Abstract
The impact of obesity on health-related quality of life (HRQoL) in chronic kidney disease (CKD) population has not been elucidated, despite the impairment of HRQoL in the obese among general population. We hypothesized that the impact of obesity on HRQoL might be confounded by impaired renal function in CKD population, and that CKD would attenuate the impact of obesity on HRQoL. To compare the impact of obesity on HRQoL according to kidney function, 17,001 subjects from Korea National Health and Nutrition Examination Survey (2008–2011) were categorized by estimated glomerular filtration rate (eGFR), as follows: group 1, eGFR ≥ 90 mL/min/1.73 m2; group 2, eGFR of 60–89 mL/min/1.73 m2; group 3, eGFR < 60 mL/min/1.73 m2. The association between obesity parameters (body mass index, waist circumference and, truncal fat mass) and HRQoL parameters (EQ-5D index and EQ-VAS) were cross-sectionally analyzed. Despite robust correlations between obesity parameters and low EQ-5D index or EQ-VAS in general population, no significant association was observed in group 3 population. Impact of obesity on HRQoL in CKD population was only limitedly observed in the mobility domain of EQ-5D, as mobility limitation was associated with increased body mass index or waist circumference regardless of kidney function. Therefore, the impact of obesity on HRQoL seems significantly attenuated in CKD population, suggesting that the risk of obesity should not be over-estimated in patients with CKD, especially with respect to HRQoL.
Similar content being viewed by others
Introduction
Obesity imposes a great burden on the public health globally, as it increases the risk of type 2 diabetes mellitus, cardiovascular disease, cerebrovascular accident, and malignancy, and is subsequently associated with high cardiovascular and all-cause mortalities1,2,3. Especially, central obesity, defined by waist circumference (WC) or WC to height ratio, more precisely predicts cardiovascular risk factors than body mass index (BMI) does4. The concept, metabolically healthy obesity, introduced broad spectral phenotypes of obesity and emphasized the importance of anatomical distribution of body fat5. Indeed, various indices from direct measurement of regional fat mass6 or from calculation of more than 2 anatomical fat deposits7 have proved their superiority over BMI on estimating the risk of cardiovascular events. On the basis of conceptual advances, novel links between obesity and human diseases are now being uncovered.
Kidney is a target of obesity8, as evidences suggest that conditions related to obesity, such as insulin resistance9, up-regulation of leptin10, or down-regulation of adiponectin11, promote glomerular injury and albuminuria, leading to the decline of kidney function. Nevertheless, clinical studies have not concluded the impact of obesity on CKD progression. A cohort study including more than 450,000 nondialysis-dependent subjects reported U-shaped association of BMI and clinical outcomes with the best in overweight and mildly obese patients, rather than in patients with ideal body weight12. Another cohort study including 1,940 participants with CKD presented quite conflicting results that obesity (defined as BMI ≥ 25 kg/m2) and/or concurrent metabolic abnormalities accelerate CKD progression13. These inconsistent results strongly imply the complex nature of CKD, where findings drawn from the general population should not be easily extrapolated.
Health-related quality of life (HRQoL) has emerged as an indispensable clinical outcome14,15. Despite the vague definition and essentially subjective nature of HRQoL16, several questionnaires have been developed to quantify HRQoL and have been validated in various clinical settings17,18. Previous studies reported relatively consistent results that obesity impairs HRQoL19,20,21, while the association of CKD with poor HRQoL has been an issue of debate22,23,24,25, until a recent study including more than 46,000 participants reported a significant association between CKD and poor HRQoL26, suggesting that the impact of obesity on HRQoL might be confounded by impaired renal function in CKD population. Yet, the association of obesity and HRQoL in subjects with kidney dysfunction has never been studied. We, therefore, hypothesized that CKD would attenuate the impact of obesity on HRQoL. In the present study, taking advantage of a nationwide, community-based population study, we analyzed and compared the impact of obesity on HRQoL among the subgroups stratified by eGFR.
