Abstract
Urban expansion threatens biodiversity worldwide, therefore urban spaces need to be amenable to biodiversity conservation. On trees in urban environments, natural colonisation and successful translocation of epiphytic orchids are necessary to enhance urban biodiversity, and depend on the availability of compatible orchid mycorrhizal fungi (OMF). However, the extent of OMF presence and distribution, as well as niche requirements for the OMF, remain poorly studied. To identify and quantify OMF on urban trees as well as assess their suitability for native epiphytic orchids, we conducted high-throughput sequencing on tree bark and orchid root samples. OMF were detected at 60% of the study sites on 16% of 270 bark samples (from stem, fork, and branch microsites within each tree). OMF presence and richness on bark samples were related to multiple biophysical factors; in general, humus presence and precipitation levels were positively predictive of OMF presence and richness. We found Ceratobasidiaceae- and Serendipitaceae-associated OMF both on bark and within roots. Orchid species also showed differing mycorrhizal specificity. Sites associated with fungal genera Ceratobasidium, Rhizoctonia, and Serendipita were considered suitable habitats for seven orchid species. The results suggest that urban trees support OMF and are therefore suitable for native orchid species; however, OMF availability are largely constrained by biophysical factors. To maximise the likelihood of translocation success and consequent natural establishment, we propose that (micro)sites are screened for compatible OMF prior to any intervention.
Similar content being viewed by others
Introduction
Urbanisation is continually imposing extensive pressures on biodiversity worldwide. With the increase in global population size and an increased tendency towards residence in urban areas, the risk of biodiversity loss continues to rise1,2. Urban expansion is projected to occur near protected areas and biodiversity hotspots, notably in developing regions such as Southeast Asia, China, and South America3. Urbanisation may lead to habitat degradation, fragmentation, and/or loss, which consequently lead to demographic or genetic isolation as well as species extirpation4,5. Indeed, urbanisation has induced substantial biodiversity loss; therefore careful management and conservation strategies in urban spaces are required to contribute to biodiversity conservation6,7,8,9. With urban areas becoming increasingly widespread, the need to assess the capacity of these spaces for biodiversity conservation becomes more urgent, necessitating knowledge of taxa and sites that are amenable to conservation in non-forest habitats.
Because of habitat loss and over-collection, one of the richest plant families worldwide, Orchidaceae, is also one of the most endangered10,11,12. Orchids are sensitive to anthropogenic environmental changes, chiefly because of their strong reliance on other taxa such as unique pollinators and mycorrhizal fungi13,14,15,16. Despite this, orchids have been found to colonise urban trees, indicating potential suitability for conservation in non-natural landscapes17,18,19. Furthermore, some orchids are amenable to ex situ conservation in non-forest habitats20,21,22,23. To improve the likelihood of successful ex situ efforts, detailed assessments regarding habitat limitations and requirements are necessary.
Orchid mycorrhizal fungi (OMF) play a pivotal role in determining orchid establishment, survival, and development13,24, especially for epiphytic species that often establish under water and nutrient-scarce conditions25,26. By forming obligate relationships with compatible fungi, orchid hosts gain carbon, nutrients, and water at one or more life history stages13,27. The majority of orchids associate with fungal taxa belonging to the polyphyletic/“rhizoctonia” group of Basidiomycetes, specifically Ceratobasidiaceae, Serendipitaceae (Sebacinales), and Tulasnellaceae13,28,29. Identifying specific OMF may be advantageous for orchid conservation—e.g., orchid propagation, ex situ seeding, seedling translocation—as contrasting fungal taxa may differ in nutrient uptake efficiency or stimulation of germination30,31,32,33.
Even though orchids show strong dependence on mycorrhizal fungi, OMF presence and distribution are independent of orchid distribution34,35. Indeed most research has inferred OMF presence and spatial distribution from orchid roots35,36,37,38. While root-based inferences can help determine species-specific mycorrhizal associations, they do not directly inform the extent of OMF presence and distribution13. With the advent of high-throughput sequencing technologies, microbial communities, diversity, and biogeography can be assessed at high resolutions39,40. High-throughput sequencing allows recovery of microbial amplicons, including unculturable microorganisms, from environmental samples such as soil and tree bark41. In this context, high-throughput sequencing can facilitate large-scale screening for known OMF on potential (micro)sites. Variation in biophysical conditions are also reported to influence OMF presence and distribution (i.e. niche requirements)31,42. Moreover, effects of specific biophysical factors (e.g., ambient temperature, relative humidity) may vary between micro/local and macro/landscape scale43,44. Fundamentally, the availability of suitable OMF and niche conditions are imperative for both in situ as well as ex situ conservation to succeed24,45,46,47,48.
Singapore has undergone massive land transformation (>99% of its original forest cover transformed), making it one of the most modified countries in the world49,50. To address vegetation and species loss, the city-state adopts multifarious policies and programmes that are dedicated to urban greening51,52,53. The progressive nature of the country’s urban-green matrix makes Singapore an apt study model for developing nations and cities in relation to biodiversity conservation in the urban landscape (e.g., habitat enrichment, managed relocation). The “orchid conservation programme” was one of many schemes administered by the Singapore government to address vegetation and species loss; this programme entailed experimental as well as restoration plantings of native epiphytic orchids—including nationally Endangered and Presumed Nationally Extinct species—on multiple sites, mainly urban parks23,52. Between 2009 and 2011, seedlings were propagated asymbiotically and planted on microsites (i.e. stem, fork, and branch) of various host trees, mostly Albizia saman (Jacq.) Merr. trees21. Following translocation, various orchid species formed symbioses with compatible OMF that were available on the host trees20,24. These translocated orchids provide an opportunity for the detection and identification of potential orchid-fungal associations. This is especially valuable for native species that are naturally rare or extinct. Unique circumstance such as this can provide both qualitative and empirical data on relevant OMF, and thereby, help devise decision-making frameworks that can facilitate the identification of alternative (micro)sites that may be applicable to future conservation attempts54,55.
In this study, we investigated whether OMF are available on urban roadside trees. To get an overview of the presence and distribution of OMF on urban sites as well as their suitability for native epiphytic orchids (i.e. presence of compatible OMF), we assessed the mycorrhizal communities of both the tree bark (microsites) and roots of translocated orchid species (from the abovementioned “orchid conservation programme”) by using DNA barcodes and high-throughput (Illumina® MiSeq) sequencing technology. We also investigated possible biophysical factors that could influence OMF presence and richness on urban sites. As previous studies have reported orchid colonisations in urban environments, we expected OMF to be present on urban trees18,19. We also expected variations in OMF presence to be related to specific biophysical factors. Knowledge of OMF availability and associated niche requirements can help with the selection of suitable (micro)sites for future orchid conservation efforts.
