Abstract
Neonates of different species are born with a set of predispositions that influence their early orienting responses toward the first stimuli encountered in their life. Human neonates and domestic chicks exhibit several similarities in the predisposition for attending to objects that move with speed changes, face-like stimuli and biological motion. Although early predispositions are connected to physiological development, little is known on the temporal course of early predispositions (whether they are stable or change in time) and on the associated genetic variability. To address these issues, we tested the preference for objects that change in speed vs. linear motion in three chicken breeds (Padovana, Polverara and Robusta maculata) within one day after hatching and three days after hatching. We found that the predisposition to preferentially attend to changes in speed is shared by different breeds on the first day of life and that it disappears by day three. These results indicate the existence of a short and transient time window of early predispositions that does not depend on visual experience.
Similar content being viewed by others
Introduction
For human adults, objects that move with visible speed changes appear animate, as if they move through an internal energy source1,2. Experiments with very young and inexperienced subjects show that the relevance of dynamic changes in speed does not need to be learned. In fact, both human neonates3 and visually naïve chicks1 are more attracted to objects that move with changing speed compared to objects that move linearly. These data suggest that this early unlearned preference might be widespread among vertebrate species. The aim of this work is to shed light on the temporal course and genetic variability associated with this predisposition.
The preference for speed changes has been described as a predisposition to attend to “animacy” cues, the properties associated with living beings4,5. Other animacy cues that are attractive for neonates include face-like stimuli5,6,7,8,9,10, biological motion11,12 and self-propulsion13. The initial orienting response towards animate objects, in turn, enhances learning and social interactions. This phenomenon has been described in domestic chicks, that quickly develop affiliative responses towards the first objects they are exposed to (especially for moving objects), through the mechanism of filial imprinting14,15,16,17. Imprinting can also modulate predisposed responses18. While chicks are a convenient model to investigate early predispositions – because they are a precocial19 species that can be easily tested in the absence of previous visual experience – human babies show striking similarities in the response to the stimuli preferentially attended and approached by chicks7,11,12,13,20,21,22,23,24,25,26. Interestingly, both human neonates at high familial risk of autism23 and chicks24,25 exposed to drugs (the histone deacetylases inhibitor valproic acid) that increase the risk of developing autism in humans27,28,29 exhibit an impairment (or a temporal shift) in early predispositions for animacy. This is another piece of evidence that points at a possible conserved origin of early predispositions in vertebrates. Comparative studies are important to clarify the origins and neurobiological mechanisms of early predispositions.
Some works have started to shed light on the temporal course of early predispositions. The spontaneous preference for biological motion documented in two-day old chicks was not identified in five-day old chick30. Morever, previous studies on chicks’ predisposition to preferentially approach a stuffed-hen vs. a scrambled version of the same stimulus showed that 24 hours after motor stimulation (walking in a wheel) chicks exhibited a much stronger preference for the stuffed-hen than after 2 hours after motor stimulation8,31. The preference for the stuffed hen is linked to the head and neck region of the model, suggesting that this predisposition is connected to the preference for the inverted triangular shape of the face-like stimuli6,8. While even human foetuses have been shown to preferentially orient towards this pattern32, it is known that the preference for face-like patterns is not stable during development, declining at around two months of age33,34,35. Shultz and colleagues36 made the interesting point of a general pattern of transitions from reflexive behaviours to volitional actions in human infants. A working hypothesis is that early predispositions are transient not only in human neonates but in other species as well, and that they decline at an age in which volitional control in approach responses takes over. Such a mechanism would be fixed at the species level, showing little genetic variability.
