Abstract
During pregnancy, substantial alterations in cerebral plasticity, vascular remodeling and neuronal growth occur in the maternal brain. We investigated whether concentrations of selected neurodiagnostic biomarkers in the cerebrospinal fluid of women with preeclampsia/HELLP syndrome differ from those in healthy controls using enzyme-linked immunosorbent assay technique. We found that tau protein concentrations (p = 0.016) and phospho-tau/tau ratio (p < 0.001) in cerebrospinal fluid were significantly lower in 39 preeclamptic women compared to 44 healthy controls during third trimester of pregnancy. Beta-amyloid(1-40)/(1-42) ratio was significantly higher in HELLP syndrome than in severe preeclampsia (8.49 + 2.73 vs. 4.71 + 1.65; p = 0.007). We conclude that beta-amyloid(1-40)/(1-42) ratio in cerebrospinal fluid can discriminate severe preeclampsia and HELLP syndrome. High beta-amyloid peptide and low tau protein concentrations are associated with impaired development of the materno-feto-placental unit and correlate with placental dysfunction.
Similar content being viewed by others
Introduction
During pregnancy, the invasion of trophoblast cells into maternal tissue of the uterus and the conversion of spiral arteries into wide sinusoids with low resistance and high flow are paramount for normal placental development1. In preeclampsia (PE) placental development is impaired by defective deep placentation, platelet and thrombin activation, intravascular inflammation, endothelial dysfunction and imbalanced angiogenesis2. Altered expression of proteins comes along with excess of antiangiogenic substances such as soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) and decreased levels of proangiogenic substances like placental growth factor (PlGF) and vascular endothelial growth factor A (VEGF-A)3. Ciampa et al. observed in 13 patients with PE altered concentrations of proteins related to signaling pathways important for vascular remodeling, inflammation, and neuronal growth4.
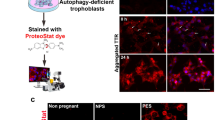
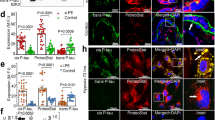
Recent studies have shown that PE shares pathophysiologic features with recognized misfolding disorders and aggregation of proteins4,5,6,7,8. There are several dysregulated proteins in PE but it is not clear whether aggregated proteins induce defective trophoblast invasion4. D’Souza et al. reported that neurotrophic factors influence the development of the materno-feto-placental unit during pregnancy9. Altered blood-brain barrier and impaired cerebral autoregulation may affect erebral blood flow in the maternal brain10. Aggregated beta-amyloid peptides were observed in PE as well as in Alzheimer’s disease7. The presence of beta-amyloid aggregates in placentas of women with PE and intrauterine growth restriction (IUGR) further supports the notion that this condition goes with protein conformational disorders6,11. Moreover, it was observed that short peptides occupying the self-recognition sites of beta-amyloid inhibit beta-amyloid aggregation8.
The aim of this study was to determine whether CSF concentrations of beta-amyloid peptides and tau protein differ between women with PE and women with HELLP syndrome as compared to healthy pregnant women during the third trimester of pregnancy.
Results
Patient characteristics
A total of 105 pregnant women who underwent spinal anesthesia participated; 39 women with PE/HELLP were consecutively assigned to a prospective research cohort. Forty-four of 66 pregnant women served as controls (Fig. 1). Demographic data including age, height, current weight and BMI were comparable between study group and controls. The number of previous pregnancies, miscarriages and parities did not differ between the two groups, but weeks of gestation were significantly fewer in women with PE/HELLP syndrome than in controls (33.4 + 3.4 vs. 38.1 + 1.1; p < 0.001).
Cardiorespiratory parameters and blood chemistry
In the study group we distinguished mild PE (n = 18), severe PE (n = 13) and HELLP syndrome (n = 8). According to the underlying pathomechanism systolic blood pressure (167.1 + 16.9 vs. 121.4 + 8.4; p < 0.001), diastolic blood pressure (103.9 + 20.4 vs. 77.8 + 8.4; p < 0.001) and pulse rate (82.5 + 17.0 vs. 89.8 + 13.4; p = 0.023) differed significantly between PE and HELLP syndrome and controls. In HELLP syndrome platelet count (85.6 × 109/L + 25.5) was significantly lower than for mild PE (171.3 × 109/L + 54.8; p = 0.002) or severe PE (178.0 × 109/L + 47.1; p = 0.001). In addition, sGOT in HELLP syndrome (263.9 U/L + 470.4) was significantly higher than in mild PE (29.3 U/L + 16.4; P < 0.001) or severe PE (33.5 U/L + 15.0; p = 0.005).