Results
Overall, ~41.7 million reads were obtained and assigned to 270 bark samples and 65 root samples (average number of reads per sample = 80,396 for bark samples and 308,289 for root samples). After quality-filtering, each bark and root sample contained 0–36 and 0–48 unique sequences respectively. Clustering sequences to OTUs yielded 593 OTUs (6,270 unique sequences), of which 26 were assigned to putative OMF-OTUs in three families: Ceratobasidiaceae (8 OMF-OTUs), Serendipitaceae (10 OMF-OTUs), and Tulasnellaceae (8 OMF-OTUs). OMF-OTUs were found on 43 of 270 (16%) of bark samples/microsites (Table 1), or more broadly, 18 out of 30 sites (Fig. 1). We also successfully retrieved OMF-OTUs from roots of 8 of the 11 orchid species sampled (Table 2; see also Supplementary Table S1 The Ceratobasidiaceae- and Serendipitaceae-related sequences were present across both bark and root samples, whereas Tulasnellaceae-related sequences were detected only in orchid roots. The ranges of OMF-OTUs were 0–3 per microsite (mean = 0.2), 0–6 per site (mean = 1.6), and 0–10 per orchid species (mean = 2.0). Non-rhizoctonia OTUs encountered on bark samples and in orchid roots were largely Ascomycetes, e.g., genera Fusarium (Hypocreales), Lachnum (Helotiales), and Curvularia (Pleosporales); basidiomycete representatives such as genera Mycena (Agaricales) and Marasmius (Agaricales) were also detected.
The composition of OMF varied between sites. The more common Ceratobasidiaceae was present at 18 sites, of which five had Serendipitaceae present as well (Fig. 1). Both “grassland” and “urban” sites had the highest frequency of OMF occurrence while the former had the highest OMF richness (Fig. 2). “Secondary forest” sites had the lowest frequency of OMF occurrence and richness. The NMDS showed differentiation in fungal community composition between the three habitat-types, particularly “secondary forest” and “grassland” sites (Fig. 3), which was statistically supported by ANOSIM test (Bray-Curtis; R = 0.16, P = 0.04).
The overall OMF richness across microsites was 12 OMF-OTUs (RichnessChao2 = 17.0 ± 8.1); the closeness of the OMF richness estimate (Chao2 diversity estimator) suggested that our sampling effort (n = 126) was adequate. The fork microsites had the highest frequency of OMF occurrence whereas the branch microsites had the highest OMF richness (Fig. 2). The stem microsites had the lowest frequency of OMF occurrence and richness.
Members of Ceratobasidiaceae (4 OMF-OTUs), Serendipitaceae (5 OMF-OTUs), and Tulasnellaceae (8 OMF-OTUs) occurred in the roots of eight orchid species (Table 2). Three species were associated with representatives of Ceratobasidiaceae, six with Serendipitaceae, and two with Tulasnellaceae. In addition, two orchid species were associated with more than one rhizoctonia family: Dendrobium leonis (Lindl.) Rchb. f. with Serendipitaceae and Tulasnellaceae, and Cymbidium bicolor Lindl. ssp. pubescens (Lindl.) Du Pay & Cribb with Ceratobasidiaceae and Serendipitaceae. Five orchid species were associated with one OMF-OTU except D. leonis (11 OMF-OTUs), Coelogyne rochussenii de Vr. (3 OMF-OTUs), and C. bicolor (2 OMF-OTUs) (Table 2). Based on genus similarity between bark- and root-associated OMF-OTUs (i.e. orchid-site suitability assessment), sites associated with members of Ceratobasidium (Ceratobasidiaceae) were considered as potential habitats for the orchid species C. bicolor and C. rochussenii, Rhizoctonia-associated (Ceratobasidiaceae) sites for C. rochussenii and Phalaenopsis cornu-cervi (Breda) Blume & Rchb. f., and Serendipita-associated (Serendipitaceae) sites for Bulbophyllum medusae (Lindl.) Rchb. f., C. bicolor, Cymbidium finlaysonianum Lindl., Dendrobium aloifolium (Blume) Rchb. f., and D. leonis (see Table 1).
At the microsite scale, habitat-type corresponded to OMF presence (Table 3). Mean daily rainfall and presence of humus were positively predictive of both OMF presence and richness. Similarly, humus presence positively influenced presence and richness of Ceratobasidiaceae-related OMF. For Serendipitaceae, OMF presence showed a positive association with humus presence while OMF richness positively corresponded to presence of moss and mean daily rainfall. At the site scale, the overall model indicated the positive relationship between frequency of OMF occurrence and mean daily rainfall. The same trend held true when considering only Serendipitaceae-related OMF. By contrast, no biophysical factor was predictive of Ceratobasidiaceae-related OMF occurrence. No spatial autocorrelation was detected in all data sets.
Discussion
Fungal communities associating with tree microsites and orchid roots
Knowing the presence as well as identity of OMF on microsites and within orchid roots can facilitate the process of selecting both suitable microsite and compatible orchid species for translocation attempts. By employing Illumina® ITS-targeted sequencing approach on bark samples, we found several Ceratobasidiaceae- and Serendipitaceae-related taxa that are commonly associated with both terrestrial and epiphytic orchids13,37. Similarly, we detected both fungal taxa in the roots of native epiphytic orchids, as well as Tulasnellaceae-associated OMF. However, the latter was not detected on bark samples; this shortfall was likely due to the downstream effects of inhibitory compounds such as polysaccharides and phenolics, which are typically found in recalcitrant materials such as tree bark56. These inhibitors are known to negatively affect primer efficacy by disrupting the annealing process of the primer to the DNA template during amplification and/or sequencing process, i.e. competitive binding57. In this case, it is likely that the ITS1/ITS4-TUL primer combination is highly susceptible to this inhibitory effect. Nevertheless, these findings suggest that OMF that are associated with the study species may be present on urban roadside trees as well.
Non-rhizoctonia OMF were also discovered on microsites; although largely unknown, this fungal group may potentially form mycorrhizal associations with native epiphytic orchid species. For instance, the basidiomycetous fungal genera—Mycena (Agaricales; sequence identity = 99%) and Marasmius (Agaricales; sequence identity = 90%)—were previously reported to form mycorrhizal relationships with epiphytic orchids, including species from the genus Dendrobium25,58,59,60. A specific ascomycete genus, Fusarium (sequence identity = 99%), was also detected on bark samples. Although ascomycetes are rarely involved in orchid mycorrhizas13, Salifah et al.61 demonstrated that Fusarium sp. can establish functional mycorrhizae with Grammatophyllum speciosum Blume seeds. In this study, we chose a conservative approach of considering only rhizoctonia fungi (i.e. Ceratobasidiaceae, Serendipitaceae, and Tulasnellaceae) for analyses and excluded other possible OMF. Nevertheless, we hope to see more studies implicate OMF beyond the three rhizoctonia taxa, especially since increasingly more OMF, both basidiomycetous and ascomycetous fungi, are being discovered37,62. We also encourage future studies to include other primer sets that may improve the detection as well as coverage of OMF on bark samples.