Results
Baseline characteristics
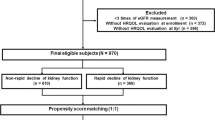
To compare the baseline characteristics according to the kidney function, all study subjects were divided into 3 groups (Fig. 1 and Table 1). Among the entire study participants, 2.74% belonged to group 3, while 62.12% and 32.14% did to group 1 and group 2, respectively. The study subjects were older in age as kidney function deteriorated. The surrogates of HRQoL, EuroQoL-5 dimension-3 level (EQ-5D) index and EuroQoL-visual analogue scale (EQ-VAS) scores, significantly decreased as kidney dysfunction progressed. Compared to group 1, the proportion of subjects with mobility limitation sharply increased in in groups 2 and 3 (10.42% vs. 26.34% and 49.68%, respectively). Obesity parameters also showed robust distinctions according to the renal function. Compared to the subjects at groups 1, BMI and other indices regarding central obesity such as waist circumference (WC), truncal fat mass (TF) as well as ratio of TF to whole body fat mass (TF/WF) increased in the subjects of groups 2 and 3. The ratio of leg fat mass to WF (LF/WF) decreased as kidney function deteriorated, indicating the predominance of central obesity in CKD populations. The subjects of groups 2 and 3 also had a higher prevalence of comorbidities, and showed remarkable distinction in laboratory findings. Compared to the other two groups, hemoglobin level decreased, while serum ferritin level increased in group 3. Metabolic derangement became apparent as eGFR declined, as serum triglyceride, fasting glucose, alkaline phosphatase, and parathyroid hormone levels increased in groups 2 and 3, compared to group 1. The incidence of proteinuria also increased as kidney function deteriorated.
Modification of association between obesity and HRQoL by kidney function
To investigate the association between obesity and HRQoL, survey-weighted generalized linear model was analyzed (Table 2). In the analyses of crude and adjusted models 1–3 including all study subjects, BMI was negatively associated with EQ-5D index score, which trend was substantially attenuated as estimated glomerular filtration rate (eGFR) decreased. In the subgroup analysis of group 1 subjects, the similar negative association was still observed, even after full adjustments with co-variates (Coefficients, −0.002; 95% CI, −0.004–-0.001; P = 0.0033). This association was marginally significant in group 2 subjects (Coefficients, −0.002; 95% CI, −0.004–0; P = 0.0185), but was no more valid in group 3 subjects (Coefficients, −0.005; 95% CI, −0.011–0.001; P = 0.0985). Further, neither of WC, TF, nor TF/WF was significantly associated with EQ-5D index among group 3 population either in crude or adjusted models, although the statistical significance was observed in the analyses including all study population. To figure out whether differential anatomical fat deposit other than central obesity may be linked to HRQoL in CKD, the effect of LF/WF on EQ-5D index was also analyzed. Despite a significant association between LF/WF and EQ-5D index was robustly present even after full adjustment of co-variates in the analysis of all study subjects and in subgroup analyses of group 1 and 2 populations., the association of LF/WF with EQ-5D index no longer existed in the analysis of adjusted model 3 for group 3 population. As the number of subjects in group 3 were substantially smaller than those of the others, the study subjects were dichotomized by incidence of CKD for propensity score-matching analysis (Table S1), where none of BMI, WC or TF was significantly associated with EQ-5D, regardless of incident CKD (Table S2). EQ-VAS score in the analysis of fully adjusted model including group 3 population was not associated with either of BMI, WC, or TF (Table S3). Taken together, these indicate that the association between obesity and HRQoL is modified by kidney function.
To test the impact of obesity parameters on HRQoL in the elderly and in the relative young, we additionally analyzed the effect of BMI, WC or TF on EQ-VAS score in subgroups stratified by age categories (Table S4), as the subgroup with impaired renal function was significantly older in the ages (Table 1). Only BMI in the subgroup with age <60 proved a statistically significant association with EQ-VAS score in the fully adjusted model, albeit the trend was inversed from the crude analysis model, suggesting the beneficial effect of high BMI in the subjects with chronic illness.
