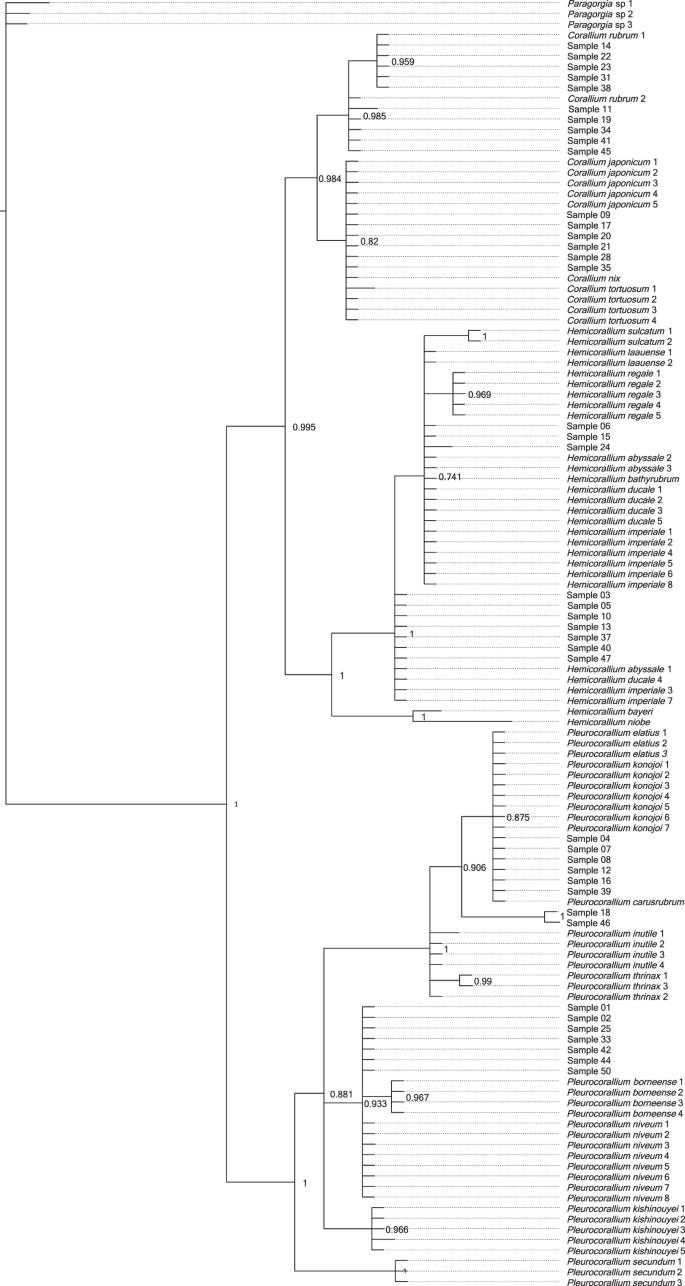
The lengths of the concatenated LR and MSH sequences were between 264 base-pairs (bp) and 290 bp long per coral sample (Supplementary Results S2). Bayesian phylogenetic analysis identified 10 samples (11, 14, 19, 22, 23, 31, 34, 38, 41, 45) as Corallium rubrum, of which nine had sequences identical to either of two the reference C. rubrum sequences, and one (11) had a single variable site (Fig. 4). Six samples (9, 17, 20, 21, 28, 35) were identical with reference samples of Corallium japonicum, but also with the reference samples of C. nix and C. tortuosum.
Three samples (6, 15, 24) formed a polytomic clade with Hemicorallium reference sequences. Two of these (6, 15) had sequences identical to Hemicorallium laauense, but also to samples of H. abyssale, H. bathyrubrum, H. ducale and H. imperiale. The third sample (24) was one bp different from these sequences. Seven samples (3, 5, 10, 13, 37, 40, 47) with identical sequences appeared as an unresolved clade basal to the formerly mentioned samples. These had identical sequences with H. abyssale, H. ducale and H. imperiale.
Six samples (4, 7, 8, 12, 16, 39) had identical sequences with Pleurocorallium carusrubrum, P. elatius and P. konojoi reference samples. Two samples (18, 46) formed a sister clade to the former group with the posterior probability value 1. Finally, seven identical samples (1, 2, 25, 33, 42, 44, 50) were same as sequences of Pleurocorallium niveum. These were grouped together as an unresolved tree branch.