Some orchid species may exhibit differing mycorrhizal specificity. We found five orchid species associated with only one OMF-OTU, suggesting high mycorrhizal specificity25,63. Conversely, three orchid species associated with multiple OMF-OTUs, of which two formed associations with more than one rhizoctonia taxa, suggesting low mycorrhizal specificity. We also discovered a pair of orchid species from the genus Cymbidium associating with the same Serendipita OMF-OTU. Possible similarities in orchid mycorrhizal fungal preference among closely related epiphytic species were also reported in Martos et al.25. Overall, knowledge on mycorrhizal specificity can influence a species’ suitability for ex situ conservation. It is likely that species with low mycorrhizal specificity are preferable due to their less restrictive mycorrhizal preference25. Our evaluation of orchid-mycorrhizal specificity was based on roots sampled from translocated orchids only and thus may be limited. As the study species were part of Singapore’s “orchid conservation programme”, root sampling was constrained as well. Therefore, to improve the assessment of orchid-mycorrhizal specificity as well as the detection and coverage of OMF, we encourage prospective studies to involve more root samples collected from multiple sites, ideally (if possible), both natural and non-natural areas.
We also discovered non-mycorrhizal taxa that may benefit orchid development. Members of saprotrophic Hypocreales, Helotiales, and Pleosporales were identified on bark and root samples. These non-mycorrhizal taxa, which are frequently found within orchid roots, have been reported to indirectly amplify nutrient access to orchids via decomposition of local substrates64,65,66. However, the exact functions and effects of non-OMF on orchid physiology remain largely unknown and warrant further research attention13,67.
Mycorrhizal distribution and niche requirements
OMF were widespread, occurring at majority of the study sites. We found that surrounding land use influenced OMF presence, with grassland habitat-types having the highest frequency of OMF occurrence and richness. This occupancy pattern may imply that dispersal and subsequent establishment of orchid mycorrhizal fungal spores/inocula are largely unhindered at sites that are surrounded by large, open areas—in this case, managed turf—unlike forests, in which wind velocities are typically reduced68,69.
In general, OMF presence and richness were correlated with presence of humus, a substrate derived from decomposed organic matter such as dead plant parts, stemflow leachates, and plant exudates70,71. Humus commonly accumulates at tree forks, forming vital sources of nutrients and water for mycelial survival and growth23,72. This may be the primary reason why the fork microsite, which is structurally ideal for aggregation of organic debris, had the highest frequency of OMF occurrence. On the other hand, we observed OMF richness to be highest at the branch microsite. Given that tree branches tend to be exposed and structurally angled (cf. fork and vertical stem), it is possible that this part of the tree has the highest capacity for interception of diverse fungal spores/inocula and rain73. The open structure and orientation of tree branches however may not be ideal for humus accumulation (cf. fork); this may perhaps be the reason why the frequency of OMF occurrence on branch microsites was slightly lower than fork microsites.
Our results denote the central role of precipitation levels in determining OMF presence and richness at both microsite and site scale. Fungal establishment and mycelial development are particularly dependent on moisture conditions72; Querejeta et al.74 noted that fungal mortality increases with lower moisture, particularly during dry periods. Presence of mosses can enhance the water-holding capacity of microsites75,76. The association between richness of Serendipitaceae-associated OMF and both rainfall and moss presence suggests that members of this fungal taxon may be more sensitive to moisture limitation than that of Ceratobasidiaceae-associated OMF. The additional dependence on moisture conditions may explain their lower frequency of occurrence and richness, as well as limited distribution, as compared to Ceratobasidiaceae-associated OMF.
Overall, our findings indicate that OMF presence and richness vary in accordance to precipitation levels and humus presence. Both biophysical factors are also known to influence orchid germination and development25,26,77, suggesting possible overlap between niche requirements of OMF and orchid species. Such OMF-orchid correspondence can benefit the microsite selection process, therefore improving the likelihood of a successful translocation.
Potential applications in orchid conservation
Our findings demonstrate the widespread presence and distribution of OMF-associated (micro)sites as well as their potential as orchid habitats. Based on the orchid-site suitability assessment, the similarities in orchid mycorrhizal fungal associations between bark and orchid root samples may suggest high amenability of native orchids towards translocation efforts, especially for species with low mycorrhizal specificity20,78. Such species are able to associate with different fungal lineages, and possibly multiple OMF partners, which can exploit various nutritional resources, and as a consequence, enhance the nutrient uptake of host orchid66,79,80. Orchid species that showed high mycorrhizal specificity may be advantageous for orchid conservation as well. Unique orchid-fungal affiliations may induce spatial differentiation among establishment sites (at tree or site scale), leading to possible co-occupancy of multiple species81,82.
The geographic distribution of OMF at landscape scale highlights the prevalence of sites suited for orchid species that associated with Ceratobasidiaceae-related OMF; thus, it might be sensible to focus on these species in future translocation attempts. Although more limited, Serendipitaceae-associated sites appear to have greater potential for orchid conservation as this fungal taxon was linked with five study species. To maximise this potential, we propose fungal inoculations on microsites that meet the niche requirements of Serendipitaceae-associated OMF, i.e. presence of humus, moss, and high precipitation levels (see McCormick et al.47). In fact, this may be a good pre-translocation practice for any applicable species. Since high-throughput sequencing-based quantification of species abundance is prone to overestimation83, we did not quantify the orchid mycorrhizal fungal density/potential (abundance and vitality) of each site. Nevertheless, fungal density/potential can influence a microsite’s capacity to support orchid establishment and development and therefore should be considered in future research24,47.
This study demonstrates that translocated orchids can form relationships with compatible OMF on host trees in non-forest habitats, in this case, urban parks (root sampling sites). De hert et al.84, Keel et al.85, and Waud et al.78 observed mycorrhizal associations in germinated seeds that were sowed in novel, unoccupied habitats, i.e. sites without adults. Similarly, Downing et al.20 detected OMF in orchids that were translocated beyond their natural ranges. These evidence suggest that communities of OMF in non-natural habitats, including urban landscapes, can support orchid establishment, survival, as well as development and thus should be considered in future ex situ conservation efforts. Ideally, these fungal communities should have the capacity to support both germinating seeds and mature orchids86.