Health status of the newly born
Mean baby weight differed significantly between mild PE (2,362 g + 546.9) and severe PE (1,095 g + 359.2; p < 0.001), as well as between severe PE and HELLP syndrome (1,993 g + 543.7; p = 0.018). In a post hoc subgroup analysis placental weight (p = 0.021) and baby weight (p = 0.001) were significantly lower in cases with beta-amyloid(1-40) exceeding 5000 pg/mL compared to beta amyloid(1-40) beyond 5000 pg/mL.
Biomarkers
CSF concentrations of tau protein were significantly lower in 39 women with PE and HELLP syndrome than in controls (p = 0.016). Phospho-tau-181/tau ratio was significantly higher in women with PE/HELLP (p < 0.001). In the study group median concentrations of beta-amyloid peptides(1-40) were higher in HELLP syndrome (9,683 pg/mL ± 5,643; p = 0.023) than for severe PE (4,836 pg/mL ± 2,004), and the beta-amyloid(1-40)/(1-42) ratio differed significantly (8.49 ± 2.73 vs. 4.71 ± 1.65; p = 0.007) (Table 1).
Beta-amyloid(1-40) correlated with serum markers sFlt-1 (rs = −0.536; p = 0.001) and PlGF (rs = 0.357; p = 0.042). Tau protein correlated with serum markers sFlt-1 (rs = −0.330; p = 0.040) and PlGF (rs = 0.356; p = 0.028). Beta-amyloid(1-40)/(1-42) ratio was significantly higher in HELLP syndrome than in severe PE (8.49 + 2.73 vs. 4.71 + 1.65; p = 0.007) (Table 1). Using post-hoc analysis with the Dunn-Bonferroni test, the beta-amyloid(1-40)/(1-42) ratio differed significantly between HELLP syndrome and severe PE (p = 0.007) and between HELLP syndrome and controls (p = 0.049) (Table 2).
Discussion
In our study mean concentrations of beta-amyloid peptides(1-40) were higher in women with HELLP syndrome than in preeclamptic women and healthy controls. Human placenta and thrombocytes abundantly express amyloid precursor protein (APP)5,6. Presumably, thrombocytopenia influences metabolism of beta-amyloid(1-42) and (1-40)12. Although the histopathologic profile and the range of placental lesions differ between PE and HELLP syndrome13, it remains a matter of ongoing debate whether HELLP syndrome is a severe form of PE or a separate disease. We found that beta-amyloid(1-40)/(1-42) ratio can discriminate between severe PE and HELLP syndrome. The findings of our study support the hypothesis that HELLP syndrome is a distinct disease pattern and not simply a variety of severe PE.
In our study, median CSF concentrations of tau protein and the phospho-tau/tau ratio were significantly lower in women with PE and HELLP syndrome than in healthy controls. This is in agreement with recently reported diminished CSF tau and phospho-tau-181 protein concentrations in patients with placental dysfunction14,15. During normal pregnancy there is an increase in tau protein concentrations16. Presumably, diminished CSF concentrations in PE and HELLP syndrome imply fewer maternal brain adaptations. Virtanen et al. observed an inhibitory effect on tubule formation in third trimester preeclamptic women17. Serum concentrations of angiogenic factors sFlt-1 levels were higher and PlGF were lower in third trimester preeclamptic women compared to healthy controls with increasing sFlt-1/PlGF ratio between the first and the third trimester18. Unfortunately, sFlt-1 and PlGF have limited sensitivity for stratification of women with suspected PE19. PE still lacks a reliable, early means of diagnosis or prediction, and a safe and effective therapy20, So far, the diagnosis of PE and HELLP syndrome is mostly based on clinical findings and increasing sFlt-1/PlGF ratio. In particular, the onset threshold of plasma levels of the sFlt-1/PlGF ratio proved to be a valuable screening tool for detecting the imminent onset of PE within four weeks after blood sampling during the second trimester, namely at 19 to 31 weeks21. Potentially, detection of amyloid-targeting fluorophores in the urine may contribute to early identification of PE patients during pregnancy22. Affintiy for conformational antibodies raised against aggregated beta-amyloid peptides and dysregulation in the APP proteolytic pathway may offer future diagnostic and therapeutic options6,8. Neuron-specific enolase and S100B were reported to be increased before clinical development of PE can be verified23. This supports the hypothesis that altered neural remodeling in the maternal brain precedes impaired development of the placenta.