Control experiments with occluders that hid the change in speed have shown that the preference disappears when the speed change is not visible, showing that this predisposition is not simply a preference for a particular speed11. Moreover, chicks’ spontaneous preferences extended to stimuli that changes direction, which is another animacy cue1. The temporal course of the predisposition for changes in speed has not been previously investigated. To address this issue, we tested chicks in the first day after hatching (post-hatching day 0, p0) and three days after hatching (post-hatching day 2, p2). Previous studies showed that while the very first orienting responses to the stuffed hen are fixed across different breeds, the subsequent responses of sustained attention have a genetic basis that differs between breeds7. To investigate whether the predisposition to orient towards changes in speed and its temporal course have genetic variability, we conducted the study in three breeds of domestic chickens that have been kept genetically isolated for 20 years in the same farm: Padovana, Polverara and Robusta maculata. This arrangement ensures that differences between breeds are not due to environmental differences.
Methods
Ethics statement
All experiments comply with the current Italian and European Union laws for the ethical treatment of animals and the experimental procedures were approved by the Ethical Committee of University of Trento and licensed by the Italian Health Ministry (permit number 1138/2015 PR).
Subjects and rearing conditions
Fertilized eggs of three breeds of domestic fowl (Gallus gallus) kept genetically isolated for 20 years, avoiding interbreeding, in the conservation programme CO.VA37,38 were obtained from the Agricultural High School “Duca degli Abruzzi”, Padova (Italy). Details on this population, breeds and their early predisposition are available in previous publications7,39,40. Briefly, each flock contained about 54 individuals for each breed (34 females and 20 males), divided in two groups. To avoid inbreeding, the groups were alternately mated with the females. Each year the progeny replaced the parents as breeding livestock and males were exchanged among the flocks in a circular scheme. Eggs were incubated in darkness at a constant temperature of 37.7 °C, humidity 40% until 3 days before hatching (day 17 of incubation). Three days before hatching, the humidity was increased at 60% and eggs of each breed were located on the same tray for hatching. Until the moment of the test, chicks were kept in constant darkness inside the incubator in a dark room, making sure they did not receive any visual stimulation until the moment of the test. Each chick was tested only once.
In Experiment 1, we tested 117 domestic chicks (Gallus gallus) and analysed the performance of the 88 chicks that moved during the test (it was possible to calculate a preference index only for these chicks): 31 chicks of Padovana breed (PD), 30 chicks of Polverara breed (PL), 27 chicks of Robusta maculata breed (RB). Chicks were tested at day post-hatching 0 (p0, 12–24 hours after hatching) and were kept in the incubator until the moment of the test.
In Experiment 2, we tested 77 chicks and analysed the performance of the 51 domestic chicks that moved during the test (it was possible to calculate a preference index only for these chicks): 31 chicks of Padovana breed (PD) and 20 chicks of Polverara Breed (PL). Chicks were tested at day post-hatching 2 (p2, 3 days after hatching) and were kept in the incubator until the moment of the test. In this experiment, only two breeds were used because there no more eggs of the Robusta breed were available.
After the test, chicks were housed in groups and maintained with food and water available ad libitum in standard rearing conditions until donated to local farmers.
Apparatus
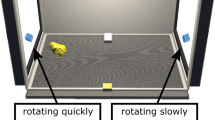
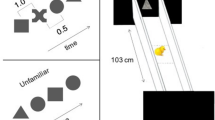
The test apparatus was a rectangular black box (85 cm length × 62 cm width × 58 cm height). At the opposite sides of the apparatus, two monitors (LCD Monitor BenQ XL2410T, resolution 1920 × 1080 pixels, refresh frequency 120 Hz, 48.7 cm width × 36.5 height) played the stimuli. One monitor showed the stimulus with changes in speed (animate stimulus), the other one showed the linearly moving stimulus (see section Stimuli), see Fig. 1.
A running wheel (30 cm diameter, 13 cm width, covered with 1 cm of opaque foam on both sides) was located in the middle of the apparatus at a distance of 40 cm from each monitor). Above the wheel, a video-camera (Windows Lifecam) at a distance of 77 cm from the bottom of the running wheel recorded the experimental sessions. We recorded the centimetres run by the chicks in the wheel in both directions using magnetic sensors located on the sides of the wheel. In the running wheel, subjects could easily change the direction of movement. The experiment was run in darkness except for the stimuli played on the monitors.