Disappearance of inverted U-shaped correlation between BMI and HRQoL in CKD stages 3–5 population
To examine whether a non-linear correlation between BMI and HRQoL score may be present, such as U-shaped or inverted J-shaped correlation, the study subjects were divided into quintiles according to BMI (Fig. 2, Tables 3, and S5). In the crude analysis including all subjects, inverted U-shaped correlation was clearly observed between BMI and EQ-VAS score, where the score was lowest in the 1st quintile BMI group, peaked in the 2nd and 3rd quintile BMI groups, and then dropped in 4th and 5th quintile BMI groups. In the analysis of fully adjusted model including all subjects, however, the inverted U-shaped correlation no more existed, where the score was lowest in the 1st quintile BMI group, peaked in 3rd quintile BMI groups, and plateaued in in 4th and 5th quintile BMI groups. The similar pattern was observed in the subgroup analyses of group 1 subjects. Intriguingly, in the subgroup analyses of crude model including group 2 subjects, EQ-VAS score continued to rise as BMI increased with its peak in the 5th quintile BMI group, although the statistical significance disappeared after adjustment of covariates. Moreover, no significant differences of EQ-VAS scores along the entire BMI ranges either in the crude or adjusted analysis including group 3 population. To additionally address the probable non-linear correlation of HRQoL with other obesity-related parameters, the study subjects were divided into quintiles according to WC or TF. The baseline characteristics of the study population stratified by WC and TF quintile categories are shown in Tables S6 and S7, respectively. In the analyses of crude and adjusted model including all study subjects or group 1 subjects, inverted U-shaped correlation was observed between WC and EQ-VAS score (Fig. 3 and Table S8). In the analyses including group 2 and 3 populations, however, no remarkable differences in the EQ-VAS score were observed along the entire range of WC quintiles. On the other hand, inverted J-shaped correlation was observed between TF and EQ-VAS score in the analysis of crude model including all study subjects or group 1 subjects, with a sharp decline of EQ-VAS score in the 4th and/or 5th TF quintiles (Fig. 4 and Table S9). Of note, after full adjustment of covariates, such a sharp decline of EQ-VAS score was markedly blunted with a peak of EQ-VAS score in the 4th quintile. Again, no distinct alterations in the EQ-VAS score were observed along the entire range of TF quintiles from the analysis of adjusted model including group 3 subjects. Therefore, these collectively suggest that, despite a unique association between central obesity and HRQoL in general populations, even a non-linear correlation does not exist in the subjects with impaired kidney function.
Comparison of least-square means of EQ-5D index among WC quintiles in all study subjects and in subgroups stratified by eGFR categories. Abbreviations: WC, waist circumference; Group 1, ≥90 mL/min/1.73 m2; Group 2, eGFR of 60–89 mL/min/1.73 m2; Group 3, eGFR <60 mL/min/1.73 m2; WC, waist circumference.
Impact of obesity on mobility limitation in subjects with renal dysfunction
To unveil the association between obesity and mobility, a component of HRQoL, which has been suggested in certain populations27,28, odd ratios (ORs) of obesity-related parameters for mobility limitation were analyzed (Table 4). In the analyses of crude and adjusted models including all study subjects or group 1 and 2 subjects, the association of mobility limitation with BMI or WC was significantly observed. Interestingly, the significant correlation still existed between BMI (OR, 1.152; 95% CI, 1.054–1.259; P = 0.0021) or WC (OR, 1.05; 95% CI, 1.02–1.081; P = 0.0013) and mobility limitation even in the analysis of adjusted model including group 3 subjects, although the statistical significances were not observed in the analyses of crude models. The association between increased TF and mobility limitation was significantly observed only in the analyses of adjusted models for group 1 and group 3 subjects, despite the significant associations in the crude analyses of all study participants and all subgroups according to eGFR. These results suggest that the impact of obesity on HRQoL is only limited to mobility among subjects with renal dysfunction.
Discussion
In the present study, we analyzed the association of obesity and HRQoL in general population and in subgroups categorized by kidney function. Contrary to the findings in general population, the impact of obesity on HRQoL was markedly attenuated as kidney function declined. Even a non-linear, inverted U-shaped association between obesity and HRQoL was not valid in subjects with kidney dysfunction. The association of obesity with HRQoL in CKD population was only limited to the mobility dimension, a component of EQ-5D, where obesity significantly impaired mobility both in general and CKD populations.