Discussion
Technical advancements and the growing body of reference DNA data have made genetic analyses a powerful tool to combat poaching, illegal trading and mislabeling of animal products49. Application of genetic barcoding was suggested by Ledoux, et al.50 as a forensic tool to identify species of corals. Acknowledging that the discriminatory power of standard species barcoding markers (e.g. the cytochrome c oxidase subunit I gene) is poor to distinguish the closely related precious coral species, these authors suggested development of custom designed species identification markers. Moreover, if the aim is to distinguish coral skeletal samples, then the high portion of fragmented DNA will call these markers to be as short as possible. A further challenge is if sampling of the coral sample is to be done with minimal material loss. As a consequence, the chosen DNA extraction method has to be capable of recovering DNA from a small sample amount.
In our quest to find an optimal method to recover DNA from worked coral samples, we tested the performance of five DNA extraction methods, each on equal amounts of coral material from the same set of 25 worked coral samples. We found two methods, protocol “E” and “Y” that yielded DNA that was successfully amplified and sequenced from all of the 25 tested corals. Methods “E” and “Y” are two similar techniques developed for the extraction of DNA from ancient eggshells and ancient bones. They only slightly differ in their lysis buffer ingredients and the type of DNA-binding silica column used for the purification of the recovered DNA molecules45,46. These methods produced similar amounts of DNA, however method “Y” produced slightly higher DNA yield, particularly in the samples that had <50 DNA copies per mg of coral powder. The three other tested DNA extraction methods did not result in amplifiable DNA from all samples, which may be due to their inability to recover DNA coupled with PCR-inhibitory effect of co-extracted substances, which was detected in some extracts, PCR inhibition was not detected in any extracts produced with methods “W”, “E” and “Y”. By using these methods, PCR inhibition seems to be overcome in precious corals, unlike in other types of corals, where it led to technical challenges51.
DNA concentration of the extracts differed largely; while in certain samples <10 copies per mg of material was recovered, in some others this reached up to the order of magnitude of 103 copies per mg of material. The large variation in DNA preservation of the samples may be determined by several factors; the age of the coral when fished, whether the coral was fished dead or alive30,31,32 and the time since the coral was fished6. However, without specific knowledge about the age of the samples this remains hypothetical.
Our test to choose the best DNA extraction protocol from potential methods was based on 100 mg of coral skeleton material, which is a standard amount used for extracting DNA from pulverized material with the applied protocols. The essence of precious material testing would be to use as little material as possible, ideally using a “quasi non-destructive” sampling method. This means that the sampling area is not visible and the sampling does not cause significant weight loss of the coral object. Worked coral samples can be separated into two main types; the ones that have a hole drilled through the item (generally those that are strung as beads for bracelets or necklaces) and the ones that do not have a hole, instead generally have flat reverse or bottom sides (those that are mounted to a frame and used as pendants, i.e. cabochons, or the carved figures used as ornaments). We performed “quasi non-destructive” sampling using a drill with a 0.8 mm diameter diamond engraver head taking care not to heat up the sampled object (no hard pressing of the drill and regular pauses to let the drill head cool down). With careful handling, it was possible to take sample material by slightly widening the internal surface of the ca. 1 mm wide drill-holes, which ensures that this material extraction is invisible by eye upon subsequent inspection of the sample. From the cabochons, a thin layer was removed from the reverse side; therefore the visible front side remains unaffected by the sampling. Assuming approximately 3.8 kg/dm3 density of the precious corals9, the removed 2.3–13.1 (on average 7.9) mg powder per sample corresponds to a 0.7–3.5 (2.1) mm3 volume loss of the items.
We were able to repeatedly produce PCR products for 16 out of the 25 “quasi non-destructively” sampled worked coral samples. We could not determine a threshold for the minimum amount of material necessary for successful genetic testing; the two samples processed with the lowest weight of coral powder, 2.3 mg and 2.6 mg, respectively, both produced results. Although it was not possible to genetically analyze all samples with the minimally destructive method, there might be a good chance that when analyzing several samples from a batch of samples, at least some will produce results.
We expected that all of the DNA sequences we generated will be identical with at least one reference sequence of the eight species listed by CIBJO as relevant in the jewelry industry9. However, against our expectations, we found a much higher diversity within our samples, with several of our sequences not grouping together with any of the reference sequences of the eight species. Hence, we performed an other phylogenetic analysis with a more extended reference sample set. The results of this analysis show that samples could clearly be identified as Corallium rubrum. The samples grouping together with Corallium japonicum also grouped together with two other species, C. nix and C. tortuosum, which, however, have white and pink color, respectively, unlike the dark red color of C. japonicum44,52. Hence, we can confidently identify these red corals as C. japonicum based on the combination of genetic and morphological characteristics.
Samples that grouped together with the Hemicorallium references all had identical sequences with multiple Hemicorallium species. As a consequence, these samples could be identified only to the genus level as Hemicorallium. A part of these samples (i.e. 3, 5, 10, 13, 37, 40, 47) did not cluster with the three reportedly fished Hemicorallium species (H. laauense, H regale, H. sulcatum), but instead had identical sequences to other species (H. abyssale, H. ducale and H. imperiale) that all occur around the Hawaii islands, a historically important fishing area44,45,47. This result strongly suggests that H. laauense, H regale and H. sulcatum are not the only Hemicorallium species present in the jewelry trade.