Stimuli
We played the same stimuli used in a previous study for the measurement of spontaneous preferences for speed changes in visually naïve chicks1. Briefly, the stimuli consisted of video animations of a red disk (3 cm diameter) on a black background moving horizontally. A horizontal grey band was used as a floor on which the stimulus moved. The 30 cm wide screen was bounded by lateral grey bars at the sides (3 cm wide), but placed on the screen in order to occupy only 2.5 cm of visible screen space, so that the visible trajectory of the red stimulus was 24.6 cm long, see1 for further details).
The two stimuli were presented simultaneously on the opposite monitors. The stimulus always entered the visible scene already in motion and then disappeared from view while still moving. One stimulus always exhibited visible speed changes along its trajectory (the “animate stimulus”), whereas the other stimulus moved at a constant speed (the “non-animate stimulus”). The non-animate stimulus moved at constant 4.64 cm/s speed. The animate stimulus had an initial speed of 3.37 cm/s, at one-third of its way it abruptly accelerated to 19.64 cm/s up to two-thirds of the route, where abruptly decelerated at the initial speed. Both stimuli moved at the same average speed. Previous control experiments showed that the response to change in speed, and not a generic preference for the presence of different speeds presented in subsequent displays, drive the preference exhibited by chicks1. The stimuli appeared and disappeared simultaneously, and appeared again after one second of delay on the same side from where they disappeared. The stimuli were presented to the chick as moving, and continued looping for the whole duration of the test (30 minutes).
Test procedure
At the beginning of the test, each chick was taken from the incubator and transported to the test room, constantly kept in dark. Each individual was placed in the running wheel facing the long side of the apparatus, oriented with the right eye facing the right screen. In this way, the chick could simultaneously see both stimuli.
In the wheel, the chick could walk towards each stimulus, and the distance run (in centimetres) by every chick towards each side (right/left) was recorded every 10 minutes (at 600, 1200, 1800 seconds), for 3 time periods (30 minutes overall). Besides investigating the overall motor activity as number of centimetres run, we calculated a preference index for the animate stimulus as
In this index, 1 indicates a complete preference for the animate stimulus, 0 a complete preference for the non-animate stimulus and 0.5 no preference.
Statistical analysis
To analyse the spontaneous preference for the animate stimulus, we performed an ANOVA using Breed (Padovana, Polverara, Robusta) as between subjects variable and Time (minutes 0–10, 10–20, 20–30) as a within subjects variable. For the comparison between chicks tested at day post-hatching 0 (Experiment 1) and day post-hatching 2 (Experiment 2), we performed an ANOVA using Breed and Day post-hatching (p0, p2) as between subjects variables and Time (minutes 0–10, 10–20, 20–30) as within subjects variable. We used one sample t-tests to assess departures from the chance level (0.5).
Because the assumptions of parametrical tests were not satisfied, to analyse the differences between Breeds in motor activity (centimetres run) we used the Kruskall-Wallis test with Breed as independent variable. We used the Mann-Whitney test for post-hoc comparisons, including the comparison between Experiment 1 and Experiment 2.
For all tests, significance was set to p < 0.05. Statistical analyses were performed with the software R (version 3.5.2).
Results
Spontaneous preference for the animate stimulus
In Experiment 1 (test at post-hatching day 0), we observed no significant main effect of Breed (F2,85 = 0.265, p = 0.768) and Time (F2,170 = 0.279, p = 0.757) but a significant interaction Breed x Time (F4,170 = 4.227, p = 0.003), with a stable performance for Padovana and Robusta and an increasing preference for the Polverara breed, see Fig. 2a. Post-hoc t tests between breeds at each time point revealed only one significant difference, between Polverara and Padovana breed at 0–10 minutes (t55.19 = −2.042, p = 0.046). Since all data points were located above the chance level and there was no main effect of Breed, we also tested the overall performance of the population against the chance level. The overall population exhibited a significant preference for the animate stimulus (t87 = 2.917, p = 0.004, Mean = 0.590, SD = 0.289).