This is the first study to explore OMF presence and distribution, as well as assess the biophysical factors that influence both aspects, on epiphytic microsites directly via bark samples, rather than indirectly via orchid roots. By employing metabarcoding and high-throughput sequencing, we were able to screen a large number of environmental samples and characterise fungal communities at a much higher resolution than before, thus making it an efficient and valuable management tool for large-scale detection of OMF, i.e. potential orchid colonisation sites. This tool may be particularly useful in dynamic or disturbed habitats whereby OMF are stochastically available87,88,89. Absence of obligate OMF must be dealt with if orchid conservation programmes are to be successful with lasting conservation benefits90. Applicable countermeasures include fungal isolation, preservation, and propagation as well as on-site inoculation of relevant fungal cultures20,47,91,92. Cevallos et al.93 noted the influence of keystone species: a core OMF species in which mycorrhizal communities are built around. Given their fundamental role in mycorrhizal assembly, future conservation strategies should perhaps prioritise keystone OMF and regularly monitor (e.g., via molecular detection) for their continued presence13.
Ultimately, knowledge on specific OMF—primarily presence, geographical distribution, and niche requirements—is imperative for future plans of establishing orchids, especially endangered species, in either natural or urban landscapes13,24,94. Knowledge on another biotic dependency of orchids, specific pollinator(s), is equally crucial for orchid propagation and therefore continuity15. Hence we encourage future studies to focus on orchid-pollinator interaction as well, especially in urban habitats. With appropriate planning, urban environments can serve as viable habitats for epiphytic orchid species, and accordingly, help mitigate their extinction risks.
Methods
Species selection
We focussed on 11 native species (planted as part of the “orchid conservation programme”; see Yam et al.23 for descriptions); except for Bulbophyllum vaginatum (Lindl.) Rchb. F. (nationally Endangered), C. bicolor (nationally Critically Endangered), and C. finlaysonianum (nationally Critically Endangered), all other species—Bulbophyllum blumei (Lindl.) J. J. Sm., B. medusae, Coelogyne mayeriana Rchb. f., C. rochussenii, D. aloifolium, D. leonis, G. speciosum, and P. cornu-cervi—are Presumed Nationally Extinct95 (see Supplementary Table S1). The average number of years since planting of all orchid species is 4.2 years. These species were selected primarily for three reasons: (1) differing conservation statuses, (2) easy access by bucket crane/ladder for root sampling, and (3) consistency in host tree, as all individuals were planted on Albizia saman trees. Albizia saman—native to South America—is the most widely planted urban tree in Singapore96. It has distinctive characteristics such as wide, umbrella-shaped crown, leaf-free inner branches, and flaky bark that make this species favourable host trees for epiphyte colonisation19.
Sample collection
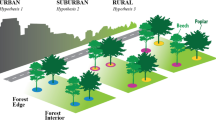
Through collaboration with Singapore’s National Parks Board, we generated a comprehensive list of roads planted with Albizia saman trees. From this list, 30 roads/sites were randomly selected, ten in each habitat stratum (“habitat-type”) defined by the dominant surrounding land use type17 (Fig. 1): urban (surrounded by man-made structures), grassland (surrounded by large areas of manicured turf grass), or secondary forest (surrounded by tree-dominated urban parks, lowland tropical forest, or young secondary forest patches occurring on previously degraded or cleared land)97. The dominant land use was defined as >50% of the area within an octagon of ~ 200 m diameter, drawn in Google Earth around the selected site (see Supplementary Fig. S1). At each site, three individual trees were randomly selected and the location of each tree was recorded using a Garmin® GPSMAP 60CSx GPS receiver (Garmin International, Inc., Olathe, KS, USA).
To quantify the presence of OMF on roadside trees, we sampled bark (~25 cm2 per sample) from 90 urban trees in September 2016. For each tree, sample collection points within each of the three microsites—stem, fork, and branch—were randomly selected (total microsites = 270). We then employed a grid system and random number generator to select a specific quadrat for bark collection. Each individual sample was placed in a storage bag and stored at -80 °C prior to DNA extraction.
We sampled roots of translocated orchids to identify species-specific OMF (independent of bark samples). Approximately 5–6 orchid roots were collected from six different individuals per species. To avoid cross-contamination, root samples were collected using a sterilised scalpel and rubber gloves, placed in separate storage bags, and stored at -80 °C until DNA extraction. In total, 65 samples were collected from four different urban parks (see Supplementary Table S1); one B. medusae individual was not collected during the sampling period due to tree pruning.
Several biophysical variables were recorded at each tree: microsite location (stem/fork/branch), substrate (presence/absence of humus and moss), tree DBH (diameter at breast height; measured with a diameter tape, at 1.3 m above the base), mean ambient temperature, mean rainfall, and mean wind speed. Climate data were 5-year daily averages, calculated from collated weather records provided by Meteorological Service Singapore98. We also measured the nearest distance between individual trees located on the same road and distance of trees from nearest forest vegetation via Google Earth. Bark chemistry (e.g., pH, hydrocarbon content) was not quantified and analysed in this study.
Molecular analyses
To extract fungal DNA from tree bark, the material of each sample was removed by scraping the surface with sterile scalpel. Approximately 50 mg of this material was ground using liquid nitrogen, sterile mortar, and pestle, followed by bead-based homogenisation in Bead Ruptor 24 (Omni International, Kennesaw, GA, USA). To extract fungal DNA from orchid roots, the root material was cut into 5 cm pieces, washed with Milli-Q® water (Merck & Co., Inc., Kenilworth, NJ, United States) for 2 min, surface-sterilised in 1% sodium hypochlorite for 1 min, and rinsed with Milli-Q® for 2 min. Six to eight root sections per plant were pooled (~50 mg) and homogenised using Bead Ruptor 24.
Total DNA was extracted from both homogenised bark and root samples using a modified CTAB protocol99. The fungal nuclear ribosomal internal transcribed spacer (ITS) sequences were amplified using primer combinations ITS86F/ITS4100 and ITS1/ITS4-TUL101. Each primer was tagged with a 9 bp long sequence for specimen-to-sequence association and a dual indexing strategy was used. Two replicates of polymerase chain reaction (PCR) amplifications were conducted for each DNA extract. PCRs were conducted in 25 µl reaction volumes containing 10 µl of 100-times diluted DNA extract and 15 µl of GoTaq® Colorless Master Mix (Promega Corporation, Madison, WI, USA). PCR temperature profile for ITS86F/ITS4 primer-pair was as follows: initial denaturation step at 94 °C for 3 min, followed by 30 cycles of denaturation at 94 °C for 45 s each, annealing at 59 °C for 45 s, and extension at 72 °C for 2 min. For ITS1/ITS4-TUL primer-pair, PCR conditions were as follows: initial denaturation step at 96 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s each, annealing at 60 °C for 40 s, and extension at 72 °C for 1 min. The final cycle of both amplifications was followed by a 7-min extension at 72 °C. PCR products were verified on 1% agarose gel, quantified using Qubit® 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MS, USA), pooled in equimolar quantities, and cleaned using MinElute PCR Purification Kit (Qiagen, Hilden, Germany). The pooled products were subjected to paired-end (2 × 300 bp) sequencing on an Illumina® MiSeq sequencer (Illumina, Inc., San Diego, California, USA). Libraries were prepared using NEB Ultra II DNA Library Preparation and amplification-free protocol (New England BioLabs, Massachusetts, USA).