Pregnancy renders substantial changes in maternal brain, primarily reduction in gray matter volume in regions subserving social cognition24. Decrease in brain size begins after placental implantation25. It reaches maximum at term and it endures for at least 2 years post-partum25,26. Structural and functional changes accompany fundamental behavioural adaptations, stimulating to progress from self-involved individuality to carying motherhood26. Changes in the maternal brain during pregnancy cannot be explained by endocrine and environmental factors alone27. Van Dijk et al. reported that the PE-susceptibility gene STOX1 controls a conserved pathway shared between placenta and brain28. STOX1A correlated with severity of disease and was associated with increased amyloid-beta protein precursor processing in neural cells and trophoblast cells28. Vaiman and Miralles hypothesized that selective inhibition of one of the two isoforms STOX1A and STOX1B could possibly improve outcome in severe PE29.
In our study angiogenic factors in serum correlate with CSF beta-amyloid(1-40) and tau protein. Our findings question the current perspective on etiology of PE and of HELLP syndrome and support the hypothesis that neurotrophic factors influence the development of the materno-feto-placental unit during pregnancy. However, there are some limitations. First and foremost, the number of enrolled women with PE and HELLP syndrome is small as data were only available on women who have already developed clinically evident disease and who underwent surgical or obstetrical operations in spinal anesthesia. We are aware that routine lumbar puncture for diagnosis of PE and HELLP syndrome is not practical during pregnancy. Furthermore, treatment is limited mostly to maternal stabilization and timely delivery by medically induced labor or cesarean section2. In women with PE and HELLP syndrome delivery by cesarean section was on average four weeks of gestation earlier than in controls, thus partly explaining the low values for placental weight, baby weight and Apgar score. Duration of storage of samples varied among women enrolled and might have biased measurement results. Sampling of patient CSF was performed mostly during the daytime. Observer bias among pediatricians and midwives might have influenced assessment of Apgar Scores and placental weight. Furthermore, rating of perceived stress and physical activity depended on subjective justification of participating women and might have been influenced by current events. Larger trials focusing on placental morphology and function are needed to draw definitive conclusions.
In conclusion we observed that beta-amyloid(1-40)/(1-42) ratio in cerebrospinal fluid can discriminate severe PE and HELLP syndrome. High beta-amyloid peptide and low tau protein concentrations are associated with impaired development of the materno-feto-placental unit and correlate with placental dysfunction.
Materials and methods
Women with PE/HELLP syndrome during pregnancy who underwent spinal puncture for regional anesthesia were consecutively enroled during normal work time in a prospective cross-sectional research at the Department of Gynecology, Innsbruck Medical University Hospital. Healthy women with uncomplicated pregnancies who underwent a cesarean section under spinal block served as controls.
Ethical approval & patients consent
The Ethics Committee of Innsbruck Medical University approved this study (AN2017-0073 371/4.25). Written informed consent was obtained from all patients before participation with the understanding that anonymized data would be published in a scientific journal. All methods were performed in accordance with the relevant guidelines and regulations.
Study design and study population
Anesthetists from obstetrical anesthesia screened patients by medical history before participation. Blood chemistry (blood sugar, hemoglobin, serum protein) and kidney function parameters (blood urea nitrogen, creatinine, glomerular filtration rate, and urinary protein) were obtained from medical records. Circulatory and respiratory status (systolic blood pressure, pulse rate and oxygen saturation) were recorded before and during anesthesia. Perceived stress and physical activity were inquired, as they may affect incidence and severity of PE30. We used a 5-point Likert scale with (1, very much; 2, much; 3, moderate; 4, poor; 5, very poor) for individual grading of conceptualization of estimated stress. Demographics, clinical characteristics and laboratory findings for participating women were recorded on a working chart.