Considering the contribution of obesity to CKD progression13, loss of correlation between obesity and HRQoL in CKD population was unexpected. Of interest is that a similar trend has been reported from the studies of serum uric acid level in CKD population29,30. Based on the studies devoid of specific subgroup analyses for CKD population31,32, it has been widely accepted that hyperuricemia is a risk factor for cardiovascular and all-cause mortality. Although hyperuricemia accelerates CKD progression33, higher uric acid levels were associated with lower risk of all-cause and cardiovascular mortality in the hemodialysis population29. This paradoxical association is an example of reverse epidemiology, which is well-described among patients on hemodialysis in respect of hypertension34, total cholesterol level35, plasma total homocysteine level36, and leptin37. While the precise mechanism has not been established, statistical analyses are indicating the role of systemic inflammation and/or malnutrition as a cause of these paradoxical associations29,35,36,37. For example, increment of total cholesterol level was associated with a decreased risk of all-cause mortality in all HD patients and in HD patients with inflammation/malnutrition, whereas serum cholesterol level was associated with an increased risk in the absence of inflammation/malnutrition35. Accordingly, given that low BMI and total body fat percentage are components of suggested criteria for malnutrition in CKD patients, and that obesity does not necessarily mean well-nourished status38, it is speculated that the mild obesity in CKD population might be a proof of adequate nutrition without active inflammation, and might substantially abolish the drawbacks of obesity observed in subjects with normal kidney function, leading to preserved HRQoL outcomes. Another explanation for loss of correlation between obesity and HRQoL in CKD population is the possible role of metabolic abnormalities. A recent study39 reported the association of obesity phenotypes and HRQoL in patients with CKD stages 3–4, which revealed that HRQoL was impaired in the metabolically unhealthy obese, but not in the metabolically healthy obese, patients. The results clearly suggest that obesity defined by anthropometric measurement has its limitation in the predictability of HRQoL in CKD patients, and emphasize the impact of metabolic status based on the laboratory findings. To summarize, the findings from the current study and others suggest that obesity per se might not impair HRQoL in CKD population, and that, rather, inflammation or metabolic abnormalities would be truly associated with low HRQoL, both of which conditions are prevalent and easily accompanied in patients with CKD13,38.
Various parameters other than BMI, such as WC, TF, TF/WF, and LF/WF, were comprehensively analyzed to reveal the association of obesity or anatomical fat deposit status with HRQoL in general population and in subgroups with kidney dysfunction, as distinct features of each index might be reported regarding HRQoL7. While the overall trend was similar regarding the correlation with HRQoL, the individual parameters also had distinct features in detail (Figs. 2–4). The shape of correlation curves between HRQoL and each of BMI, WC, and TF in adjusted models was unique among group 3 population, with inverted U-shapes in BMI and TF, and relatively flat shape in WC. Although none of them were statistically significant, these results imply that a certain index related to obesity or anatomical fat deposit, but not analyzed in this study, might be superior to the prediction of HRQoL and the guidance of nutritional support in CKD population, which should be further highlighted in the future.
Contrary to the overall EQ-5D index, the mobility limitation, a dimension of EQ-5D questionnaire, was significantly associated with obesity in group 3 population as well as in general population. A previous study reported the associations of body size and composition with functional ability and HRQoL in patients undergoing HD40, where no statistical significance was observed between HRQoL index and adiposity measures, such as BMI, WC, and intra-abdominal fat. These conflicting findings may result from the different study population, as the previous study included subjects with end-stage renal diseases only, while the subjects in the current study are not restricted to dialysis-dependents individuals. Considering the trend of association between overall EQ-5D index and obesity according to CKD stages, it seems quite reasonable that impact of obesity on the mobility also attenuates as CKD progresses form earlier stages to end stage. Hence, our results collectively suggest that medical interventions targeting obesity control should be recommended in a subset of CKD patients who have physically active lifestyle for better HRQoL outcomes.
There are some limitations to our study. First, we cannot elucidate causal relationships, as a cross-sectional survey data was analyzed. Second, eGFR value may not accurately reflect the actual status of kidney function, since serum creatinine and other laboratory values for each participants were measured only once. Third, no data is available regarding renal replacement therapy in this survey. Therefore, CKD population in the present study may include both nondialysis-dependent and dialysis-dependent subjects. Fourth, the number of study participants in each subgroup is considerably disproportional, although the numbers represent all of non-institutionalized general population in South Korea. Finally, because the cohort of this survey consists of only Koreans dwelling in South Korea, caution is required if our results are to be generalized to other ethnic groups or other nations.