The sequence reads were merged using PEAR version 0.9.11102 and processed using OBITools version 1.2.11103. Samples and replicates were demultiplexed using the ngsfilter command; only amplified regions remained for subsequent analyses. We then employed the obiuniq command to identify and cluster strictly identical sequences. Sequences with <1% sample-specific percentage occurrence were removed (potential amplification/sequencing errors). Sequences shorter than 100 bp were also omitted via the obigrep command. Given that there were two replicates of PCRs per sample, only sequences that passed the various filtering criteria in both replicates were retained for further analyses. The sequences were then aligned and clustered into operational taxonomic units (OTUs) at 97% sequence similarity criterion using VSEARCH version 2.6.0104. To identify the different OTUs, BLAST searches were performed on representative sequences to determine the closest match represented in GenBank (nt database). No ties—i.e. same OTU sequence giving hit to multiple species at same best identity—were encountered. A taxonomic assignment was given for a match of ≥85% identity (as done in Xing et al.105,106). Based on current orchid mycorrhizal fungal knowledge, only OTUs related to rhizoctonia taxa (i.e. Ceratobasidiaceae, Sebacinales/Serendipitaceae, Tulasnellaceae; see Dearnaley et al.13 and Jacquemyn et al.27) were considered potentially mycorrhizal (i.e. OMF-OTU). Other OTUs were considered as non-rhizoctonia OTUs, which are endophytic fungi that associate with orchid roots that may or may not provide benefit to the host107. Note that we also tested sequence identification using ecotag in OBITools and EMBL database as well as BLAST to UNITE database, but overall, GenBank yielded better matches.
Additionally, orchid-site suitability was assessed based on similarity of fungal taxa at genus-level, i.e. generic correspondence between bark- and root-associated OMF-OTUs. Thus, if a specific OMF genus was detected on a bark sample—a representative of site—as well as in an orchid root sample, we considered the site to be a potential habitat for the orchid species. We chose to compare fungal genera rather than species because (1) based on a recent large-scale study by Martos et al.25, tropical epiphytic orchids tend to have low mycorrhizal specificity, mainly associating with typical genera/species found in the three rhizoctonia families and (2) the fungal databases consist of numerous poor-quality fungal sequences—particularly species-level sequences—with low-resolution taxonomic annotations and subpar technical quality108,109. Hence to achieve a reasonable yet conservative compromise, a genus-based comparative approach was adopted.
Statistical analyses
A non-metric multidimensional scaling (NMDS) analysis based on the Bray-Curtis dissimilarity matrix was used to visualize variation in orchid mycorrhizal fungi between habitat-types110; we then performed a variance analysis of these distances using analysis of similarities (ANOSIM)111. We also used the nonparametric diversity estimator Chao2112 to estimate the OMF richness of the microsite-level data set.
We applied either binomially/Poisson-distributed generalised linear models (GLMs) or generalised linear mixed models (GLMMs)113 to explore the correspondence of biophysical factors with (1) the presence of OMF (any OMF-OTU) at the microsite level, (2) OMF richness (i.e. number of OMF-OTUs) at the microsite-level, and (3) frequency of OMF occurrence (i.e. total number of microsites with OMF-OTUs per site) at the site-level. Multicollinearity was assessed and highly collinear variables were removed from the models. All models were conducted at two levels: (1) overall (i.e. all orchid mycorrhizal fungal families) and (2) family-specific. When overdispersion was evident, the model was fitted with quasi-binomial or quasi-Poisson error structure. The explanatory variables for the models were all recorded biophysical variables. “Tree” was a random factor for all GLMMs. Akaike’s information criterion (AIC)114 was employed for step-wise simplification and model evaluation. Additionally, GLM deviance was estimated as goodness-of-fit. Spatial autocorrelation was examined using correlograms and variograms115.
NMDS, ANOSIM, and diversity estimator test were conducted on PAST Version 3.17116. All other analyses were conducted using R version 3.4.2117, including the packages “spatial”118 and “car”119.
Data availability
The datasets generated during the current study are available in the figshare repository, https://doi.org/10.6084/m9.figshare.8063366.v1.
References
Angel, S., Parent, J., Civco, D. L., Blei, A. & Potere, D. The dimensions of global urban expansion: estimates and projections for all countries, 2000–2050. Prog. Plann. 75, 53–107 (2011).
Seto, K. C., Fragkias, M., Güneralp, B. & Reilly, M. K. A meta-analysis of global urban land expansion. Plos One 6, e23777 (2011).
Güneralp, B. & Seto, K. C. Futures of global urban expansion: uncertainties and implications for biodiversity conservation. Environ. Res. Lett. 8, 1–10 (2013).
Gardner, T. A. et al. Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 12, 561–582 (2009).
Ricketts, T. H. The matrix matters: effective isolation in fragmented landscapes. Am. Nat. 158, 87–99 (2001).
Fischer, L. K., Rodorff, V., Lippe, Mvd & Kowarik, I. Drivers of biodiversity patterns in parks of a growing South American megacity. Urban. Ecosyst. 19, 1231–1249 (2016).
Goddard, M. A., Dougill, A. J. & Benton, T. G. Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol. Evol. 25, 90–98 (2010).
Lin, H. L. Land use planning, environmental management, and the Garden City as an urban development approach in Singapore In Land Use Law For Sustainable Development (eds. Chalifour, N. J., Kameri-Mbote, P., Lin, H. L. & Nolon, J. R.) 374–396 (Cambridge University Press, (2007).
Wang, J. W. et al. Building biodiversity: drivers of bird and butterfly diversity on tropical urban roof gardens. Ecosphere 8, 1–22 (2017).
Cribb, P. J., Kell, S. P., Dixon, K. W. & Barrett, R. L. Orchid conservation: a global perspective In Orchid Conservation (eds. Dixon, K. W., Kell, S. P., Barrett, R. L. & Cribb, P. J.) 1–24 (Natural History Publications, (2003).
Kull, T. & Hutchings, M. J. A comparative analysis of decline in the distribution ranges of orchid species in Estonia and the United Kingdom. Biol. Conserv. 129, 31–39 (2006).
Phelps, J. & Webb, E. W. “Invisible” wildlife trades: Southeast Asia’s undocumented illegal trade in wild ornamental plants. Biol. Conserv. 186, 296–305 (2015).