Inclusion criteria for the study group were: pregnant women, 18 years of age or older, with evidence of PE/HELLP syndrome verified by clinical signs of hypertension, proteinuria and edema and, if available, positive sFlt-1/PlGF ratio31. Exclusion criteria for the study group were: lacking written consent. Inclusion criteria for the control group were: pregnant women, 18 years of age or older, in good general health. Participants were on no chronic medication other than multivitamins, magnesium and iron supplementation. Subjects were in particular free from past or present major psychiatric disorders other than mild depression, major neurological disease, gestational diabetes, and chronic renal and hepatic disease. Exclusion criteria for the control group were: women suffering from hypertension in pregnancy, thrombocytopenia, proteinuria, evidence of placental dysfunction, IUGR, as well as women with a history of PE or HELLP syndrome, cases lacking written consent. All study findings and documents were handled in strictest confidence. Enrolment of patients, data collection and analysis followed the CONSORT 2010 checklist of information to include when reporting a randomized trial32. Recruitment of patients depended on pre-determined sample sizes (quota sampling according to power analysis) stratified by clinical diagnosis and voluntary participation.
Sample collection
All pregnant women undergoing a spinal block with direct access to CSF and meeting the inclusion criteria were eligible as study subjects. Spinal anesthesia was performed according to standard operating procedures of the Department of Anesthesiology and Critical Care Medicine33. Following skin disinfection, a 20-gauge introducer needle (B Braun, 34209 Melsungen, Germany) was inserted into the mid-line lumbar region and directed towards the interspinous ligament. Then, a 25-gauge needle (Spinocan® pencil-point spinal needle, B Braun, 34209 Melsungen, Germany) was inserted through the introducer into the subarachnoid space and the trocar was removed. Before administration of the intrathecal local anesthetic one milliliter CSF was collected in a sterile polypropylene tube34. Immediately after transport to the psychiatric laboratory, CSF samples were frozen and stored at minus 80 °C until the assays were performed.
Definitions
-
Body mass index (BMI), calculated by dividing weight in kilograms by the square of height in meters.
-
Physical activity, defined as (>30 minutes, very much; up to 30 minutes, much; 20 minutes, moderate; 10 minutes, poor; and <10 minutes, very poor) considering the World Health Organisation recommendations for daily physical activity for adults aged 18–64 years35.
-
Placental dysfunction, abnormal uteroplacental and fetoplacental circulation assessed by Doppler sonography using Grannum classification graded as 0 (homogeneous), I (subtle), II (marked), III (confluent)36.
-
HELLP syndrome, characterized by haemolysis, elevated liver enzymes and low platelets37.
-
PE, systemic syndrome with hypertension exceeding 140/90 mmHg presenting beyond 20 weeks of gestation and proteinuria >300 mg protein in a 24 h urine collection or 1 + (0.3 g/L) on urine dipstick37.
-
Early-onset PE, hypertension, proteinuria, placental dysfunction due to poor placental perfusion and reduction of placental volume, IUGR and low birth weight occurring before 34 weeks of gestation37.
-
Mild PE, proteinuria and systolic blood pressure between 140 and 160 mmHg or diastolic blood pressure between 90 and 110 mmHg.
-
Severe PE, proteinuria and blood pressure exceeding 160 mmHg systolic or 110 mmHg diastolic, frequently combined with elevated liver enzymes37.
-
sFlt-1/PlGF ratio, calculated dividing antiangiogenic factor sFlt-1 by proangiogenic factor PlGF33.
-
Thrombocytopenia in pregnancy, defined as a platelet count <100 × 109/L38.
Processing and analytical techniques
CSF biochemical markers were detected in cell-free samples using enzyme-linked immunosorbent assay (ELISA) assays (Fujrebio, formerly Innogenetics) that can reliably detect even low levels of CSF biomarkers based on several well-defined monoclonal tau antibodies for normal and abnormal forms of CSF tau protein. For analysis, all CSF samples were thawed and concentrations of beta-amyloid(1-42) and (1-40), total tau and phospho-tau-181 proteins (Assay Innotest®, Fujirebio Europe, B-9052 Ghent, Belgium) measured by ELISA according to the manufacturer’s instructions.