In conclusion, we demonstrated that, compared to that in general population, the association of obesity with HRQoL was significantly modified by kidney function, suggesting that CKD attenuates the impact of obesity on HRQoL. The results in the present study indicate that the risk of obesity should not be over-estimated in patients with CKD, especially with respect to HRQoL.
Methods
Study design and participants
The Korea National Health and Nutrition Examination Survey (KNHANES) is a nationwide population-based cross-sectional study of the health and nutritional status of the noninstitutionalized Korean population. It consists of a health questionnaire, physical/laboratory examinations, and nutrition survey. The present study analyzed data obtained from KNHANES 2008 to 2011 (Fig. 1), because the measurement of anatomical fat distribution using dual-energy X-ray absorptiometry (DXA) is included in data from that period. The raw data of KNHANES is open to the registered investigators online (https://knhanes.cdc.go.kr). Written informed consent was obtained from each participant in KNHANES at the time of enrollment. Of 37,753 participants in the health questionnaire and physical/laboratory examination of KNHANES 2008 to 2011, participants lack of DXA measurement (n = 16,448), younger than 20 years (n = 452) were excluded. Participants lack of body fat mass measurement (n = 462) or lack of EQ-5D index (n = 1,433) were also excluded. After excluding participants whose kidney function is not accurately estimated (n = 1,955), finally 17,001 participants were included in the analyses. This study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-EXP-2019-205), and was conducted in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards.
Assessment of HRQoL
HRQoL was assessed using the Korean version of the EQ-5D health questionnaire modified from its original version in English18 and the EQ-VAS, which are included in KNHANES. The EQ-5D descriptive system comprises 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each of which are answered with 3 levels: no problem, some problem, and extreme problem (Fig. S1). The possible combination results in 243 (=35) unique health states. Using a scoring algorithm, EQ-5D index scores are calculated from the combination of answers to the questionnaire, ranging from 1 (optimal health) to −0.171 (worst health state or death)41. The EQ-5D index has been widely used to quantify HRQoL both in both general18 and specific disease populations, such as subjects with CKD populations42. Its validity and reliability have been also demonstrated in Koreans26. In this study, subjects with mobility limitation was defined as those who answered with “some problem” or “extreme problem” in mobility dimension of EQ-5D health questionnaire. In EQ-VAS, study participants marked their subjective health status using a visual scale ranging from 0 (the worst imaginable health state) to 100 (the best imaginable health state).
Measurement of regional fat distribution using DXA
DXA is a gold standard method for the measurement of regional fat distribution43. Fat mass and muscle mass were measured using DXA (QDR 4800 A; Hologic Inc., Bedford, MA, USA). TF, LF, and WF as well as the ratio of TF/WF and LF/WF, were analyzed in the present study to define regional fat distribution.
Anthropometric and clinical measurement
Trained staff members measured the height and weight of study participants. BMI was calculated as weight divided by the height squared. Comorbidities with physician diagnosis were analyzed. Blood samples were collected after at least an 8-hour fast, properly processed, immediately refrigerated, and transported in cold storage to the central laboratory (Neodin Medical Institute, Seoul, Korea) within 24 hours. Proteinuria was defined as albuminuria (≥1+) determined by dipstick urine test.
Assessment of kidney function
eGFR was calculated from serum creatinine level using the CKD-EPI (CKD Epidemiology Collaboration) equation44. The CKD-EPI equation more accurately categorizes risk for death than the MDRD (Modification of Diet in Renal Disease) Study equation45 and has also demonstrated its accuracy in Asian populations46, including Koreans47. All study subjects were classified according to eGFR, as follows: Group 1, ≥90 mL/min/1.73 m2; Group 2, eGFR of 60–89 mL/min/1.73 m2; Group 3, eGFR <60 mL/min/1.73 m2. CKD was defined as the presence of proteinuria or eGFR <60 mL/min/1.73 m2.