In Experiment 2 (test at post-hatching day 2), we observed no significant main effect of Breed (F1,49 = 0.255, p = 0.616) and Time (F2,98 = 01.458, p = 0.238) and a non-significant interaction Breed x Time (F2,98 = 5.639, p = 0.057), see Fig. 2b. The overall population exhibited no significant difference from the chance level (t50 = −0315, p = 0.754, Mean = 0.489, SD = 0.313). Comparing Experiment 1 and Experiment 2 we observed significant decline in the preference for the changes in sped in day post-hatching 2 (W = 5214, p = 0.022).
Motor activity
In Experiment 1 we observed a significant difference in motor activity between breeds (Kruskal-Wallis chi-squared = 16.071, p < 0.001; see Fig. 3a. Padovana chicks ran significantly more than Polverara (U = 667.5, p = 0.004) and Robusta (U = 174, p < 0.001) chicks, while there was no significant difference between Robusta and Polverara (U = 350.5, p = 0.388). In Experiment 2 there was no significant difference between Padovana and Polverara chicks (Kruskal-Wallis chi-squared = 2.265, p = 0.132), although Padovana had a trend for running more than Polverara (see Fig. 3b), as in Experiment 1. Overall, chicks ran more in Experiment 2 than in Experiment 1 (U = 2892, p = 0.005).
The boxplots show for each Breed the median, interquartile range, minimum, maximum and outliers for the motor activity (centimetres run) in (a) Experiment 1 and7 (b) Experiment 2.
Discussion
Studying the early responses of chicks of three different chicken breeds (Padovana, Polverara, Robusta maculata), we found that the predisposition to preferentially approach an object that moves changing speed vs. a linearly moving object is a trait shared at the species level in the first day of life (post-hatching day 0). The presence of a this shared predisposition, as well as the shared predisposition for approaching a hen-like stimulus previously documented7, suggests that early predispositions for animacy have been selected for. The preference for changes is speed, though, is not present two days later (p2). This decline is in line with other reports of human neonates that lose an initial predisposition9,10,21,22 (in the absence of previous experience) for orienting towards face-like stimuli while moving to a voluntary control of the attention (reviewed here36), as well as a peak in the predisposition to approaching a stuffed hen vs. control stimuli in chicks at around 24 hours of age8,31.
While the study of predispositions in human neonates is constrained by practical and ethical issues, it is possible to deprive domestic chicks from any visual experience until the moment of the test to understand whether the course of early visual predisposition depends on experience. Here, we kept chicks visually naïve until the moment of the test to excluded that the decline in the predisposition is due to experience. The increase of general motor activity (distance run) with time, when the predisposition was not apparent anymore, constitutes strong evidence that the decline in the predisposition is not due to a reduced interest in approaching the stimuli at post-hatching day 2. On the contrary, older chicks were more motivated in approaching the experimental stimuli, in spite of the lack of a predisposed preference for the object that changed speed. These findings suggest that the decline of the predisposition to attend to speed changes does not depend on experience but rather on developmental processes of maturation. It has been previously shown that, although chicks are a precocial species with developed motor and sensory abilities at hatching, their nervous system undergoes a maturation process in the first stages of life41, similar to that which happens in human neonates36. The presence of a transient time window associated with the preference for speed changes triggers new questions on the role of predispositions for imprinting. It remains to be clarified how early predispositions and their disappearance affect filial imprinting responses, given that at post-hatching day 2 chicks are still within the sensitive period for imprinting (that lasts at least 3 days in darkness42) when the predisposition for changes in speed declines. A possibility is that both phenomena are connected to the brief period of high sensitivity to thyroid hormones that characterize the maturation of body organs and regulate periods of heightened plasticity42,43,44.