Statistical analysis
Primary study endpoint was to evaluate selected CSF neurodiagnostic biomarkers in women with PE/HELLP syndrome. Secondary study endpoint was to detect differences in CSF biomarkers between preeclamptic women and women with HELLP syndrome. Our H0 hypothesis was: there are no differences in CSF concentrations of beta-amyloid(1-42) or (1-40), total tau or phospho-tau-181 proteins between women with PE and HELLP syndrome and normal pregnancies. In order to compensate for individual variations in beta-amyloid production the beta-amyloid(1-42)/(1-40) ratio was calculated.
The sample size was calculated for an alpha error of 0.05 and a power of 80% (beta error of 0.2) to detect significant differences between CSF biomarkers of women with PE/HELLP syndrome and women with normal pregnancy using nQueryAdvisor v.7.0 software. Calculations were conducted with SPSS 25 (IBM SPSS Statistics Standard) using the T test or the Mann-Whitney U test, as indicated. For multiple pairwise comparisons between more than two subgroups, the Kruskal-Wallis test was applied with Bonferroni correction. A post hoc subgroup analysis was performed for beta-amyloid(1-40) <5000 pg/mL and for beta amyloid(1-40) >5000 pg/mL. Group comparisons of the beta-amyloid(1-42)/(1-40) ratio were assessed by analysis of variance followed by the Dunn-Bonferroni test39. Pearson’s correlations (two-tailed, bivariate) were calculated for CSF marker levels and blood sugar, proteinuria, systolic blood pressure and body weight as expressed in BMI. Correlations with either stress or physical exercise values were established using the Spearman’s rank correlation coefficient. Statistical significance was deemed when p < 0.05.
Data availability
Supplementary Information in an Additional File 1 is available in persistent web link to datasets. Further data and materials are available from the corresponding author on reasonable request.
References
Latendresse, G. & Founds, S. The fascinating and complex role of the placenta in pregnancy and fetal well-being. J. Midw Womens Health. 60, 360–370, https://doi.org/10.1111/jmwh.12344 (2015).
Rana, S., Lemoine, E., Granger, J. & Karumanchi, S. A. Preeclampsia. Circ. Res. 124, 1094–1112, https://doi.org/10.1161/CIRCRESAHA.118.313276 (2019).
Dymara-Konopka, W., Laskowska, M. & Błażewicz, A. Angiogenic Imbalance as a Contributor of Preeclampsia. Curr. Pharm. Biotechnol. 19, 797–815, https://doi.org/10.2174/1389201019666180925115559 (2018).
Ciampa, E. et al. Cerebrospinal Fluid Protein Changes in Preeclampsia. Hypertension. 72(1), 219–226, https://doi.org/10.1161/HYPERTENSIONAHA.118.11153 (2018).
Cheng, S. B., Nakashima, A. & Sharma, S. Understanding Pre-Eclampsia Using Alzheimer’s Etiology: An Intriguing Viewpoint. Am. J. Reprod. Immunol. 75, 372381, https://doi.org/10.1111/aji.12446 (2016).
Buhimschi, I. A. et al. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci. Transl. Med. 6, 245ra92, https://doi.org/10.1126/scitranslmed.3008808 (2014).
Cater, J. H. et al. Human pregnancy zone protein stabilizes misfolded proteins including preeclampsia- and Alzheimer’s-associated amyloid beta peptide. Proc. Nat. Acad. Sci. USA 116, 61016110, https://doi.org/10.1073/pnas.1817298116 (2019).
Kouza, M., Banerji, A., Kolinski, A., Buhimschi, I. A. & Kloczkowski, A. Oligomerization of FVFLM peptides and their ability to inhibit beta amyloid peptides aggregation: consideration as a possible model. Phys. Chem. Chem Phys. 19, 29902999, https://doi.org/10.1039/c6cp07145g (2017).
D’Souza, V. et al. Levels of brain derived neurotrophic factors across gestation in women with preeclampsi. a. Int. J. Dev. Neurosci. 37, 36–40, https://doi.org/10.1016/j.ijdevneu.2014.06.008 (2014).
Bergman, L. et al. Investigating Maternal Brain Alterations in Preeclampsia: the Need for a Multidisciplinary Effort. Curr. Hypertens. Rep. 21, 72, https://doi.org/10.1007/s11906-019-0977-0 (2019).
Gerasimova, E. M., et al Protein Misfolding during Pregnancy: New Approaches to Preeclampsia Diagnostics. Int J Mol Sci. 20, https://doi.org/10.3390/ijms20246183 (2019).