Some samples had identical sequences as the three genetically and morphologically, very similar species, Pleurocorallium, P. carusrubrum, P. elatius and P. konojoi47,53. Of these species, the latter two are well known in the jewelry industry, while the former is a recently described species known from a single area of the West Pacific53. To distinguish these species, the coloration of the skeletal axis may provide a partial solution. In particular, the color of P. carusrubrum is red, P. elatius varies from pale to dark pink, while P. konojoi is always pure white4,6,9,10,17. Consequently, our specimens identified as one of these species with pink shading may be identified as P. elatius, while our samples with white color are determined as P. konojoi.
Of our multiple samples within the Pleurocorallium clade that did not group together with the species traditionally accepted as being present in the coral trade (P. elatius, P. konojoi and P. secundum), two samples (18, 46) formed an individual clade and were identified to the genus level as Pleurocorallium. DNA sequences of the other samples were all identical with the sequences of the Pleurocorallium niveum samples. This species was described from waters surrounding the Hawaii islands, which was a historically important coral fishing area54,55. The 41 samples that we managed to genetically analyze from 50 samples of a single collection is not representative enough to be able to draw conclusions about the entire jewelry industry, but it indicates that there may be more species present in the trade than the eight precious coral species commonly listed as part of the jewelry industry (cf9,10,16.). This is conceivable, if we consider that in the Pacific Ocean different precious coral species may co-occur and coral fishing does not seek to individually separate them based on species. The presence of more than the previously anticipated eight species also implies that accurate species identification in all cases will only be possible using markers that can differentiate among all species within the Coralliidae family.
Conclusions
This study is a proof of concept demonstrating that genetic analysis can be an effective tool to taxonomically identify precious corals worked for jewelry. We demonstrated that while 100 mg coral skeletal material is sufficient for successful DNA extraction in all cases, DNA sequencing and taxonomic assignment were possible with minute amounts of “quasi non-destructive” samples in more than half of the cases. Among the worked precious corals examined in this study, DNA sequence analyses revealed several samples very likely belonging to precious coral species previously not considered to be present in the jewelry industry. Future research should focus on broadening the reference data by sequencing multiple specimens for each species identified by experts in order to substantiate their intra- and interspecific genetic diversity. Additionally, the development of more specific markers will allow for the identification of coral samples with higher accuracy. These will be essential steps in developing genetic tests that can become a reliable and standardized method to promote transparency, traceability and sustainable use of precious corals in the jewelry industry.
Materials and Methods
Studied species
The precious corals relevant to the high-end jewelry industry are Octocorallid Anthozoans that belong to the Alcyonacea order and Coralliidae family. Recent phylogenetic studies confirmed the existence of three genera in the family; Corallium, Hemicorallium and Pleurocorallium56,57. Of the eight species listed by CIBJO as significant in the precious coral industry, a single, Corallium rubrum, is distributed in the Mediterranean Sea and has been fished since antiquity6. Four other species, Corallium japonicum, Hemicorallium sulcatum, Pleurocorallium elatius and Pleurocorallium konojoi have been fished in the Western Pacific ocean since the early 19th century12. The remaining three species, Hemicorallium laauense, Hemicorallium regale and Pleurocorallium secundum were discovered on seamounts surrounding the Hawaii archipelago and were fished in large quantities during the second half of the 20th century58. Distribution, CITES listing and trade names of the eight precious coral species relevant to the jewelry industry are summarized in Table 1, while further details on their distribution, taxonomy, harvesting and conservation are available in Cannas, et al.59.
Genetic markers used in the study
We expected that the DNA extracted from the coral skeletal samples would be highly degraded. Therefore, we used markers developed on the mitochondrial genome, which is present in each cell in multiple copies and offers the best chances of achieving positive results for fragmented DNA. Octocoral mitochondrial genomes have an exceptionally low rate of evolution and standard taxonomic markers are unable to distinguish closely related species34,38,60. Hence, we designed primers for two genetic markers with the criteria that the resulting amplicon sequences are short enough to be suitable for degraded DNA and highly variable in order to maximize our ability to identify the precious coral species to the lowest possible taxonomic level. We expected each analyzed sample to originate from one of the eight precious coral species listed by CIBJO, thus chose our markers with the aim that they should be capable of distinguishing these eight species. The two mitochondrial markers were developed based on DNA sequence data of Tu, et al.57, which is the most detailed study on precious coral phylogeny to date. Marker selection and procedures for designing PCR primers are detailed in Supplementary Methods S3.