The implications of delayed or impaired early predispositions for the physiological development have still to be clarified. Yet, a generalised pattern of absent or delayed predispositions has been already documented both in human neonates at high risk of autism23 and newborn chicks exposed to autism-inducing drugs24,25. The domestic chick appears to be an ideal model for studying early predispositions for several reasons: the study of early predispositions is easy in avian species, whose experience can be manipulated more easily from embryonic development; extensive parallels in early predispositions between neonate chicks and humans have been extensively documented, and our results confirm that early predispositions are widespread among vertebrate species and fixed in the domestic chicken; both physiological and pathological patterns appear to converge in human neonates and chicks in the first hours after birth; our findings point towards a basis of early predispositions that might not depend on visual experience, and whose neurobiological basis has already clarified several target areas in the chick brain such as the lateral septum, thee preoptic area and the nucleus taeniae of the amygdala45,46,47,48,49. Further studies should clarify the developmental mechanisms (e.g. the role of the thyroid hormones T3 and Dio242,44) that sustain the transient presence of early predispositions in neonate animals.
Data availability
All data presented in this study will be included in the Supplementary Information Files.
References
Rosa-Salva, O., Grassi, M., Lorenzi, E., Regolin, L. & Vallortigara, G. Spontaneous preference for visual cues of animacy in naïve domestic chicks: the case of speed changes. Cognition 157, 49–60 (2016).
Tremoulet, P. D. & Feldman, J. Perception of animacy from the motion of a single sound object. Perception 29, 943–951 (2000).
Di Giorgio, E., Lunghi, M., Simion, F. & Vallortigara, G. Visual cues of motion that trigger animacy perception at birth: The case of self-propulsion. Dev. Sci. 1–12, https://doi.org/10.1111/desc.12394 (2016).
Di Giorgio, E. et al. Filial responses as predisposed and learned preferences: Early attachment in chicks and babies. Behav. Brain Res. 325, 90–104 (2017).
Rosa Salva, O., Mayer, U. & Vallortigara, G. Roots of a social brain: Developmental models of emerging animacy-detection mechanisms. Neurosci. Biobehav. Rev. 50, 150–168 (2015).
Rosa-Salva, O., Regolin, L. & Vallortigara, G. Faces are special for newly hatched chicks: evidence for inborn domain-specific mechanisms underlying spontaneous preferences for face-like stimuli. Dev. Sci. 13, 565–77 (2010).
Versace, E., Fracasso, I., Baldan, G., Dalle Zotte, A. & Vallortigara, G. Newborn chicks show inherited variability in early social predispositions for hen-like stimuli. Sci. Rep. 7, 40296 (2017).
Johnson, M. H. & Horn, G. Development of filial preferences in dark-reared chicks. Anim. Behav. 36, 675–683 (1988).
Goren, C. C., Sarty, M. & Wu, P. Y. K. Visual Following and Pattern Discrimination of Face-like Stimuli by Newborn Infants. Pediatrics 56, 544–549 (1975).
Buiatti, M. et al. Cortical route for facelike pattern processing in human newborns. Proc. Natl. Acad. Sci. USA 116, 1–6 (2019).
Vallortigara, G., Regolin, L. & Marconato, F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 3, e208 (2005).
Simion, F., Regolin, L. & Bulf, H. A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. USA 105, 809–13 (2008).
Mascalzoni, E., Regolin, L. & Vallortigara, G. Innate sensitivity for self-propelled causal agency in newly hatched chicks. Proc. Natl. Acad. Sci. USA 107, 4483–5 (2010).
Miura, M. & Matsushima, T. Biological motion facilitates imprinting. Anim. Behav. 116, 171–180 (2016).
Versace, E., Martinho-Truswell, A., Kacelnik, A. & Vallortigara, G. Priors in Animal and Artificial Intelligence: Where Does Learning Begin? Trends Cogn. Sci. 22, 963–925 (2018).
Bolhuis, J. J. Early learning and the development of filial preferences in the chick. Behav. Brain Res. 98, 245–52 (1999).