Dearnaley, J. D. W., Martos, F. & Selosse, M. A. Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects In Fungal Associations (ed. Hock, B.) 207–230 (Springer-Verlag, (2012).
Dixon, K. W. & Phillips, R. D. The orchid conservation challenge. Lankesteriana 7, 11–12 (2007).
Gravendeel, B., Smithson, A., Slik, F. J. W. & Schuiteman, A. Epiphytism and pollinator specialization: drivers for orchid diversity? Philos. T. R. Soc. B 359, 1523–1535 (2004).
Bower, C. C. Specific pollinators reveal a cryptic taxon in the bird orchid, Chiloglottis valida sensu lato (Orchidaceae) in south-eastern Australia. Aust. J. Bot. 54, 53–64 (2006).
Bhatt, A., Gairola, S., Govender, Y., Baijnath, H. & Ramdhani, S. Epiphyte diversity on host trees in an urban environment, eThekwini Municipal Area, South Africa. New Zeal. J. Bot. 53, 24–37 (2015).
Izuddin, M. & Webb, E. L. The influence of tree architecture, forest remnants, and dispersal syndrome on roadside epiphyte diversity in a highly urbanized tropical environment. Biodivers. Conserv. 24, 2063–2077 (2015).
Wee, Y. C. Vascular epiphytes of Singapore’s wayside trees. Gard. Bull. Singap. 31, 114–126 (1978).
Downing, J. L. et al. Contrasting changes in biotic interactions of orchid populations subject to conservation introduction vs. conventional translocation in tropical China. Biol. Conserv. 212, 29–38 (2017).
Izuddin, M., Yam, T. W. & Webb, E. L. Specific niche requirements drive long-term survival and growth of translocated epiphytic orchids in an urbanised tropical landscape. Urban. Ecosyst. 21, 531–540 (2018).
Scade, A., Brundrett, M. C., Batty, A. L., Dixon, K. W. & Sivasithamparam, K. Survival of transplanted terrestrial orchid seedlings in urban bushland habitats with high or low weed cover. Aust. J. Bot. 54, 383–389 (2006).
Yam, T. W., Tay, F., Ang, P. & Soh, W. Conservation and reintroduction of native orchids of Singapore—the next phase. Eur. J. Environ. Sci. 1, 38–47 (2011).
Rasmussen, H. N., Dixon, K. W., Jersáková, J. & Těšitelová, T. Germination and seedling establishment in orchids: a complex of requirements. Ann. Bot. 116, 391–402 (2015).
Martos, F. et al. The role of epiphytism in architecture and evolutionary constraint within mycorrhizal networks of tropical orchids. Mol. Ecol. 21, 5098–5109 (2012).
Zotz, G. & Hietz, P. The physiological ecology of vascular epiphytes: current knowledge, open questions. J. Exp. Bot. 52, 2067–2078 (2001).
Rasmussen, H. N. Terrestrial Orchids From Seed To Mycotrophic Plant (Cambridge University Press, 1995).
Rasmussen, H. N. & Rasmussen, F. N. Seedling mycorrhiza: a discussion of origin and evolution in Orchidaceae. Bot. J. Linn. Soc. 175, 313–327 (2014).
Weiß, M., Waller, F., Zuccaro, A. & Selosse, M. A. Sebacinales—one thousand and one interactions with land plants. New Phytol. 211, 20–40 (2016).
Brundrett, M. C. Scientific approaches to Australian temperate terrestrial orchid conservation. Aust. J. Bot. 55, 293–307 (2007).
Jacquemyn, H., Waud, M., Merckx, V., Lievens, B. & Brys, R. Mycorrhizal diversity, seed germination and long-term changes in population size across nine populations of the terrestrial orchid Neottia ovata. Mol. Ecol. 24, 3269–3280 (2015).
van der Heijden, M. G. A., Wiemken, A. & Sanders, I. R. Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plants. New Phytol. 157, 569–578 (2003).
Zi, X. M., Sheng, C. L., Goodale, U. M., Shao, S. C. & Gao, J. Y. In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 24, 487–499 (2014).
Jersáková, J. & Malinová, T. Spatial aspects of seed dispersal and seedling recruitment in orchids. New Phytol. 176, 237–241 (2007).
McCormick, M. K. et al. Abundance and distribution of Corallorhiza odontorhiza reflect variations in climate and ectomycorrhizae. Ecol. Monogr. 79, 619–635 (2009).
Jacquemyn, H. et al. Nonrandom spatial structuring of orchids in a hybrid zone of three Orchis species. New Phytol. 193, 454–464 (2012).
Jacquemyn, H., Duffy, K. J. & Selosse, M. A. Biogeography of orchid mycorrhizas In Biogeography Of Mycorrhizal Symbiosis (ed. Tedersoo, L.) 159–177 (Springer International Publishing, 2017).
Swarts, N. D., Sinclair, E. A., Francis, A. & Dixon, K. W. Ecological specialization in mycorrhizal symbiosis leads to rarity in an endangered orchid. Mol. Ecol. 19, 3226–3242 (2010).
Lentendu, G. et al. Assessment of soil fungal diversity in different alpine tundra habitats by means of pyrosequencing. Fungal Divers. 49, 113–123 (2011).
Öpik, M., Metsis, M., Daniell, T. J., Zobel, M. & Moora, M. Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 184, 424–437 (2009).
Davison, J. et al. Communities of arbuscular mycorrhizal fungi detected in forest soil are spatially heterogeneous but do not vary throughout the growing season. Plos One 7, e41938 (2012).
Jacquemyn, H. et al. Co-existing orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytol. 202, 616–627 (2014).
Philips, R. D., Barrett, M. D., Dalzeill, E. L., Dixon, K. W. & Swarts, N. D. Geographical range and host breadth of Sebacina orchid mycorrhizal fungi associating with Caladenia in south-western Australia. Bot. J. Linn. Soc. 175, 140–151 (2016).
Voyron, S., Ercole, E., Ghignone, S., Perotto, S. & Girlanda, M. Fine-scale spatial distribution of orchid mycorrhizal fungi in the soil of host-rich grasslands. New Phytol. 213, 1428–1439 (2016).
Bidartondo, M. I. & Read, D. J. Fungal specificity bottlenecks during orchid germination and development. Mol. Ecol. 17, 3707–3716 (2008).
McCormick, M. K. & Jacquemyn, H. What constrains the distribution of orchid populations? New Phytol. 202, 392–400 (2014).
McCormick, M. K. et al. Limitations on orchid recruitment: not a simple picture. Mol. Ecol. 21, 1511–1523 (2012).
Reiter, N. et al. Orchid re-introductions: an evaluation of success and ecological considerations using key comparative studies from Australia. Plant Ecol. 217, 81–95 (2016).