Following examination of the phylogenetic resolution of multiple short mitochondrial genome fragments, we developed two sets of primers for the large ribosomal RNA gene subunit (LR gene, LR-F 5′TTCATCACAGTGAGGGTTTGT3′ and LR-R 5′TGCAAAGAAGGAGAACAAAAGG3′) and the putative mismatch repair protein (MSH gene, MSH-F 5′CGAAAGCGGATAAAAGCTACC3′ and MSH-R 5′CCTCACTGTCAGGCTAATGAG3′), respectively. The LR marker was used for the assessment of DNA purity and DNA quantification. Phylogenetic analysis using the combined LR and MSH markers showed that these two short markers were able to reconstruct the phylogenetic relationships obtained by much longer sequences, and they allowed the distinction of each of the eight precious coral species from each other, except for Pleurocorallium elatius and P. konojoi, It is not possible to conclusively distinguish these two species based on the data of Tu, et al.57 (Supplementary Methods S3).
Comparison of DNA purity and quantity extracted with different methods
DNA extraction
All laboratory work was carried out at the Forensic Genetics department of the Zurich Institute of Forensic Medicine, University of Zurich, in the laboratory facility dedicated to human and animal forensic casework. We strictly adhered to the ISO 17025 guidelines throughout the laboratory workflow with stringent rules to avoid contamination and authenticate our results (Supplementary Methods S4). Precious coral samples used in this study originated from the collection of the Swiss Gemmological Institute SSEF, Basel, Switzerland.
Twenty-five worked coral samples were selected for the experiment (named samples 1–25, Supplementary Table S5). The samples were cleaned as described in Supplementary Methods S4 and crushed in a metal mortar with a metal pistil to produce crude coral powder, which was then transferred to a porcelain mortar and ground to fine powder. The coral skeleton powder was divided into five aliquots of equal weight, 100 mg ± 1 mg in general, except for four samples that had less available powder (Supplementary Table S5). The powder aliquots were used to extract DNA using five different extraction methods, which have proven to be effective in successfully recovering DNA from biomineralized material (Table 2). For each method, we followed the protocols cited in Table 2. All DNA extracts were eluted in 100 µl and stored at −20 °C.
Assessment of the purity of the DNA extracts
We used qPCR to compare the purity of the DNA extracts produced from worked precious coral samples with five different extraction protocols. DNA purity was measured by testing the PCR inhibiting effect of the coral extracts during amplification of an internal positive control DNA fragment. We used 103 copies of a synthetic oligonucleotide (gBlocks Gene Fragments; International DNA Technologies, Coralville, IA, USA61) as internal amplification control (IAC, Supplementary Methods S6). The 197 bp sequence of the IAC matched 151 bp of the C. rubrum LR gene fragment (with manual introduction of five unique mismatches for contamination detection purpose) flanked by potato-specific sequences as primer sites following Nolan, et al.62.
Following optimization (see Supplementary Methods S6), reactions were conducted in 20 µl volumes containing 1 × PowerUp SYBR Green Master Mix (Thermo Fisher), 1 µl of both 15 uM concentration primers, 103 copies of the AIC in 3 µl and 3 µl coral DNA extract. Alongside the samples containing coral DNA extracts, we run three positive standard reactions that did not contain coral DNA. Following the manufacturer’s recommendation, reactions commenced with 50 °C for 2 minutes, which was followed by initial denaturation at 95 °C for 2 minutes and 50 cycles of denaturation at 95 °C for 15 seconds, primer annealing at 60 °C for 15 seconds and elongation at 72 °C for 1 minute. A melting-curve analysis was performed at the end of the reaction by heating the PCR products from 60 °C to 95 °C with 1% ramping speed. Each coral extract was run in triplicates on an ABI 7500 qPCR instrument (Thermo Fisher).
The quantification cycle (Cq) value of each reaction containing coral DNA extract was compared to the average Cq value of the three positive standard reactions and then the three Cq shift values of each sample were averaged. The intensity of PCR inhibition in each reaction was determined as follows: we considered inhibition to be present if there was a 0.5< cycle Cq shift compared to the positive standard Cq. Four categories of PCR inhibition were considered: 0.5–1, 1–2, 2<cycle shifts and complete inhibition in case at least one out of the three reactions produced no PCR product.
Absolute DNA quantification of the coral DNA
Absolute quantification of the coral LR gene fragment was conducted by qPCR of the coral DNA using a calibration curve prepared as a series of standard reactions with a known template DNA amount. The standards contained seven different 10-fold diluted template inputs (107–101 copies) of a GBlocks synthetic oligonucleotides of the 154 bp long sequence of the LR gene fragment characteristic to C. rubrum (with manual introduction of three unique mismatches for the purpose of contamination detection) flanked by the LR primer sequences (Supplementary Methods S6). Following optimization of the reaction setup (Supporting Methods S6), reactions were carried out in 20 µl volumes containing 1 × PowerUp SYBR Green Master Mix (Thermo Fisher), 1 µl of both 15 µM concentration primers and 3 µl coral DNA extract. The cycling conditions were identical to those of the DNA extract purity test.