Statistical analysis
Data are presented as the mean ± standard deviation for continuous variables, and as number, or percent for categorical variables. To compare the difference in the baseline characteristics according to kidney function, one-way analysis of variance and χ2 test were used for continuous and categorical variables, respectively. The composite sample weight was introduced in the analyses to estimate the noninstitutionalized Korean population (Table S10). Survey weight for the participants in the health questionnaire and physical/laboratory examinations was computed using the sampling rate, response rate, and age/sex proportion of the Korean population. Differences in HRQoL according to various indices related to obesity and regional fat distribution were evaluated in models with different variables in all or subsets of study participants. Starting with a crude model, blocks of variables were sequentially introduced to evaluate their effects on the association between HRQoL and obesity. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for comorbidities (diabetes, hypertension, dyslipidemia, coronary artery disease, stroke, chronic obstructive pulmonary disease, and smoking). Model 3 was further adjusted for laboratory finding (eGFR, hemoglobin, ferritin, fasting plasma glucose and triglyceride, 25-hydroxyvitamin D, parathyroid hormone, alkaline phosphatase, and proteinuria). Concerned with the co-linearity between the independent variables, hemoglobin and ferritin, we revealed only a weak correlation, with a correlation coefficient of 0.29 (Fig. S2). To minimize bias resulted from the relatively small number of subjects with renal dysfunction, the subjects were dichotomized by incidence of CKD, and were propensity score-matched at a 1:1 ratio, where propensity scores were based on age and sex. Statistical analyses were performed using R software version 3.6.0, with a survey package (https://cran.r-project.org/package=survey) for analyses of complex survey data. P < 0.05 was considered statistically significant.
References
Mokdad, A. H. et al. Prevalence of Obesity, Diabetes, and Obesity-Related Health Risk Factors, 2001. JAMA 289, 76–79, https://doi.org/10.1001/jama.289.1.76 (2003).
Kelly, T., Yang, W., Chen, C. S., Reynolds, K. & He, J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 32, 1431–1437, https://doi.org/10.1038/ijo.2008.102 (2008).
Jee, S. H. et al. Body-Mass Index and Mortality in Korean Men and Women. New England Journal of Medicine 355, 779–787, https://doi.org/10.1056/NEJMoa054017 (2006).
Lee, C. M., Huxley, R. R., Wildman, R. P. & Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol 61, 646–653, https://doi.org/10.1016/j.jclinepi.2007.08.012 (2008).
Ahima, R. S. & Lazar, M. A. The Health Risk of Obesity—Better Metrics Imperative. Science 341, 856–858, https://doi.org/10.1126/science.1241244 (2013).
Neeland, I. J. et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging 6, 800–807, https://doi.org/10.1161/CIRCIMAGING.113.000532 (2013).
Han, E. et al. Anatomic fat depots and cardiovascular risk: a focus on the leg fat using nationwide surveys (KNHANES 2008-2011). Cardiovasc Diabetol 16, 54, https://doi.org/10.1186/s12933-017-0536-4 (2017).
Camara, N. O., Iseki, K., Kramer, H., Liu, Z. H. & Sharma, K. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol 13, 181–190, https://doi.org/10.1038/nrneph.2016.191 (2017).
Ostergaard, M. V. et al. DBA2J db/db mice are susceptible to early albuminuria and glomerulosclerosis that correlate with systemic insulin resistance. Am J Physiol Renal Physiol 312, F312–F321, https://doi.org/10.1152/ajprenal.00451.2016 (2017).
Wolf, G. et al. Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [seecomments]. Kidney Int 56, 860–872, https://doi.org/10.1046/j.1523-1755.1999.00626.x (1999).
Fang, F. et al. Deletion of the gene for adiponectin accelerates diabetic nephropathy in the Ins2 (+/C96Y) mouse. Diabetologia 58, 1668–1678, https://doi.org/10.1007/s00125-015-3605-9 (2015).
Johansen, K. L. et al. Association between body composition and frailty among prevalent hemodialysis patients: a US Renal Data System special study. J Am Soc Nephrol 25, 381–389, https://doi.org/10.1681/ASN.2013040431 (2014).
Yun, H. R. et al. Obesity, Metabolic Abnormality, and Progression of CKD. Am J Kidney Dis 72, 400–410, https://doi.org/10.1053/j.ajkd.2018.02.362 (2018).
Kaplan, R. M. & Bush, J. W. Health-related quality of life measurement for evaluation research and policy analysis. Health Psychology 1, 61–80, https://doi.org/10.1037/0278-6133.1.1.61 (1982).