Hammer, E. S. & Cipolla, M. J. Cerebrovascular Dysfunction in Preeclamptic Pregnancies. Curr. Hypertens. Rep. 17, 64, https://doi.org/10.1007/s11906-015-0575-8 (2015).
Vinnars, M. T. et al. Severe preeclampsia with and without HELLP differ with regard to placental pathology. Hypertension. 51, 12951299, https://doi.org/10.1161/HYPERTENSIONAHA.107.104844 (2008).
Bergman, L. et al. Blood-based cerebral biomarkers in preeclampsia: Plasma concentrations of NfL, tau, S100B and NSE during pregnancy in women who later develop preeclampsia - A nested case control study. PLoS One 13(5), e0196025, https://doi.org/10.1371/journal.pone.0196025 (2018).
Fisher, S. J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 213, 115–22, https://doi.org/10.1016/j.ajog.2015.08.042. (2015).
González-Arenas, A. et al. Expression pattern of Tau in the rat brain during pregnancy and the beginning of lactation. Brain Res. Bull. 89, 108114, https://doi.org/10.1016/j.brainresbull.2012.07.011 (2012).
Virtanen, A. et al. Angiogenic capacity in pre-eclampsia and uncomplicated pregnancy estimated by assay of angiogenic proteins and an in vitro vasculogenesis/angiogenesis test. Angiogenesis. 22(1), 6774, https://doi.org/10.1007/s10456-018-9637-2 (2019).
Bednarek-Jędrzejek, M. et al. The sFlt-1/PlGF ratio values within the <38, 38–85 and >85 brackets as compared to perinatal outcomes. J. Perinat. Med. 47(7), 732740, https://doi.org/10.1515/jpm-2019-0019 (2019).
Askie, L. M., Henderson-Smart, D. J. & Stewart, L. A. & Antiplatelet agents for the prevention of preeclampsia: a meta-analysis of individual patient data. Lancet. 369, 17911798, https://doi.org/10.1016/S0140-6736(07)60712-0 (2007).
McCarthy, F. P., Ryan, R. M. & Chappell, L. C. Prospective biomarkers in preterm preeclampsia: A review. Pregnancy Hypertens. 14, 7278, https://doi.org/10.1016/j.preghy.2018.03.010 (2018).
Ohkuchi, A. et al. Onset threshold of the plasma levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio for predicting the imminent onset of preeclampsia within 4 weeks after blood sampling at 19–31 weeks of gestation. Hypertens. Res. 36, 1073–1080, https://doi.org/10.1038/hr.2013.95 (2013).
Do, J. P. et al. Identification of Patients with Preeclampsia by Measuring Fluorescence of an Amyloid-Binding Aryl Cyano Amide in Human Urine Samples. Anal. Chemistry. 90, 1431614320, https://doi.org/10.1021/acs.analchem.8b03649 (2018).
Bergman, L. & Åkerud, H. Plasma Levels of the Cerebral Biomarker, Neuron-Specific Enolase, are Elevated During Pregnancy in Women Developing Preeclampsia. Reprod. Sci. 23, 395400, https://doi.org/10.1177/1933719115604732 (2016).
Hoekzema, E. et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20(2), 287296, https://doi.org/10.1038/nn.4458 (2017).
Oatridge, A. et al. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. Am J Neuroradiol. 23, 1926, PMID:11827871 (2002).
Barba-Müller, E., Craddock, S., Carmona, S. & Hoekzema, E. Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch. Womens Ment. Health. 22(2), 289299, https://doi.org/10.1007/s00737-018-0889-z (2019).
Brunton, P. J. & Russell, J. A. Endocrine induced changes in brain function during pregnancy. Brain Res. 1364, 198215, https://doi.org/10.1016/j.brainres.2010.09.062 (2010).
van Dijk, M. et al. The pre-eclampsia gene STOX1 controls a conserved pathway in placenta and brain upregulated in late-onset Alzheimer’s disease. J. Alzheimers Dis. 19, 673679, https://doi.org/10.3233/JAD-2010-1265 (2010).
Vaiman, D. & Miralles, F. Targeting STOX1in the therapy of preeclampsia. Expert. Opin. Ther. 20, 14331443, https://doi.org/10.1080/14728222.2016.1253682 (2016).