For each sample, PCR was considered successful if at least two reactions of the triplicates amplified. The Ct values were averaged for each sample and the mean Ct values were transformed to number of DNA molecules per mg of coral sample based on the volume of the DNA template in the PCR reaction, the DNA extract elution volume and the amount of coral powder used for the DNA extraction. We compared the DNA quantities gained with the extraction methods for which DNA was successfully amplified for all 25 samples with a correlation test and paired t-test in R63.
“Quasi non-destructive” sampling, DNA extraction and quantification
We define “quasi non-destructive” sampling as taking material for analysis from the worked objects without compromising its market value. A new set of 25 worked coral samples were selected from the SSEF coral collection for this experiment (named samples 26–50, Supplementary Table S7), and each was thoroughly cleaned as described in Supporting Methods S4. Two main types of samples were sampled differently: (i) beads with drill-holes: the inner surface of the drill-hole was carefully widened (Fig. 2a); (ii) worked items with no existing drill-hole: a small layer of the surface of the back side of a cabochon was removed (Fig. 2b). We used 0.8 mm diameter diamond engraver bit heads attached to a Dremel 4000–4 rotary tool (Dremel, Racine, WI, USA). The rotation speed was set to 10,000 rpm and the extracted coral powder was left to drop in 1.5 ml collection tubes.
DNA was extracted from the quasi non-destructively sampled drill-powder of the 25 samples with the “Y” method. The material amount obtained by the “quasi non-destructive” sampling was far lower than the 100 mg used in the experiment comparing extraction methods, therefore we slightly modified the “Y” protocol to accommodate it to the low material amount. In particular, 200 µl lysis buffer was added to the coral powder and incubated at 56 °C for one hour with mixing, then another 100 µl lysis buffer was added. The lysis-mixture was incubated again with mixing at 56 °C for one hour and then at 37 °C for an additional 65 hours. In the next step, the lysate was mixed with 450 µl 1 × TE buffer and 3750 µl PB buffer (Qiagen) and the entire volume of the mixture was centrifuged through a MinElute (Qiagen) column. The column was washed with PE buffer and the DNA was eluted in 35 µl EB buffer (Qiagen).
Taxonomic identification
DNA amplification and sequencing
We sequenced PCR products of DNA samples extracted with the “Y” method. For the LR fragment, qPCR products generated for the DNA quantity assessment were sequenced: from each sample one of the triplicate qPCR was selected for sequencing. The MSH region was amplified and sequenced for altogether 41 DNA samples: all 25 DNA samples from the DNA extraction test and those 16 DNA extracts from the “quasi non-destructive” sampling that gave amplification products for the LR region. The MSH was amplified in singlicate for each sample with identical reaction setup and cycling conditions as described above for the LR region.
The 16S and MSH PCR products were purified with the AMPure bead system (Beckman Coulter, Brea, CA, USA) and quantified with a Qubit 4 Fluorimeter (Thermo Fisher). The two amplicons of each DNA sample were pooled with equimolar concentrations, and sequencing libraries were constructed with the Ion Plus Fragment Library Kit (Thermo Fisher) according to the vendor’s protocol. The libraries were quantified with the Ion Library TaqMan Quantitation Kit (Thermo Fisher) and all samples were pooled with equimolar concentrations. Sequencing was carried out on an Ion S5 (Thermo Fisher) instrument at the Zurich Institute of Forensic Medicine, University of Zurich.
Analysis of the amplicon sequence data
Raw DNA sequence read data was exported to fastq files according to sequencing barcodes with the FileExporter plugin of the Torrent Suite software version 5.10. Primer sequences were removed from the end of the sequences of each fastq file using the cutadapt algorithm64 implemented on the Galaxy server65. Trimmed sequences were quality-filtered using Usearch66 with a maximum expected error threshold of 100 and clustered into operational taxonomic units (OTUs) with Uparse67 at 97% minimal identity threshold and minimal OTU size of 10 sequence reads, as default settings. In some cases, these settings were slightly modified for more relaxed quality filtering and clustering to allow OTU creation for samples with lower quality sequence reads. Sequences of the resulting LR and MSH OTUs were aligned and the alignments were concatenated in Geneious version 11.1.5 (https://www.geneious.com). Our concatenated LR-MSH sequence alignment was added to the LR-MSH alignment of reference samples of the eight precious coral species listed in Table 1. The taxonomic identity of our sequences was determined by constructing a Bayesian phylogenetic tree as described in Supporting Methods S2. We noticed that several of the DNA sequences obtained from the coral samples were not identical with any of the reference sequences of the eight precious coral species described to be found in the international trade. We therefore performed an additional phylogenetic analysis with identical settings, which included the orthologous LR-MSH DNA sequences of all Coralliidae specimens from Tu, et al.47 that were identified to the species level (Supplementary Table S8).