John E. Ware, J. The Status of Health Assessment 1994. Annual Review of Public Health 16, 327–354, https://doi.org/10.1146/annurev.pu.16.050195.001551 (1995).
Karimi, M. & Brazier, J. Health, Health-Related Quality of Life, and Quality of Life: What is the Difference? Pharmacoeconomics 34, 645–649, https://doi.org/10.1007/s40273-016-0389-9 (2016).
Brazier, J., Roberts, J. & Deverill, M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 21, 271–292 (2002).
Rabin, R. & de Charro, F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 33, 337–343 (2001).
Seidell, J. C. et al. The relation between overweight and subjective health according to age, social class, slimming behavior and smoking habits in Dutch adults. American Journal of Public Health 76, 1410–1415, https://doi.org/10.2105/ajph.76.12.1410 (1986).
Jia, H. & Lubetkin, E. I. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf) 27, 156–164, https://doi.org/10.1093/pubmed/fdi025 (2005).
Bentley, T. G. et al. Race and gender associations between obesity and nine health-related quality-of-life measures. Qual Life Res 20, 665–674, https://doi.org/10.1007/s11136-011-9878-7 (2011).
Chow, F. Y. et al. Health-related quality of life in Australian adults with renal insufficiency: a population-based study. Am J Kidney Dis 41, 596–604, https://doi.org/10.1053/ajkd.2003.50121 (2003).
Chin, H. J. et al. Moderately decreased renal function negatively affects the health-related quality of life among the elderly Korean population: a population-based study. Nephrology Dialysis Transplantation 23, 2810–2817, https://doi.org/10.1093/ndt/gfn132 (2008).
Lee, H. et al. The association of moderate renal dysfunction with impaired preference-based health-related quality of life: 3rdKorean national health and nutritional examination survey. BMC Nephrology 13, 19, https://doi.org/10.1186/1471-2369-13-19 (2012).
Campbell, K. H. et al. Association between estimated GFR, health-related quality of life, and depression among older adults with diabetes: the Diabetes and Aging Study. Am J Kidney Dis 62, 541–548, https://doi.org/10.1053/j.ajkd.2013.03.039 (2013).
Park, J. I., Baek, H. & Jung, H. H. CKD and Health-Related Quality of Life: The Korea National Health and Nutrition Examination Survey. Am J Kidney Dis 67, 851–860, https://doi.org/10.1053/j.ajkd.2015.11.005 (2016).
Shadyab, A. H., Li, W., Eaton, C. B. & LaCroix, A. Z. General and Abdominal Obesity as Risk Factors for Late-Life Mobility Limitation After Total Knee or Hip Replacement for Osteoarthritis Among Women. Arthritis Care & Research 70, 1030–1038, https://doi.org/10.1002/acr.23438 (2018).
Sharma, A. Body mass index and mobility limitations: An analysis of middle-aged and older Black, Hispanic, and White women in the U.S. Obes Res Clin Pract 12, 547–554, https://doi.org/10.1016/j.orcp.2018.06.001 (2018).
Latif, W. et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol 6, 2470–2477, https://doi.org/10.2215/CJN.00670111 (2011).
Kim, C. S. et al. Relationship between serum uric acid and mortality among hemodialysis patients: Retrospective analysis of Korean end-stage renal disease registry data. Kidney Res Clin Pract 36, 368–376, https://doi.org/10.23876/j.krcp.2017.36.4.368 (2017).
Niskanen, L. K. et al. Uric Acid Level as a Risk Factor for Cardiovascular and All-Cause Mortality in Middle-aged Men: A Prospective Cohort Study. JAMA Internal Medicine 164, 1546–1551, https://doi.org/10.1001/archinte.164.14.1546 (2004).
Hoieggen, A. et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int 65, 1041–1049, https://doi.org/10.1111/j.1523-1755.2004.00484.x (2004).
Oh, T. R. et al. Hyperuricemia has increased the risk of progression of chronic kidney disease: propensity score matching analysis from the KNOW-CKD study. Sci Rep 9, 6681, https://doi.org/10.1038/s41598-019-43241-3 (2019).
Kalantar-Zadeh, K., Kilpatrick, R. D., McAllister, C. J., Greenland, S. & Kopple, J. D. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension 45, 811–817, https://doi.org/10.1161/01.HYP.0000154895.18269.67 (2005).