Vallortigara, G. & Versace, E. Filial Imprinting. Encyclopedia of Animal Behavior, 1943–1948 (2018).
Versace, E., Schill, J., Nencini, A. M. & Vallortigara, G. Naïve chicks prefer hollow objects. PLoS One 11 (2016).
Versace, E. Precocial. In Encyclopedia of Animal Cognition and Behavior 1–3, https://doi.org/10.1007/978-3-319-47829-6_459-2 (Springer International Publishing, 2018).
Bardi, L., Regolin, L. & Simion, F. Biological motion preference in humans at birth: Role of dynamic and configural properties. Dev. Sci. 14, 353–359 (2011).
Simion, F. & Di Giorgio, E. Face perception and processing in early infancy: Inborn predispositions and developmental changes. Front. Psychol. 6, 1–11 (2015).
Morton, J. & Johnson, M. H. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychol. Rev. 98, 164–181 (1991).
Di Giorgio, E. et al. Difference in Visual Social Predispositions Between Newborns at Low- and High-risk for Autism. Sci. Rep. 6, 26395 (2016).
Lorenzi, E. et al. Embryonic, Exposure to Valproic Acid impairs Social Predispositions for Dynamic Cues of Animate Motion in Newly-Hatched Chicks. Front. Physiol., https://doi.org/10.1101/412635 (2019).
Sgadò, P., Rosa-Salva, O., Versace, E. & Vallortigara, G. Embryonic Exposure to Valproic Acid Impairs Social Predispositions of Newly-Hatched Chicks. Sci. Rep. 8, 5919 (2018).
Mascalzoni, E., Regolin, L., Vallortigara, G. & Simion, F. The cradle of causal reasoning: Newborns’ preference for physical causality. Dev. Sci. 16, 327–335 (2013).
Christensen, J. et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA - J. Am. Med. Assoc. 309, 1696–1703 (2013).
Tomson, T. et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 17, 530–538 (2018).
Clayton-Smith, J. et al. Diagnosis and management of individuals with Fetal Valproate Spectrum Disorder; a consensus statement from the European Reference Network for Congenital Malformations and Intellectual Disability. Orphanet J. Rare Dis. 14, 1–21 (2019).
Miura, M. & Matsushima, T. Preference for biological motion in domestic chicks: Sex-dependent effect of early visual experience. Anim. Cogn. 15, 871–879 (2012).
Johnson, M. H., Bolhuis, J. J. & Horn, G. Interaction between acquired preferences and developing predispositions during imprinting. Anim. Behav. 33, 1000–1006 (1985).
Reid, V. M. et al. The Human Fetus Preferentially Engages with Face-like Visual Stimuli. Curr. Biol. 27, 1825–1828.e3 (2017).
Davison, A. et al. Formin Is Associated with Left-Right Asymmetry in the Pond Snail and the Frog. Curr. Biol. 26, 654–660 (2016).
Simion, F., Valenza, E., Umiltà, C. & Dalla Barba, B. Preferential orienting to faces in newborns: a temporal-nasal asymmetry. J. Exp. Psychol. Hum. Percept. Perform. 24, 1399–405 (1998).
Johnson, M. H., Dziurawiec, S., Ellis, H. & Morton, J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition 40, 1–19 (1991).
Shultz, S., Klin, A. & Jones, W. Neonatal Transitions in Social Behavior and Their Implications for Autism. Trends Cogn. Sci. 22, 452–469 (2018).
Zanetti, E., De Marchi, M., Abbadi, M. & Cassandro, M. Variation of genetic diversity over time in local Italian chicken breeds undergoing in situ conservation. Poult. Sci. 90, 2195–201 (2011).
Zanetti, E., De Marchi, M., Dalvit, C. & Cassandro, M. Genetic characterization of local Italian breeds of chickens undergoing in situ conservation. Poult. Sci. 89, 420–7 (2010).