Data availability
Raw DNA sequence data generated for this study are deposited in the NCBI Sequence Read Archive under submission number SUB6412194. Data used for the analyses is available as Supplementary Information.
References
Fürst, S. et al. Raman investigations to identify Corallium rubrum in Iron Age jewelry and ornaments. Minerals 6, 56 (2016).
Moradi, Z. The role of coral in art and architecture. An overview. International Journal of Aquatic Biology 4, 125–142 (2016).
Skeates, R. Mediterranean coral: its use and exchange in and around the alpine region during the later Neolithic and copper age. Oxford Journal of Archaeology 12, 281–292 (1993).
Shiraishi, H. Seeing red. Precious coral trade in East Asia. (TRAFFIC Office Japan) (2018).
Chang, S.-K. Precious corals become more precious in the northwestern pacific: Urgent need for integrated policy. Marine Policy 52, 103–107 (2015).
Torntore, S. J. Precious corals in a global marketplace. in Proceedings of the first international workshop on Corallium science, management, and trade. (ed AW Bruckner & GG Roberts) 34–58 (NOAA Technical Memorandum NMFS-OPR-43 and CRCP-8) (2009).
Perrin, J. et al. Block-by-block and layer-by-layer growth modes in coral skeletons. American Mineralogist 100, 681–695 (2015).
Nonaka, M., Muzik, K. & Iwasaki, N. Descriptions of two new species and designation of three neotypes of Japanese Coralliidae from recently discovered specimens that were collected by Kishinouye, and the introduction of a statistical approach to sclerite abundance and size. Zootaxa 3428, 1–67 (2012).
CIBJO. The coral book. (Coral Commission of The World Jewellery Confederation) (2015).
CIBJO. Coral guide for customes. Classification & identification of coral materials. (The World Jewellery Confederation) (2017).
Cattaneo-Vietti, R. et al. An overexploited Italian treasure: past and present distribution and exploitation of the precious red coral Corallium rubrum (L., 1758) (Cnidaria: Anthozoa). Italian Journal of Zoology 83, 443–455 (2016).
Tsounis, G. et al. The exploitation and conservation of precious corals. Vol. 48 (CRC Press) (2010).
Bruckner, A. W. Advances in management of precious corals in the family Corallidae: are new measures adequate? Current Opinion in Environmental Sustainability 7, 1–8 (2014).
CITES. Convention on International Trade in Endangered Species of Wild Fauna and Flora. Checklist of CITES Species. www.checklist.cites.org Accessed: 04.07.2019 (2019).
Cau, A., Cannas, R., Sacco, F. & Follesa, M. Adaptive management plan for red coral (Corallium rubrum) in the GFCM competence area. (University of Cagliari) (2013).
de Carvalho, R. G. Precious corals. InColor. A publication of the International Colored Gemstone Association 37, 70–78 (2018).
Cooper, E. W., Torntore, S. J., Leung, A. S., Shadbolt, T. & Dawe, C. Guide to the identification of precious and semi-precious corals in commercial trade. (TRAFFIC North America and WWF-Canada) (2011).
Hasegawa, H., Rahman, M. A., Luan, N. T., Maki, T. & Iwasaki, N. Trace elements in Corallium spp. as indicators for origin and habitat. Journal of Experimental Marine Biology and Ecology 414, 1–5 (2012).
Macchia, M., Resta, V., Quarta, G. & Calcagnile, L. Precious coral non-destructive characterization by Raman and XRF spectroscopy. X-Ray Spectrometry 45, 281–287 (2016).
Debreuil, J. et al. Specific organic matrix characteristics in skeletons of Corallium species. Marine Biology 158, 2765–2774 (2011).
Cartier, L. E., Krzemnicki, M. S., Lendvay, B. & Meyer, J. B. DNA fingerprinting of pearls, corals and ivory: a brief review of applications in Gemmology. Journal of Gemmology 36, 152–160 (2018).
Foran, D. R. & Ray, R. L. Mitochondrial DNA profiling of illegal tortoiseshell products derived from hawksbill sea turtles. Journal of Forensic Sciences 61, 1062–1066 (2016).
Dubey, B., Meganathan, P. & Haque, I. DNA mini-barcoding: an approach for forensic identification of some endangered Indian snake species. Forensic Science International: Genetics 5, 181–184 (2011).