Corlett, R. T. The ecological transformation of Singapore, 1819–1990. J. Biogeogr. 19, 411–420 (1992).
Sodhi, N. S., Koh, L. P., Brook, B. W. & Ng, P. K. L. Southeast Asian biodiversity: an impending disaster. Trends Ecol. Evol. 19, 654–660 (2004).
Ministry of National Development. An Endearing Home, A Distinctive Global City (Ministry of National Development, (2008).
Tan, P. Y., Wang, J. & Sia, A. Perspectives on five decades of the urban greening of Singapore. Cities 32, 24–32 (2013).
Yuen, B. Creating the garden city: the Singapore experience. Urban Stud. 33, 955–970 (1996).
Liu, H. et al. Potential challenges of climate change to orchid conservation in a wild orchid hotspot in southwestern China. Bot. Rev. 76, 174–192 (2010).
Lunt, I. D. et al. Using assisted colonisation to conserve biodiversity and restore ecosystem function under climate change. Biol. Conserv. 157, 172–177 (2013).
Bahnweg, G. et al. DNA isolation from recalcitrant materials such as tree roots, bark, and forest soil for the detection of fungal pathogens by polymerase chain reaction. Anal. Biochem. 15, 79–82 (1998).
Schrader, C., Schielke, A., Ellerbroek, L. & Johne, R. PCR inhibitors – occurrence, properties and removal. J. Appl. Microbiol. 113, 1014–1026 (2012).
Burgeff, H. Mycorrhizas of orchids In The Orchids (ed. Withner, K.) 361–395 (Ronald, 1959).
Ogura-Tsujita, Y., Gebauer, G., Hashimoto, T., Umata, H. & Yukawa, T. Proc. R. Soc. Lond. B 276, 761–768 (2009). Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena.
Zhang, L., Chen, J., Lv, Y., Gao, C. & Guo, S. Mycena sp., a mycorrhizal fungus of the orchid Dendrobium officinale. Mycol. Prog. 11, 395–401 (2012).
Salifah, H. A. B., Muskhazli, M., Rusea, G. & Nithiyaa, P. Variation in mycorrhizal specificity for in vitro symbiotic seed germination of Grammatophyllum speciosum Blume. Sains Malays. 40, 451–455 (2011).
van der Heijden, M. G. A., Martin, F. M., Selosse, M. A. & Sanders, I. R. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 205, 1406–1423 (2015).
Jacquemyn, H. et al. Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity in the genus Orchis (Orchidaceae). New Phytol. 192, 518–528 (2011).
Herrera, P., Suárez, J. P. & Kottke, I. Orchids keep the ascomycetes outside: a highly diverse group of ascomycetes colonizing the velamen of epiphytic orchids from a tropical mountain rainforest in Southern Ecuador. Mycology 1, 262–268 (2010).
Tedersoo, L. et al. Ascomycetes associated with ectomycorrhizas: molecular diversity and ecology with particular reference to the Helotiales. Environ. Microbiol. 11, 3166–3178 (2009).
Waterman, R. J. et al. The effects of above- and belowground mutualisms on orchid speciation and coexistence. Am. Nat. 177, 1–15 (2011).
Wang, X. et al. Influence of host tree species on isolation and communities of mycorrhizal and endophytic fungi from roots of a tropical epiphytic orchid, Dendrobium sinense (Orchidaceae). Mycorrhiza 27, 709–718 (2017).
Malloch, D. & Blackwell, M. Dispersal of fungal diaspores In The Fungal Community: Its Organization And Role In The Ecosystem, Second Edition (eds. Carroll, G. C. & Wicklow, D. T.) 147–172 (Marcel Decker, (1992).
Tisserat, N. & Kuntz, J. E. Dispersal gradients of conidia of the butternut canker fungus in a forest during rain. Can. J. Forest Res. 13, 1139–1144 (1983).
Awasthi, O. P., Sharma, E. & Palni, L. M. S. Stemflow: a source of nutrients in some naturally growing epiphytic orchids of the Sikkim Himalaya. Ann. Bot. 75, 5–11 (1995).
Böhnert, T. et al. Effects of land-use change on vascular epiphyte diversity in Sumatra (Indonesia). Biol. Conserv. 202, 20–29 (2016).
Osono, T., Ono, Y. & Takeda, H. Fungal ingrowth on forest floor and decomposing needle litter of Chamaecyparis obtusa in relation to resource availability and moisture condition. Soil Biol. Biochem. 35, 1423–1431 (2003).
Chapela, I. H. & Boddy, L. Fungal colonization of attached beech branches. New Phytol. 110, 47–57 (1988).
Querejeta, J. I., Egerton-Warburton, L. M. & Allen, M. F. Hydraulic lift may buffer rhizosphere hyphae against the negative effects of severe soil drying in a California Oak savanna. Soil Biol. Biochem. 39, 409–417 (2007).
Cascante-Marin, A. C., Wolf, J. H. D., Oostermeijer, J. G. B. & Den Nijs, J. C. M. Establishment of epiphytic bromeliads in successional tropical premontane forests in Costa Rica. Biotropica 40, 441–448 (2008).
Nadkarni, N. M. Colonization of stripped branch surfaces by epiphytes in a lower montane cloud forest, Monteverde, Costa Rica. Biotropica 32, 358–363 (2000).
Diez, J. M. Hierarchical patterns of symbiotic orchid germination linked to adult proximity and environmental gradients. J. Ecol. 95, 159–170 (2007).
Waud, M., Brys, R., Van Landuyt, W., Lievens, B. & Jacquemyn, H. Mycorrhizal specificity does not limit the distribution of an endangered orchid species. Mol. Ecol. 26, 1687–1701 (2017).
Jacquemyn, H., Deja, A., De hert, K., Bailarote, B. C. & Lievens, B. Variation in mycorrhizal associations with Tulasnelloid fungi among populations of five Dactylorhiza species. Plos One 7, e42212 (2012).
Leake, J. R. & Cameron, D. D. Physiological ecology of mycoheterotrophy. New Phytol. 185, 601–605 (2010).
Jacquemyn, H., Brys, R., Lievens, B. & Wiegand, T. Spatial variation in below-ground seed germination and divergent mycorrhizal associations correlate with spatial segregation of three co-occurring orchid species. J. Ecol. 100, 1328–1337 (2012).
Těšitelová, T., Těšitel, J., Jersáková, J., Říhová, G. & Selosse, M. A. Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am. J. Bot. 99, 1020–1032 (2012).
Medinger, R. et al. Diversity in a hidden world: potential and limitation of next-generation sequencing for surveys of molecular diversity of eukaryotic microorganisms. Mol. Ecol. 19, 32–40 (2010).