Awad, M. A., Hasanin, M. E., Taha, M. M. & Gabr, A. A. Effect of stretching exercises versus autogenic training on preeclampsia. J. Exerc. Rehabil. 15, 109113, https://doi.org/10.12965/jer.1836524.262 (2019).
Lederer, W., Dominguez, C. A., Popovscaia, M., Putz, G. & Humpel, C. Cerebrospinal fluid levels of tau and phospho-tau-181 proteins during pregnancy. Pregnancy Hypertens. 6, 384387, https://doi.org/10.1016/j.preghy.2016.08.243 (2016).
Schoonenboom, N. S. et al. Effects of processing and storage conditions on amyloid ß (1-42) and tau concentrations in cerebrospinal fluid: implications for use in clinical practice. Clin. Chemistry. 51, 189195, https://doi.org/10.1373/clinchem.2004.039735 (2005).
Hirashima, C. et al. Alteration of serum soluble endoglin levels after the onset of preeclampsia is more pronounced in women with early-onset. Hypertens. Res. 31, 1541–1548, https://doi.org/10.1291/hypres.31.1541 (2008).
CONSORT checklist of information to include when reporting a randomised trial*, http://www.consort-statement.org/media/default/downloads/CONSORT%202010%20Checklist.pdf (2010).
WHO.Global Strategy on Diet, Physical Activity and Health. 2011, http://www.who.int/dietphysicalactivity/pa/en/index.html, Accessed 02.June 2019.
McKenna, D., Tharmaratnam, S., Mahsud, S. & Dornan, J. Ultrasonic evidence of placental calcification at 36 weeks’ gestation: maternal and fetal outcomes. Acta Obstet. Gynecol. Scand. 84, 7–10, https://doi.org/10.1111/j.0001-6349.2005.00563.x (2005).
Tranquilli, A. L., Brown, M. A., Zeeman, G. G., Dekker, G. & Sibai, B. M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 3, 44–47, https://doi.org/10.1016/j.preghy.2012.11.001 (2013).
Burrows, R. F. & Kelton, J. G. Thrombocytopenia at delivery: a prospective survey of 6715 deliveries. Am. J. Obstet. Gynecol. 162, 731–734, https://doi.org/10.1016/0002-9378(90)90996-k (1990).
Zwick, R., Neuhoff, V., Marascuilo, L. A. & Levin, J. R. Statistical tests for correlated proportions: Some extensions. Psych. Bull. 92, 258–271, https://doi.org/10.1037/0033-2909.92.1.258 (1982).
Acknowledgements
The authors want to thank the laboratory personnel at the psychiatric research laboratories for their conscientious measurement work. Serological tests were financed by Experimental Anesthesia, Research Centre, Department of Anesthesiology and Critical Care Medicine, Medical University of Innsbruck. The funding source was involved neither in the collection, analysis or interpretation of data nor in the writing of the report or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
W.L. conceived the idea, designed the study, prepared proposal and working charts, discussed and wrote the manuscript. H.S. conceived the clinical investigation and sample collection, wrote and discussed the manuscript. C.A.D. conceived the clinical investigation and sample collection, prepared the working charts, wrote and discussed the manuscript. J.T. and R.F. conceived the clinical investigation and sample collection, wrote and discussed the manuscript. L.D. performed statistical analysis, wrote and discussed the manuscript. G.P. conceived the clinical investigation and sample collection, wrote and discussed the manuscript. C.H. conceived the method for this study, supervised processing, wrote and discussed the manuscript. All authors were involved in the interpretation of the results and all authors approved the revised manuscript before its submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of financial and non-financial interests including personal relationships with other persons or organizations that could inappropriately influence, or be perceived to influence, the work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lederer, W., Schaffenrath, H., Alomar-Dominguez, C. et al. Cerebrospinal beta-amyloid peptides(1-40) and (1-42) in severe preeclampsia and HELLP syndrome – a pilot study. Sci Rep 10, 5783 (2020). https://doi.org/10.1038/s41598-020-62805-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62805-2
This article is cited by
-
Beta-amyloid peptides(1–42) and (1–40) in the cerebrospinal fluid during pregnancy: a prospective observational study
Archives of Women's Mental Health (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.