Pilli, E. et al. Pet fur or fake fur? A forensic approach. Investigative Genetics 5, 7 (2014).
Janjua, S., Fakhar-I-Abbas, William, K., Malik, I. U. & Mehr, J. DNA Mini-barcoding for wildlife trade control: a case study on identification of highly processed animal materials. Mitochondrial DNA Part A 28, 544–546 (2017).
Kitpipit, T., Thongjued, K., Penchart, K., Ouithavon, K. & Chotigeat, W. Mini-SNaPshot multiplex assays authenticate elephant ivory and simultaneously identify the species origin. Forensic Science International: Genetics 27, 106–115 (2017).
Winters, M. et al. Isolation of DNA from small amounts of elephant ivory: Sampling the cementum with total demineralization extraction. Forensic Science International 288, 131–139 (2018).
Kitpipit, T., Tobe, S. S., Kitchener, A. C., Gill, P. & Linacre, A. The development and validation of a single SNaPshot multiplex for tiger species and subspecies identification—Implications for forensic purposes. Forensic Science International: Genetics 6, 250–257 (2012).
Meyer, J. B. et al. DNA fingerprinting of pearls to determine their origins. PloS One 8, e75606 (2013).
Chen, C.-S. Management of the precious coral fishery in Taiwan: progress and perspectives. Marine Policy 36, 623–629 (2012).
Huang, M.-H. & Ou, C.-H. Precious coral fisheries management in Taiwan—Past, present & future. Marine Policy 34, 1002–1009 (2010).
Okumura, T. 14C dating of precious corals in Kochi for understanding the fishing field formation processes. in International precious coral conference (Kochi, Japan) (2018).
Iwasaki, N. Precious coral fishery in Japanese history since World War II: issues and visions for sustainable use of resources. in The academic pilgrimage to sustainable social development. Vol. 1 225-258 (Rissho University) (2018).
Shearer, T., Van Oppen, M., Romano, S. & Wörheide, G. Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Molecular Ecology 11, 2475–2487 (2002).
Uda, K. et al. Complete mitochondrial genomes of the Japanese pink coral (Corallium elatius) and the Mediterranean red coral (Corallium rubrum): a reevaluation of the phylogeny of the family Coralliidae based on molecular data. Comparative Biochemistry and Physiology, Part D 8, 209–219 (2013).
Takata, K. et al. Multiplexed ISSR genotyping by sequencing distinguishes two precious coral species (Anthozoa: Octocorallia: Coralliidae) that share a mitochondrial haplotype. PeerJ 7, e7769 (2019).
McFadden, C. S. et al. Limitations of mitochondrial gene barcoding in Octocorallia. Molecular Ecology Resources 11, 19–31 (2011).
Hellberg, M. E. No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evolutionary Biology 6, 24 (2006).
Chatters, J. C. et al. Late Pleistocene human skeleton and mtDNA link Paleoamericans and modern Native Americans. Science 344, 750–754 (2014).
Villanea, F. A., Parent, C. E. & Kemp, B. M. Reviving Galápagos snails: Ancient DNA extraction and amplification from shells of probably extinct endemic land snails. Journal of Molluscan Studies 82, 449–456 (2016).
Stray, J. et al. Extraction of high quality DNA from biological materials and calcified tissues. Forensic Science International: Genetics Supplement Series 2, 159–160 (2009).
Hasap, L. et al. Comparison of two DNA extraction methods: PrepFiler® BTA and modified PCI-silica based for DNA analysis from bone. Forensic Science International: Genetics Supplement Series 7, 669–670 (2019).
Oskam, C. L. et al. Fossil avian eggshell preserves ancient DNA. Proceedings of the Royal Society of London B: Biological Sciences 277, 1991–2000 (2010).
Huynen, L., Gill, B. J., Millar, C. D. & Lambert, D. M. Ancient DNA reveals extreme egg morphology and nesting behavior in New Zealand’s extinct moa. Proceedings of the National Academy of Sciences 107, 16201–16206 (2010).
Oskam, C. L. & Bunce, M. DNA extraction from fossil eggshell. in Ancient DNA. Methods and protocols. (eds Beth Shapiro & Michael Hofreiter) 65-70 (Springer) (2012).
Gamba, C. et al. Comparing the performance of three ancient DNA extraction methods for high‐throughput sequencing. Molecular Ecology Resources 16, 459–469 (2016).
Der Sarkissian, C. et al. Ancient DNA analysis identifies marine mollusc shells as new metagenomic archives of the past. Molecular Ecology Resources 17, 835–853 (2017).
Der Sarkissian, C. et al. Unveiling the ecological applications of ancient DNA from mollusk shells. Frontiers in Ecology and Evolution 8, 37 (2020).