De hert, K., Jacquemyn, H., Provoost, S. & Honnay, O. Absence of recruitment limitation in restored dune slacks suggests that manual seed introduction can be a successful practice for restoring orchid populations. Restor. Ecol. 21, 159–162 (2013).
Keel, B. G., Zettler, L. W. & Kaplin, B. A. Seed germination of Habenaria repens (Orchidaceae) in situ beyond its range, and its potential for assisted migration imposed by climate change. Castanea 76, 43–54 (2011).
Oliveira, S. F. et al. Endophytic and mycorrhizal fungi associated with roots of endangered native orchids from the Atlantic Forest, Brazil. Mycorrhiza 24, 55–64 (2014).
Brundrett, M. C., Scade, A., Batty, A. L., Dixon, K. W. & Sivasithamparam, K. Development of in situ and ex situ seed baiting techniques to detect mycorrhizal fungi from terrestrial orchid habitats. Mycol. Res. 107, 1210–1220 (2003).
Izuddin, M., Yam, T. W. & Webb, E. L. Germination niches and seed persistence of tropical epiphytic orchids in an urban landscape. J. Plant Res. 132, 383–394 (2019).
Kartzinel, T. R., Trapnell, D. W. & Shefferson, R. P. Critical importance of large native trees for conservation of a rare Neotropical epiphyte. J. Ecol. 101, 1429–1438 (2013).
Whigham, D. F., O’Neill, J. P., Rasmussen, H. N., Caldwell, B. A. & McCormick, M. K. Seed longevity in terrestrial orchids—potential for persistent in situ seed banks. Biol. Conserv. 129, 24–30 (2006).
Batty, A. L., Dixon, K. W., Brundrett, M. & Sivasithamparam, K. Long-term storage of mycorrhizal fungi and seed as a tool for the conservation of endangered Western Australian terrestrial orchids. Aust. J. Bot. 49, 619–628 (2001).
Nontachaiyapoom, S., Sasirat, S. & Manoch, L. Isolation and identification of Rhizoctonia-like fungi from roots of three orchid genera, Paphiopedilum, Dendrobium, and Cymbidium, collected in Chiang Rai and Chiang Mai provinces of Thailand. Mycorrhiza 20, 459–471 (2010).
Cevallos, S., Sánchez-Rodríguez, A., Decock, C., Declerck, S. & Suárez, J. P. Are there keystone mycorrhizal fungi associated to tropical epiphytic orchids? Mycorrhiza 27, 225–232 (2017).
Batty, A. L., Dixon, K. W., Brundrett, M. C. & Sivasithamparam, K. Orchid conservation and mycorrhizal associations In Microorganisms In Plant Conservation And Biodiversity (eds. Sivasithamparam, K., Dixon, K. W. & Barrett, R. L.)195–226 (Kluwer Academic Publishers, (2002).
Chong, K. Y., Tan, H. T. W. & Corlett, R. T. A Checklist Of The Total Vascular Plant Flora Of Singapore: Native, Naturalised And Cultivated Species (Raffles Museum of Biodiversity Research, (2009).
Tan, P. Y., Yeo, B., Yip, W. X. & Lua, H. S. Carbon Storage And Sequestration By Urban Trees In Singapore (Centre for Urban Greenery and Ecology, (2009).
Yee, A. T. K., Corlett, R. T., Liew, S. C. & Tan, H. T. W. The vegetation of Singapore—an updated map. Gard. Bull. Singap. 63, 205–212 (2011).
Meteorological Service Singapore. Historical Daily Records. National Environment Agency, Singapore http://www.weather.gov.sg/climate-historical-daily/ (2017).
Doyle, J. J. & Doyle, J. L. Isolation of plant DNA from fresh tissue. Focus 12, 13–15 (1990).
Waud, M., Busschaert, P., Ruyters, S., Jacquemyn, H. & Lievens, B. Impact of primer choice on characterization of orchid mycorrhizal communities using 454 pyrosequencing. Mol. Ecol. Resour. 14, 679–699 (2014).
Taylor, D. L. & McCormick, M. K. Internal transcribed spacer primers and sequences for improved characterization of basidiomycetous orchid mycorrhizas. New Phytol. 177, 1020–1033 (2008).
Zhang, J., Kobert, K., Flouri, T. & Stamatakis, A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30, 614–620 (2014).
Boyer, F. et al. OBITOOLS: a UNIX-inspired software package for DNA metabarcoding. Mol. Ecol. Resour. 16, 176–182 (2016).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, 1–22 (2016).
Xing, X., Gai, X., Liu, Q., Hart, M. M. & Guo, S. Mycorrhizal fungal diversity and community composition in a lithophytic and epiphytic orchid. Mycorrhiza 25, 289–296 (2015).
Xing, X. et al. Specificity and preference of mycorrhizal associations in two species of the genus Dendrobium (Orchidaceae). Mycorrhiza 23, 317–324 (2013).
Dearnaley, J. D. W. Further advances in orchid mycorrhizal research. Mycorrhiza 17, 475–486 (2007).
Nilsson, R. H. et al. Towards standardization of the description and publication of next-generation sequencing datasets of fungal communities. New Phytol. 191, 14–318 (2011).
Nilsson, R. H. et al. Improving ITS sequence data for identification of plant pathogenic fungi. Fungal Divers. 67, 11–19 (2014).
Bray, J. R. & Curtis, J. T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349 (1957).
Clarke, K. R. Non‐parametric multivariate analyses of changes in community structure. Austral Ecol. 18, 117–143 (1993).
Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43, 783–791 (1987).
McCullagh, P. & Nelder, J. A. Generalized Linear Models: Monographs On Statistics And Applied Probability, Second Edition (Chapman and Hall, (1989).
Akaike, H. A new look at the statistical model identification. IEEE T. Automat. Contr. 19, 716–723 (1974).
Legendre, P. & Fortin, M. J. Spatial pattern and ecological analysis. Vegetatio 80, 107–138 (1989).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: paleontolological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9 (2001).
R Development Core Team. R version 3.4.2. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org (2017).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics With S, Fourth Edition. (Springer, (2002).
Fox, J. & Weisberg, S. An {R} Companion To Applied Regression, Second Edition. (Sage, 2011).
Acknowledgements
We are thankful to Peter Ang, Muhammad Noh Al, and Maryam Nadheera for their valuable assistance. The molecular work and computational resources were supported by SEABIG (grants R-154-000-648-646 and R-154-000-648-733).
Author information
Authors and Affiliations
Contributions
M.I. wrote the main manuscript text as well as conducted laboratory with A.L.L. and molecular analyses with A.S. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Izuddin, M., Srivathsan, A., Lee, A.L. et al. Availability of orchid mycorrhizal fungi on roadside trees in a tropical urban landscape. Sci Rep 9, 19528 (2019). https://doi.org/10.1038/s41598-019-56049-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56049-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.