Versace, E., Ragusa, M. & Pallante, V. Conserved abilities of individual recognition and genetically modulated social responses in young chicks (Gallus gallus). bioRxiv: https://doi.org/10.1101/743765 (2019).
De Marchi, M., Dalvit, C., Targhetta, C. & Cassandro, M. Assessing genetic diversity in indigenous Veneto chicken breeds using AFLP markers. Anim. Genet. 37, 101–5 (2006).
Rogers, L. J. The Development of Brain and Behaviour in the Chicken. (CAD International, 1995).
Yamaguchi, S. et al. Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat. Commun. 3, 1081 (2012).
Batista, G. & Hensch, T. K. Critical Period Regulation by Thyroid Hormones: Potential Mechanisms and Sex-Specific Aspects. Front. Mol. Neurosci. 12, 1–9 (2019).
Takemura, Y. et al. Gene expression of Dio2 (thyroid hormone converting enzyme) in telencephalon is linked with predisposed biological motion preference in domestic chicks. Behav. Brain Res. 2, 25–30 (2018).
Mayer, U., Rosa-Salva, O., Loveland, J. & Vallortigara, G. Selective response of the nucleus taeniae of the amygdala to a naturalistic social stimulus in visually naive domestic chicks. Sci Rep. 9(1), 9849, https://doi.org/10.1038/s41598-019-46322-5 (2019).
Lorenzi, E., Mayer, U., Rosa-Salva, O. & Vallortigara, G. Dynamic features of animate motion activate septal and preoptic areas in visually naïve chicks (Gallus gallus). Neuroscience 354, 54–68 (2017).
Mayer, U., Rosa-Salva, O., Morbioli, F. & Vallortigara, G. The motion of a living conspecific activates septal and preoptic areas in naive domestic chicks (Gallus gallus). Eur. J. Neurosci. 45, 423–432 (2017).
Mayer, U., Rosa-Salva, O. & Vallortigara, G. First exposure to an alive conspecific activates septal and amygdaloid nuclei in visually-naïve domestic chicks (Gallus gallus). Behav. Brain Res. 71–81 (2017).
Mayer, U., Rosa-Salva, O., Lorenzi, E. & Vallortigara, G. Social predisposition dependent neuronal activity in the intermediate medial mesopallium of domestic chicks (Gallus gallus domesticus). Behav. Brain Res. 310, 93–102 (2016).
Acknowledgements
This work was supported by the European Research Council (ERC) FP7/2007-2013 Grant ERC-2011-ADG_20110406, Project 461 295517, Predisposed mechanisms for social orienting (PREMESOR) (to G.V.). Support from Fondazione Caritro Grant Biomarker Disordini dello Spettro Autistico - Autism Spectrum Disorders (DSA) [40102839] and Progetti di Ricerca di Interesse Nazionale (PRIN) 2015 (Neural Bases of Animacy Detection and Their Relevance to the Typical and Atypical Development of the Brain) to G.V. is also acknowledged.
Author information
Authors and Affiliations
Contributions
E.V. and G.V. conceived the project; E.V. designed the experiments; E.V. and M.R. conducted the experiments, E.V. and M.R. analysed the data; E.V. drafted the manuscript; all the authors contributed to the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Versace, E., Ragusa, M. & Vallortigara, G. A transient time window for early predispositions in newborn chicks. Sci Rep 9, 18767 (2019). https://doi.org/10.1038/s41598-019-55255-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55255-y
This article is cited by
-
Phenotype alteration causes long-term changes to the social strategies of victimised birds
Scientific Reports (2023)
-
Life is in motion (through a chick’s eye)
Animal Cognition (2023)
-
Light-induced asymmetries in embryonic retinal gene expression are mediated by the vascular system and extracellular matrix
Scientific Reports (2022)
-
Evolution and function of neurocognitive systems in non-human animals
Scientific Reports (2021)
-
Stability and individual variability of social attachment in imprinting
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.