Iyengar, A. Forensic DNA analysis for animal protection and biodiversity conservation: a review. Journal for Nature Conservation 22, 195–205 (2014).
Ledoux, J.-B. et al. Molecular forensics into the sea: how molecular markers can help to struggle against poaching and illegal trade in precious corals? in The cnidaria, past, present and future (eds Stefano Goffredo & Zvy Dubinsky) 729-745 (Springer (2016).
Weber, L., DeForce, E. & Apprill, A. Optimization of DNA extraction for advancing coral microbiota investigations. Microbiome 5, 18 (2017).
Bayer, F. M. Three new species of precious coral (Anthozoa: Gorgonacea, genus Corallium) from Pacific waters. Proceedings of the Biological Society of Washington 109, 205–228 (1996).
Tu, T.-H., Dai, C.-F. & Jeng, M.-S. Precious corals (Octocorallia: Coralliidae) from the northern West Pacific region with descriptions of two new species. Zootaxa 3395, 1–17 (2012).
Parrish, F., Baco, A., Kelley, C. & Reiswig, H. State of deep‐sea coral and sponge ecosystems of the U.S. Pacific Islands Region. in The state of deep-sea coral and sponge ecosystems of the United States. NOAA Technical Memorandum NMFS‐OHC-4 (eds Thomas F Hourigan, Peter J Etnoyer, & Stephen Douglas Cairns) Chapter 7, 40 p. (US Department of Commerce, National Oceanic and Atmospheric Administration) (2017).
Parrish, F. A., Baco-Taylor, A., Kelley, C., Cairns, S. D. & Hourigan, T. F. Deep-sea coral taxa in the Hawaiian Archipelago and other U.S. Pacific Islands: depth and geographical distribution (Online resource: https://deepseacoraldata.noaa.gov) (2017).
Ardila, N. E., Giribet, G. & Sánchez, J. A. A time-calibrated molecular phylogeny of the precious corals: reconciling discrepancies in the taxonomic classification and insights into their evolutionary history. BMC Evolutionary Biology 12, 246 (2012).
Tu, T.-H., Dai, C.-F. & Jeng, M.-S. Phylogeny and systematics of deep-sea precious corals (Anthozoa: Octocorallia: Coralliidae). Molecular Phylogenetics and Evolution 84, 173–184 (2015).
Grigg, R. W. The precious corals. Fishery management plan of the Western Pacific Regional Fishery Management Council. (2010).
Cannas, R., Follesa, M., Cau, A., Cau, A. & Friedman, K. Global report on the biology, fishery and trade of precious corals. (FAO Fisheries and Aquaculture) (2019).
Bilewitch, J. P. & Degnan, S. M. A unique horizontal gene transfer event has provided the octocoral mitochondrial genome with an active mismatch repair gene that has potential for an unusual self-contained function. BMC Evolutionary Biology 11, 228 (2011).
Conte, J., Potoczniak, M. J. & Tobe, S. S. Using synthetic oligonucleotides as standards in probe-based qPCR. BioTechniques 64, 177–179 (2018).
Nolan, T., Hands, R. E., Ogunkolade, W. & Bustin, S. A. SPUD: a quantitative PCR assay for the detection of inhibitors in nucleic acid preparations. Analytical Biochemistry 351, 308–310 (2006).
R Core Development Team. (ed R Foundation for Statistical Computing) (2013).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. Journal 17, 10–12 (2011).
Afgan, E. et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research 46, W537–W544 (2018).
Edgar, R. C. & Flyvbjerg, H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 31, 3476–3482 (2015).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods 10, 996 (2013).
Liverino, V. Citing environmental responsibility, CIBJO Coral Commission seeks to find common ground with scientific community. in The World Jewellery Confederation Congress (Bangkok, Thailand) 4 p. (2017).
Acknowledgements
Enzo Liverino Srl (Torre del Greco, Italy) provided some of the precious coral material for the SSEF coral collection, which we used in this study. This study benefited largely from discussions with Dr. Nozomu Iwasaki (Rissho University, Japan).
Author information
Authors and Affiliations
Contributions
B.L., A.K., M.S.K., L.E.C. and N.V.M. conceived the study. M.S.K. and L.E.C. provided the coral samples. J.B.M. conducted preliminary DNA extraction and sequence analysis. B.L., N.V.M. and M.G. performed the laboratory work and analyzed the data. B.L. and L.E.C. wrote the manuscript with support from the other co‐authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lendvay, B., Cartier, L.E., Gysi, M. et al. DNA fingerprinting: an effective tool for taxonomic identification of precious corals in jewelry. Sci Rep 10, 8287 (2020). https://doi.org/10.1038/s41598-020-64582-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64582-4
This article is cited by
-
Mitochondrial genes as strong molecular markers for species identification
The Nucleus (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.