Liu, Y. et al. Association Between Cholesterol Level and Mortality in Dialysis PatientsRole of Inflammation and Malnutrition. JAMA 291, 451–459, https://doi.org/10.1001/jama.291.4.451 (2004).
Suliman, M. et al. The reverse epidemiology of plasma total homocysteine as a mortality risk factor is related to the impact of wasting and inflammation. Nephrol Dial Transplant 22, 209–217, https://doi.org/10.1093/ndt/gfl510 (2007).
Scholze, A., Rattensperger, D., Zidek, W. & Tepel, M. Low Serum Leptin Predicts Mortality in Patients with Chronic Kidney Disease Stage 5. Obesity 15, 1617–1622, https://doi.org/10.1038/oby.2007.191 (2007).
Fouque, D. et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73, 391–398, https://doi.org/10.1038/sj.ki.5002585 (2008).
Schweitzer, M. L. et al. Obesity phenotype and patient-reported outcomes in moderate and severe chronic kidney disease: a cross-sectional study from the CKD-REIN cohort study. Qual Life Res 28, 1873–1883, https://doi.org/10.1007/s11136-019-02110-2 (2019).
Martinson, M. et al. Associations of body size and body composition with functional ability and quality of life in hemodialysis patients. Clin J Am Soc Nephrol 9, 1082–1090, https://doi.org/10.2215/CJN.09200913 (2014).
Lee, Y. K. et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health 12, 1187–1193, https://doi.org/10.1111/j.1524-4733.2009.00579.x (2009).
Jesky, M. D. et al. Health-Related Quality of Life Impacts Mortality but Not Progression to End-Stage Renal Disease in Pre-Dialysis Chronic Kidney Disease: A Prospective Observational Study. PLoS One 11, e0165675, https://doi.org/10.1371/journal.pone.0165675 (2016).
Glickman, S. G., Marn, C. S., Supiano, M. A. & Dengel, D. R. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. Journal of Applied Physiology 97, 509–514, https://doi.org/10.1152/japplphysiol.01234.2003 (2004).
Levey, A. S. et al. A New Equation to Estimate Glomerular Filtration RateDevelopment and Comparison of a New Equation to Estimate GFR. Annals of Internal Medicine 150, 604–612, https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Matsushita, K. et al. Comparison of Risk Prediction Using the CKD-EPI Equation and the MDRD Study Equation for Estimated Glomerular Filtration RateCKD-EPI vs MDRD for Glomerular Filtration Rate. JAMA 307, 1941–1951, https://doi.org/10.1001/jama.2012.3954 (2012).
Teo, B. W. et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis 58, 56–63, https://doi.org/10.1053/j.ajkd.2011.02.393 (2011).
Jeong, T. D. et al. Comparison of the MDRD study and CKD-EPI equations for the estimation of the glomerular filtration rate in the Korean general population: the fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1), 2010. Kidney Blood Press Res 37, 443–450, https://doi.org/10.1159/000355724 (2013).
Acknowledgements
This research was supported by the Bio & Medical Development Program of the National Research Foundation funded by the Ministry of Science and ICT, Republic of Korea (2017M3A9E8023001), by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea (HI18C0331), and by a grant of Chonnam National University Hospital Biomedical Research Institute (BCRI18027).
Author information
Authors and Affiliations
Contributions
S.H. Suh and S.W. Kim desinged the study. S.W. Kim acquired the data. S.H. Suh analyzed the data. H.S. Choi, C.S. Kim, E.H. Bae, S.K. Ma, D.H. Lee interpreted the results. S.H. Suh prepared all figures and tables in the manuscripts. S.H. Suh and S.W. Kim wrote the main manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suh, S.H., Choi, H.S., Kim, C.S. et al. Chronic kidney disease attenuates the impact of obesity on quality of life. Sci Rep 10, 2375 (2020). https://doi.org/10.1038/s41598-020-59382-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59382-9
This article is cited by
-
Understanding health problems in people with extremely low health-related quality of life in Korea
Scientific Reports (2022)
-
Parental educational status independently predicts the risk of prevalent hypertension in young adults
Scientific Reports (2021)
-
Low serum adiponectin level is associated with better physical health-related quality of life in chronic kidney disease
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.